Direct Effects of Vitamin D Supplementation on Ultramarathon-Induced Changes in Kynurenine Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Participants
2.3. Vitamin D Supplementation
2.4. Sample Collection, and Measurements of Vitamin D and KYN Metabolite Levels
2.5. Ultramarathon Run
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joisten, N.; Kummerhoff, F.; Koliamitra, C.; Schenk, A.; Walzik, D.; Hardt, L.; Knoop, A.; Thevis, M.; Kiesl, D.; Metcalfe, A.J.; et al. Exercise and the Kynurenine pathway: Current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc. Immunol. Rev. 2020, 26, 24–42. [Google Scholar] [PubMed]
- Kurgan, S.; Onder, C.; Balci, N.; Akdogan, N.; Altingoz, S.M.; Serdar, M.A.; Gunhan, M. Influence of periodontal inflammation on tryptophan-kynurenine metabolism: A cross-sectional study. Clin. Oral Investig. 2022, 26, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. A J. Virtual Libr. 2015, 20, 1116–1143. [Google Scholar]
- Agudelo, L.Z.; Femenia, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1alpha1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Vecsei, L.; Szalardy, L.; Fulop, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Moller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Alves, M.D.J.; Silva, D.D.S.; Pereira, E.V.M.; Pereira, D.D.; de Sousa Fernandes, M.S.; Santos, D.F.C.; Oliveira, D.P.M.; Vieira-Souza, L.M.; Aidar, F.J.; de Souza, R.F. Changes in Cytokines Concentration Following Long-Distance Running: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 838069. [Google Scholar] [CrossRef] [PubMed]
- Rudarli Nalcakan, G.; Onur, E.; Oran, A.; Varol, S.R. Comparison of sprint interval and continuous endurance training on oxidative stress and antioxidant adaptations in young healthy adults. Balt. J. Health Phys. Act. 2021, 13, 27–35. [Google Scholar] [CrossRef]
- Petrus, P.; Cervantes, M.; Samad, M.; Sato, T.; Chao, A.; Sato, S.; Koronowski, K.B.; Park, G.; Alam, Y.; Mejhert, N.; et al. Tryptophan metabolism is a physiological integrator regulating circadian rhythms. Mol. Metab. 2022, 64, 29. [Google Scholar] [CrossRef] [PubMed]
- Mieszkowski, J.; Borkowska, A.; Stankiewicz, B.; Kochanowicz, A.; Niespodzinski, B.; Surmiak, M.; Waldzinski, T.; Rola, R.; Petr, M.; Antosiewicz, J. Single High-Dose Vitamin D Supplementation as an Approach for Reducing Ultramarathon-Induced Inflammation: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 1280. [Google Scholar] [CrossRef]
- Abushamma, A.A. The Effects of Vitamin D Supplementation on Athletic Performance and Injury Prevention. J. Sport. Med. Allied Health Sci. Off. J. Ohio Athl. Train. Assoc. 2022, 8, 3. [Google Scholar] [CrossRef]
- Baciur, P.; Chmura, A.; Skowrońska, K.; Białas, F.; Kondel, K. The role of Vitamin D in the prevention and treatment of inflammatory skin diseases—Atopic dermatitis and psoriasis—Literature review. J. Educ. Health Sport 2022, 12, 1156–1163. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal vitamin D spurs serotonin: 1, 25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, M.; Shimabukuro-Vornhagen, A.; Franke, A.; Theurich, S.; Wahl, P.; Hallek, M.; Schmidt, A.; Schinkothe, T.; Mester, J.; von Bergwelt-Baildon, M.; et al. Physical exercise modulates the homeostasis of human regulatory T cells. J. Allergy Clin. Immunol. 2016, 137, 1607–1610.e1608. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Obara-Michlewska, M. The tryptophan metabolism, kynurenine pathway and oxidative stress—Implications for glioma pathobiology. Neurochem. Int. 2022, 158, 3. [Google Scholar] [CrossRef]
- Isung, J.; Granqvist, M.; Trepci, A.; Huang, J.; Schwieler, L.; Kierkegaard, M.; Erhardt, S.; Jokinen, J.; Piehl, F. Differential effects on blood and cerebrospinal fluid immune protein markers and kynurenine pathway metabolites from aerobic physical exercise in healthy subjects. Sci. Rep. 2021, 11, 1669. [Google Scholar] [CrossRef]
- Puigarnau, S.; Fernàndez, A.; Obis, E.; Jové, M.; Castañer, M.; Pamplona, R.; Portero-Otin, M.; Camerino, O. Metabolomics reveals that fittest trail runners show a better adaptation of bioenergetic pathways. J. Sci. Med. Sport 2022, 25, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef] [PubMed]
- Mudry, J.M.; Alm, P.S.; Erhardt, S.; Goiny, M.; Fritz, T.; Caidahl, K.; Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Skrobot, W.; Kaczor, K.B.; Flis, D.J.; Karnia, M.J.; Libionka, W.; Antosiewicz, J.; Kloc, W.; Kaczor, J.J. Vitamin D Deficiency Is Associated with Muscle Atrophy and Reduced Mitochondrial Function in Patients with Chronic Low Back Pain. Oxidative Med. Cell. Longev. 2019, 2019, 6835341. [Google Scholar] [CrossRef]
- Louis, E.; Raue, U.; Yang, Y.; Jemiolo, B.; Trappe, S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J. Appl. Physiol. 2007, 103, 1744–1751. [Google Scholar] [CrossRef]
- Shirey, K.A.; Jung, J.Y.; Maeder, G.S.; Carlin, J.M. Upregulation of IFN-gamma receptor expression by proinflammatory cytokines influences IDO activation in epithelial cells. J. Interf. Cytokine Res. 2006, 26, 53–62. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 1998, 23, 635–644. [Google Scholar] [CrossRef]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical exercise-induced fatigue: The role of serotonergic and dopaminergic systems. Braz. J. Med. Biol. Res. 2017, 50, e6432. [Google Scholar] [CrossRef]
- Yamamoto, T.; Newsholme, E.A. The effect of tryptophan deficiency in the brain on rat fatigue levels: A rat model of fatigue reduction. Adv. Exp. Med. Biol. 2003, 527, 527–530. [Google Scholar] [CrossRef]
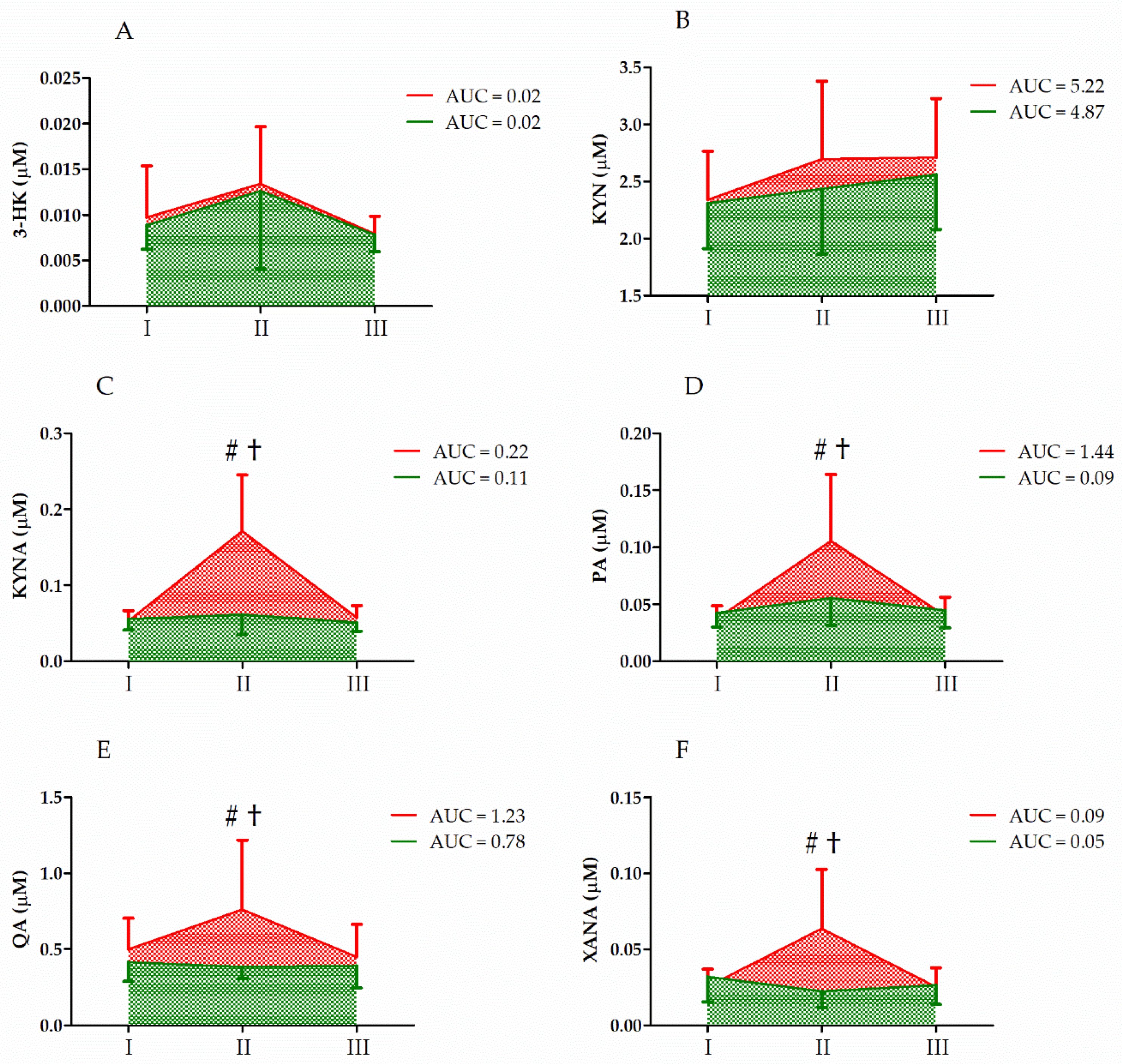
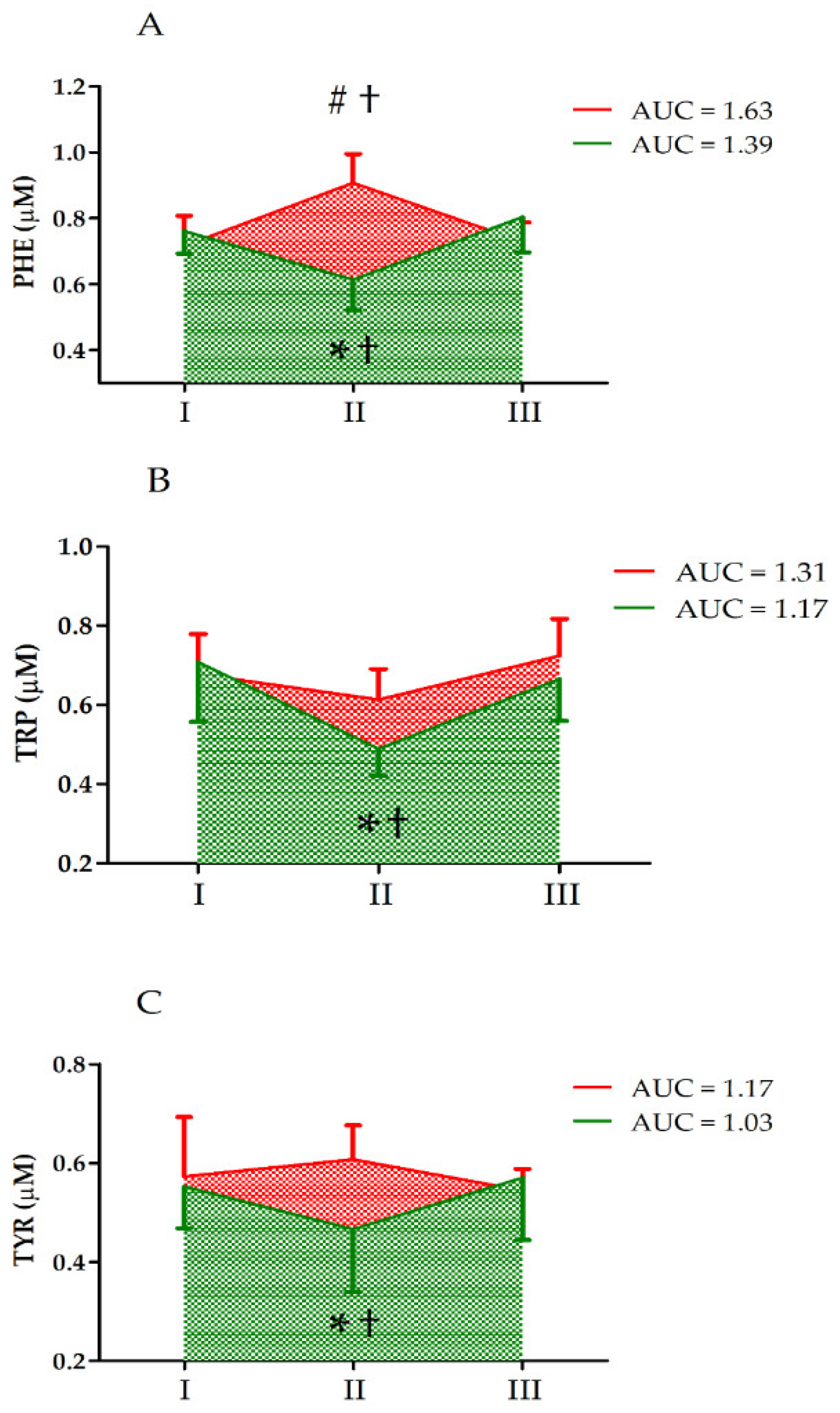
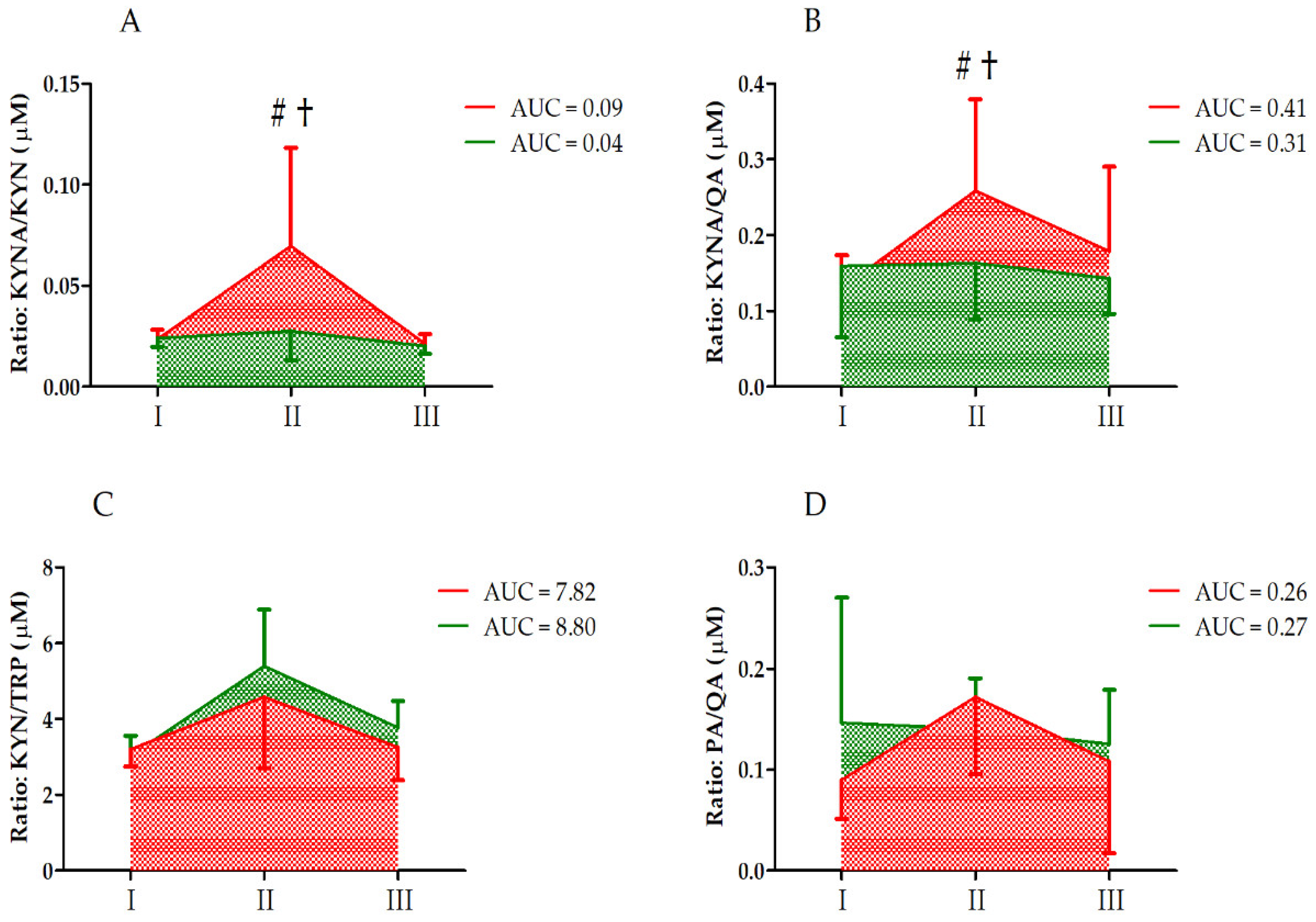
| Variable | Effect | F | Df | p | Effect Size (η2) | Post Hoc Outcome |
|---|---|---|---|---|---|---|
| 3-HK | GR UM GR × UM | 0.18 6.00 0.04 | 1, 33 2, 66 2, 66 | 0.67 <0.01 ** 0.96 | 0.01 0.16 <0.01 | II > I, III |
| KYN | GR UM GR × UM | 1.01 7.66 1.36 | 1, 33 2, 66 2, 66 | 0.32 <0.01 ** 0.27 | 0.03 0.19 0.04 | II, III > I |
| KYNA | GR UM GR × UM | 9.47 11.87 8.81 | 1, 33 2, 66 2, 66 | <0.01 ** <0.01 ** <0.01 ** | 0.23 0.27 0.22 | S < C II > I, III CII > CI, CIII CII > SII |
| PA | GR UM GR × UM | 4.99 22.39 13.31 | 1, 33 2, 66 2, 66 | 0.03 * <0.01 ** <0.01 ** | 0.13 0.41 0.25 | S < C II > I, III CII > CI, CIII CII > SII |
| QA | GR UM GR × UM | 4.46 6.50 8.51 | 1, 33 2, 66 2, 66 | <0.01 ** <0.01 ** <0.01 ** | 0.12 0.17 0.21 | S < C II > I, III CII > CI, CIII CII > SII |
| XANA | GR UM GR × UM | 4.22 4.94 10.25 | 1, 33 2, 66 2, 66 | 0.05 * 0.01 * <0.01 ** | 0.15 0.18 0.31 | S < C II > I, III CII > CI, CIII |
| Variable | Effect | F | Df | p | Effect Size (η2) | Post Hoc Outcome |
|---|---|---|---|---|---|---|
| PHE | GR UM GR × UM | 2.85 0.54 27.26 | 1, 33 2, 66 2, 66 | 0.11 0.58 <0.01 ** | 0.19 0.04 0.69 | CII > CI, CIII SII < SI, SIII CII > SII |
| TRP | GR UM GR × UM | 0.53 13.45 5.04 | 1, 33 2, 66 2, 66 | 0.47 <0.01 ** 0.01 * | 0.04 0.52 0.29 | II < I, III SII < SI, SIII CII > SII |
| TYR | GR UM GR × UM | 1.03 0.34 3.47 | 1, 33 2, 66 2, 66 | 0.32 0.71 0.04 * | 0.07 0.02 0.22 | SII < SI, SIII CII > SII |
| Variable | Effect | F | Df | p | Effect Size (η2) | Post Hoc Outcome |
|---|---|---|---|---|---|---|
| KYNA/KYN | GR UM GR × UM | 8.28 12.60 7.36 | 1, 33 2, 66 2, 66 | <0.01 ** <0.01 ** <0.01 ** | 0.21 0.28 0.19 | S < C II > I, III CII > CI, CIII CII > SII |
| KYNA/QA | GR UM GR × UM | 2.36 2.60 2.27 | 1, 33 2, 66 2, 66 | 0.13 0.08 0.11 | 0.06 0.07 0.06 | |
| KYN/TRP | GR UM GR × UM | 0.73 17.18 1.15 | 1, 33 2, 66 2, 66 | 0.40 <0.01 ** 0.33 | 0.05 0.58 0.08 | II > I, III |
| PA/QA | GR UM GR × UM | 0.24 2.15 2.40 | 1, 33 2, 66 2, 66 | 0.62 0.12 0.09 | 0.01 0.06 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieszkowski, J.; Brzezińska, P.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Reczkowicz, J.; Waldziński, T.; Kacprzak, B.; Siuba-Jarosz, N.; Petr, M.; et al. Direct Effects of Vitamin D Supplementation on Ultramarathon-Induced Changes in Kynurenine Metabolism. Nutrients 2022, 14, 4485. https://doi.org/10.3390/nu14214485
Mieszkowski J, Brzezińska P, Stankiewicz B, Kochanowicz A, Niespodziński B, Reczkowicz J, Waldziński T, Kacprzak B, Siuba-Jarosz N, Petr M, et al. Direct Effects of Vitamin D Supplementation on Ultramarathon-Induced Changes in Kynurenine Metabolism. Nutrients. 2022; 14(21):4485. https://doi.org/10.3390/nu14214485
Chicago/Turabian StyleMieszkowski, Jan, Paulina Brzezińska, Błażej Stankiewicz, Andrzej Kochanowicz, Bartłomiej Niespodziński, Joanna Reczkowicz, Tomasz Waldziński, Bartłomiej Kacprzak, Natalia Siuba-Jarosz, Miroslav Petr, and et al. 2022. "Direct Effects of Vitamin D Supplementation on Ultramarathon-Induced Changes in Kynurenine Metabolism" Nutrients 14, no. 21: 4485. https://doi.org/10.3390/nu14214485
APA StyleMieszkowski, J., Brzezińska, P., Stankiewicz, B., Kochanowicz, A., Niespodziński, B., Reczkowicz, J., Waldziński, T., Kacprzak, B., Siuba-Jarosz, N., Petr, M., & Antosiewicz, J. (2022). Direct Effects of Vitamin D Supplementation on Ultramarathon-Induced Changes in Kynurenine Metabolism. Nutrients, 14(21), 4485. https://doi.org/10.3390/nu14214485








