Development of a Caffeine Content Table for Foods, Drinks, Medications and Supplements Typically Consumed by the Brazilian Population
Abstract
1. Introduction
2. Materials and Methods
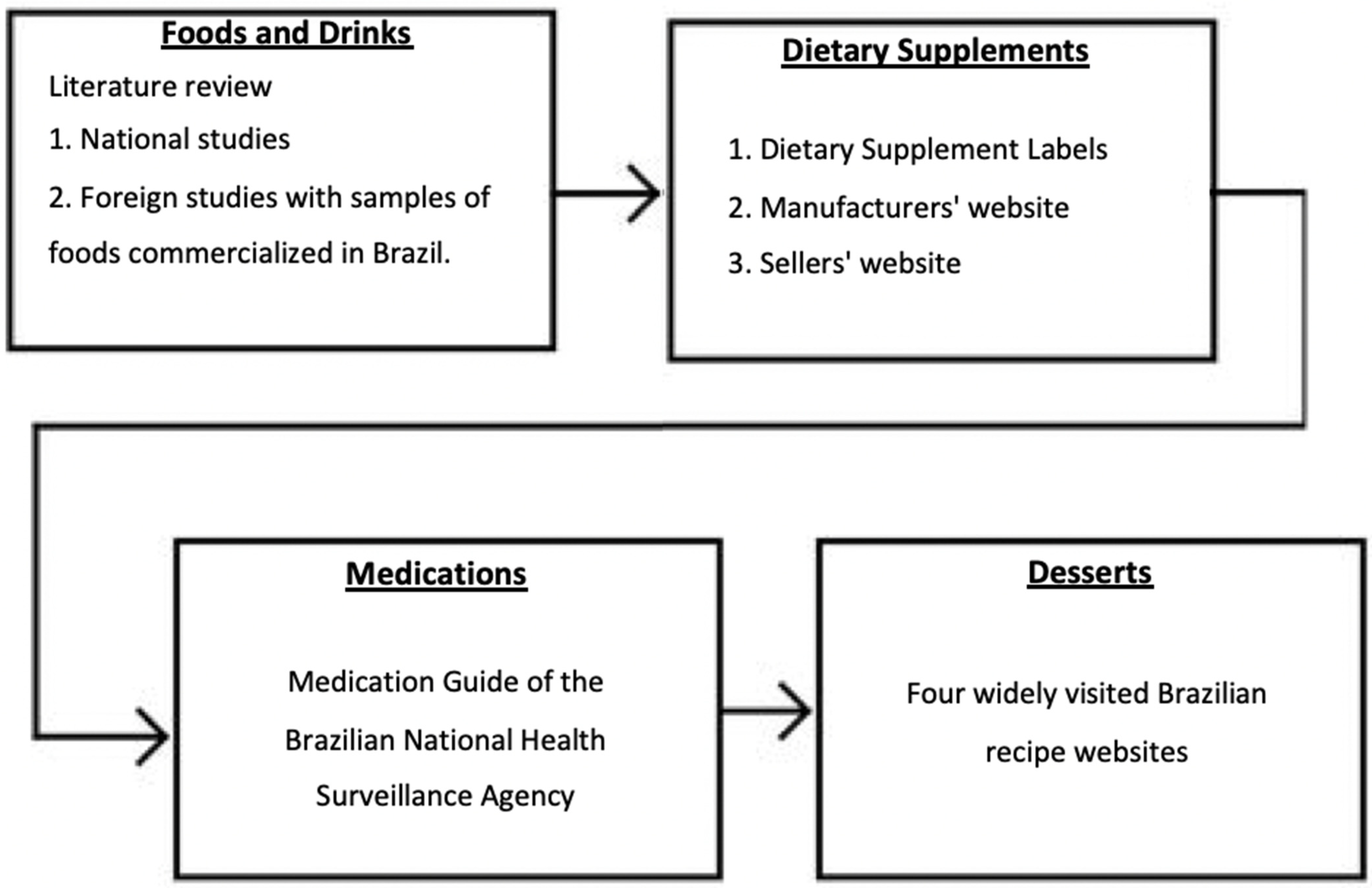
2.1. Category Development Survey
2.2. Caffeine Content in Foods, Drinks, Dietary Supplements, and Medications
Literature Search
2.3. Data Synthesis and Quantification
3. Results
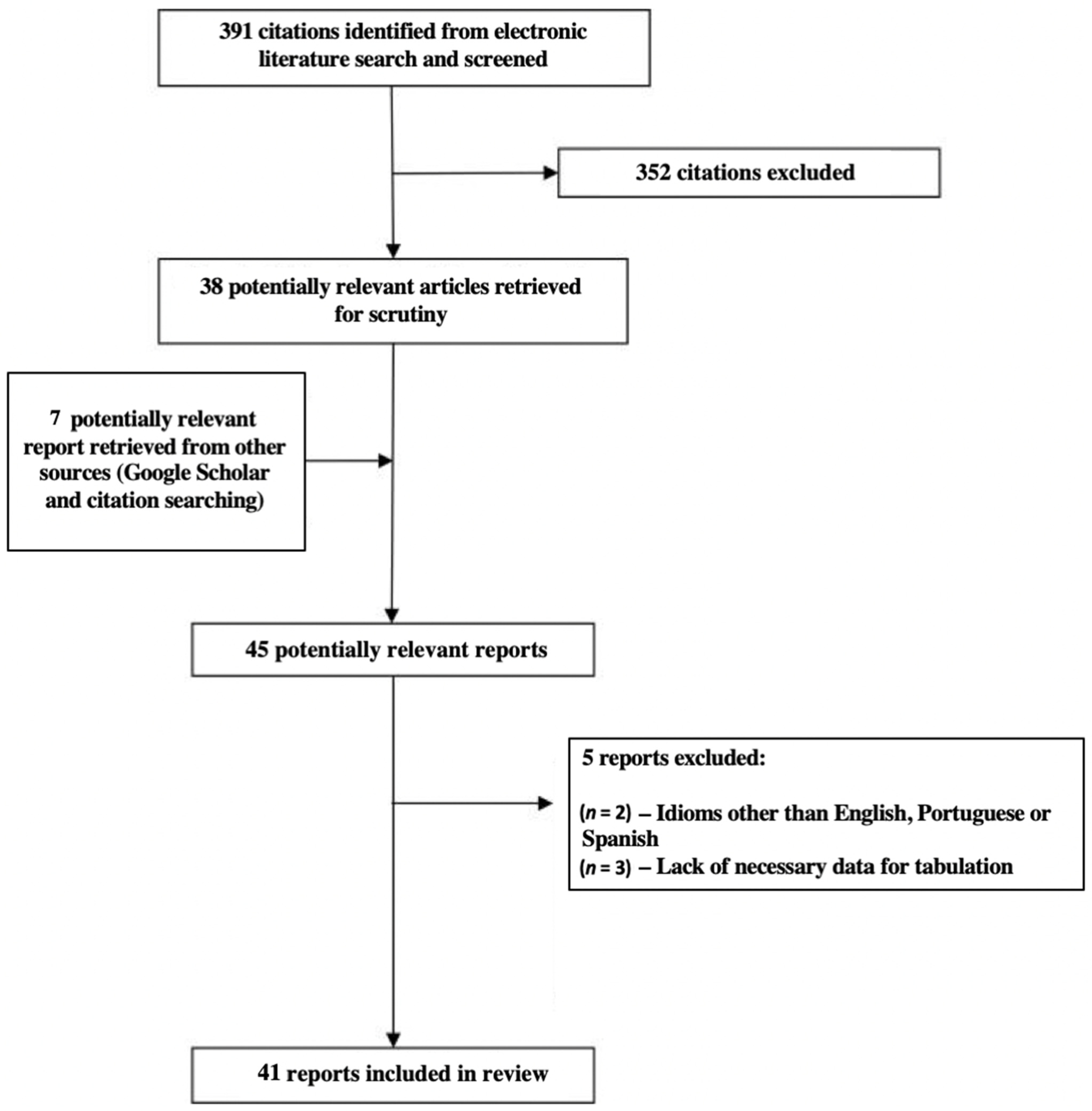
3.1. Literature Search Results
3.2. Brazilian Caffeine Content Table (BraCaffT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tfouni, S.A.V.; Camargo, M.C.R.; Vitorino, S.H.P.; Menegário, T.F.; Toledo, M.C.F. Contribuição do guaraná em pó (Paullinia cupana) como fonte de cafeína na dieta. Rev. Nutr. 2007, 20, 63–68. [Google Scholar] [CrossRef]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.R. Caffeine daily intake from dietary sources in Brazil. Food Addit. Contam. 1999, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.M.; Pereira, R.A.; Yokoo, E.M.; Levy, R.B.; Sichieri, R. Alimentos mais consumidos no Brasil: Inquérito Nacional de Alimentação 2008–2009. Rev. Saúde Pública 2013, 47, 190S–199S. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Souza, A.M.; Duffey, K.J.; Sichieri, R.; Popkin, B.M. Beverage consumption in Brazil: Results from the first National Dietary Survey. Public Health Nutr. 2014, 18, 1164–1172. [Google Scholar] [CrossRef]
- Sousa, A.G.; Da Costa, T.H.M. Usual coffee intake in Brazil: Results from the National Dietary Survey 2008–2009. Br. J. Nutr. 2015, 113, 1615–1620. [Google Scholar] [CrossRef]
- Sartori, A.G.O.; Da Silva, M.V. Caffeine in Brazil: Intake, socioeconomic and demographic determinants and major dietary sources. Nutrire 2016, 41, 11. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamento Familiar 2017–2018: Análise do Consumo Alimentar Pessoal No Brasil; IBGE: Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Ágoston, C.; Urbán, R.; Király, O.; Griffiths, M.D.; Rogers, P.J. Why Do You Drink Caffeine? The Development of the Motives for Caffeine Consumption Questionnaire (MCCQ) and Its Relationship with Gender, Age and the Types of Caffeinated Beverages. Int. J. Ment. Health Addict. 2018, 16, 981–999. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Smith, A.; Miners, J.; McNeil, J.; Proudfoot, A. Safety Aspects of Dietary Caffeine—Report from the Expert Working Group; Australia New Zealand Food Authority: Canberra, Australia; Wellington, New Zealand, 2000; pp. 20–23. [Google Scholar]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugesnholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Study of Establishment of Recommended Daily Allowance for Caffeine; MFDS: Cheongju, Korea, 2007. [Google Scholar]
- Belgium’s Superior Health Council (BSHC). The Use of Caffeine in Foodstuffs. 2012. Available online: https://www.health.belgium.be/en/use-caffeine-foodstuffs-january-2012-shc-8689 (accessed on 7 October 2022).
- EFSA—European Food Safety Authority; NDA—Nutrition and Allergies. Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102–4121. [Google Scholar] [CrossRef]
- Food Safety and Standards Authority of India. Food Safety and Standards Authority of India Proposes Regulation of Energy Drinks and Caffeine (Revised). Available online: https://fssai.gov.in/upload/uploadfiles/files/STANDARDS_OF_ENERGY_DRINKS.pdf (accessed on 7 October 2022).
- De Mejia, E.G.; Ramirez-Mares, M.V. Impact of caffeine and coffee on our health. Trends Endocrinol. Metab. 2014, 25, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberg, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 2007, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Mackus, M.; van de Loo, A.J.A.E.; Benson, S.; Scholey, A.; Verster, J.C. Consumption of caffeinated beverages and the awareness of their caffeine content among Dutch students. Appetite 2016, 103, 353–357. [Google Scholar] [CrossRef]
- McCrory, C.; White, C.M.; Bowman, C.; Fenton, N.; Reid, J.L.; Hammond, D. Perceptions and Knowledge of Caffeinated Energy Drinks: Results of Focus Groups with Canadian Youth. J. Nutr. Educ. Behav. 2017, 49, 304–311. [Google Scholar] [CrossRef]
- Khalil, M.; Antoun, J. Knowledge and consumption of caffeinated products by university students in Beirut, Lebanon. Clin. Nutr. ESPEN 2020, 37, 213–217. [Google Scholar] [CrossRef]
- McCusker, R.R.; Goldberger, B.A.; Cone, E.J. Caffeine Content of Specialty Coffees. J. Anal. Toxicol. 2003, 27, 520–522. [Google Scholar] [CrossRef]
- Heck, C.I.; De Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A Comprehensive Review on Chemistry, Health Implications and Technological Considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef] [PubMed]
- De Paula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Olechno, E.; Jakubik, A.P.; Zujko, M.E.; Socha, K. Influence of Various Factors on Caffeine Content in Coffee Brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Canela, M.D.; Bastos, D.H.M.; Pinheiro, M.M.; Ciconelli, R.M.; Ferraz, M.B.; Martini, L.A. Consumption of stimulant drinks and consequent ingestion of phenolic compounds and caffeine. Nutrire 2009, 34, 143–157. [Google Scholar]
- Barone, J.J.; Roberts, H.R. Caffeine consumption. Food Chem. Toxicol. 1996, 34, 119–129. [Google Scholar] [CrossRef]
- Irons, J.G.; Bassets, D.T.; Prendergast, C.O.; Landrum, R.E.; Heinz, A.J. Development and initial validation of the caffeine consumption questionnaire-revised. J. Caffeine Res. 2016, 6, 20–25. [Google Scholar] [CrossRef]
- Watson, E.J.; Kohler, M.; Banks, S.; Coates, A.M. Validation and reproducibility of an Australian caffeine food frequency questionnaire. Int. J. Food Sci. Nutr. 2017, 68, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Rochat, C.; Eap, C.B.; Bochud, M.; Chatelan, A. Caffeine Consumption in Switzerland: Results from the First National Nutrition Survey MenuCH. Nutrients 2020, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Brazilian National Health Surveillance Agency. Available online: https://consultas.anvisa.gov.br/#/bulario/ (accessed on 19 June 2022).
- Crispim, S.P.; Fisberg, S.P. Manual Fotográfico de Quantificação Alimentar, 1st ed.; Universidade Federal do Paraná: Curitiba, Brazil, 2017; pp. 1–147. ISBN 9788568566084. [Google Scholar]
- Landrum, R.E. College Students’ Use of Caffeine and Its Relationship to Personality. Coll. Stud. J. 1992, 26, 151–155. [Google Scholar]
- EFSA—European Food Safety Authority. The setting of nutrient profiles for foods bearing nutrition and health claims pursuant to Article 4 of the Regulation (EC) N1 1924/2006—Scientific opinion of the panel on dietetic products, nutrition and allergies. EFSA J. 2008, 644, 1–44. [Google Scholar] [CrossRef]
- Marques, A.C.; Jesus, A.A.; Giglio, B.M.; Marini, A.C.; Lobo, P.C.B.; Mota, J.F.; Pimentel, G.D. Acute Caffeinated Coffee Consumption Does Not Improve Time Trial Performance in an 800 m Run: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study. Nutrients 2018, 10, 657. [Google Scholar] [CrossRef]
- Contreras-Guillén, I.A.; Leeson, S.; Gili, R.V.; Carlino, B.; Xutuc, D.; Martins, M.C.T.; Zapata, M.E.; Segovia-Siapco, G.; Sabaté, J.; Pacheco, F.J.; et al. Development and Usability Study of an Open-Access Interviewer-Administered Automated 24 h Dietary Recall Tool in Argentina: MAR24. Front. Nutr. 2021, 8, 642387. [Google Scholar] [CrossRef] [PubMed]
- Desbrow, B.; Hughes, R.; Leveritt, M.; Scheelings, P. An examination of consumer exposure to caffeine from retail coffee outlets. Food Chem. Toxicol. 2007, 45, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, E.; Färbinger, A.; König, J. Determination of the caffeine contents of various food items within the Austrian market and validation of a caffeine assessment tool (CAT). Food Addit. Contam. Part. A 2012, 29, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.M. Methylxanthine Content in Commonly Consumed Foods in Spain and Determination of Its Intake during Consumption. Foods 2017, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Wanyika, H.N.; Gatebe, E.G.; Gitu, L.M.; Ngumba, E.K.; Maritim, C.W. Determination of caffeine content of tea and instant coffee brands in the Kenyan market. Afr. J. Food Sci. 2010, 4, 353–358. [Google Scholar]
- Nogueira, T.; Do Lago, C.L. Determination of caffeine in coffee products by dynamic complexation with 3,4-dimethoxycinnamate and separation by CZE. Electrophoresis 2007, 28, 3570–3574. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.J.; Crouzier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking. Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Redovnikovic, I.R.; Todorovic, Z.; Jankovic, G.; Dodevska, M.; Sobajic, S. Polyphenols, methylxantines and antioxidant capacity of chocolates produced in Serbia. J. Food Compos. Anal. 2015, 41, 137–143. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castle, S.M.; Siswanto, P.J.; Cifuentes-Gómez, T.; Spencer, J.P.E. Assessment of flavanol stereoisomers and caffeine and theobromine content in commercial chocolates. Food Chem. 2016, 208, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, B.R.B.; El Haddad, L.P.; De Martinis, B.S. Caffeine content of energy drinks marketed in Brazil. Drug. Test. Anal. 2022, 14, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, B.R.B.; El Haddad, L.P.; Freitas, B.T.; Marinho, P.A.; De Martinis, B.S. Pre-workout supplements marketed in Brazil: Caffeine quantification and caffeine daily intake assessment. Drug Test. Anal. 2022, 14, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.T.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; Van Dusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Steelman, S.C.; Thomas, S.L. Multi-ingredient, caffeine-containing dietary supplements: History, safety and efficacy. Clin. Ther. 2015, 37, 275–301. [Google Scholar] [CrossRef]
- Pickering, C.; Grgic, J. Caffeine and exercise: What Next? Sports Med. 2019, 49, 1007–1030. [Google Scholar] [CrossRef]
- Burns, G.; Spiller, H.A.; Pruchnicki, S.; Siegel, E.; Casavant, M.J. Acute Renal Failure and Death after Misuse of Concentrated Anhydrous Caffeine as A Pre-Work Out Supplement by Athletes. Clin. Res. 2016, 2. [Google Scholar] [CrossRef]
- Holmgren, P.; Nordén-Pettersson, L.; Ahlner, J. Caffeine fatalities—Four case reports. Forensic Sci. Int. 2004, 139, 71–73. [Google Scholar] [CrossRef]
- Rudolph, T.; Knudsen, K. A case of fatal caffeine poisoning. Acta Anaesthesiol. Scand. 2010, 54, 521–523. [Google Scholar] [CrossRef]
- Sawynok, J. Caffeine and pain. Pain 2011, 152, 726–729. [Google Scholar] [CrossRef]
- Sá, C.C.; Souza, T.M.; Favaro, E.T.; Córdoba, G.M.C.; Ramos, A.C.S.; Nobre, J.A.S. Análise comparativa entre o teor de cafeína informado no rótulo de suplementos para atletas em relação ao quantificado por cromatografia líquida de alta eficiência (CLAE). Rev. Bras. Med. Esporte 2019, 13, 265–271. [Google Scholar]
- Mirante, L.B.; da Silva Brito, M.R.; Dias, R.M.F.; Pinto, L.C. Diferenças entre o teor de cafeína identificada com a declarada nos rótulos de suplementos termogênicos e energéticos. Rev. Bras. Med. Esporte 2017, 11, 947–953. [Google Scholar]
| Foods | Caffeine (mg/100 g or mL) | SD (mg) | CV (%) | Min. (mg/100 g or mL) | Max. (mg/100 g or mL) | Serving (mg/per unit) | Common Household Serving € |
|---|---|---|---|---|---|---|---|
| Coffees ‡ | |||||||
| Brewed Coffee, Arábica | 30 | 13 | 41 | 11 | 54 | 45 | 1 coffee cup (150 mL) |
| Powder Coffee, Arábica | 1165 | 163 | 14 | 1050 | 1280 | 117 | 1 tablespoon (10 g) |
| Powder Coffee, Blend | 1444 | 283 | 20 | 1270 | 1770 | 144 | 1 tablespoon (10 g) |
| Espresso Coffee | 279 | 144 | 52 | 177 | 380 | 112 | 1 espresso cup (40 mL) |
| Capsule Coffee | 64 | 30 | 47 | 30 | 125 | 64 | 1 capsule (6 g) |
| Instant Coffee (soluble), powder | 3344 | N.A. | N.A. | N.A. | N.A. | 67 | 1 coffee spoon (2 g) |
| Instant Coffee (soluble), diluted | 36 | 14 | 39 | 20 | 45 | 54 | 1 coffee cup (150 mL) |
| Decaffeinated Brewed Coffee | 2 | N.A. | N.A. | N.A. | N.A. | 3 | 1 coffee cup (150 mL) |
| Decaffeinated Capsule Coffee, Nespresso | 3 | N.A. | N.A. | N.A. | N.A. | N.A. | 1 capsule (6 g) |
| Decaffeinated Instant Coffee | 1 | 1 | 106 | 0 | 2 | 2 | 1 coffee cup (150 mL) |
| Frappuccino Coffee, Starbucks | 25 | 2 | 9 | 23 | 26 | 88 | 1 tall cup (350 mL) |
| Cappuccino Coffee | 32 | 6 | 18 | 28 | 36 | 48 | 1 coffee cup (150 mL) |
| Brewed Coffee, Arábica with milk (80% coffee: 20% milk) | 24 | N.A. | N.A. | 9 | 43 | 48 | 1 cup (200 mL) |
| Brewed Coffee, Arábica with milk (50% coffee: 50% milk) | 15 | N.A. | N.A. | 5 | 27 | 30 | 1 cup (200 mL) |
| Brewed Coffee, Arábica with milk—Pingado (20% coffee: 80% milk) | 6 | N.A. | N.A. | 2 | 11 | 12 | 1 cup (200 mL) |
| Teas and Infusions ‡ | |||||||
| Green Tea, infused | 20 | 2 | 12 | 17 | 21 | 40 | 1 tea cup (200 mL) |
| Black Tea, infused (English breakfast; Earl Grey) | 18 | 5 | 30 | 12 | 32 | 36 | 1 tea cup (200 mL) |
| Mate Tea, infused | 5 | 2 | 47 | 3 | 6 | 10 | 1 tea cup (200 mL) |
| Yerba Mate, Chimarrão | 26 | 15 | 58 | 14 | 52 | 91 | 1 chimarrão gourd (350 mL) |
| Yerba Mate, Tereré | 24 | 12 | 45 | 17 | 36 | 84 | 1 tereré gourd (350 mL) |
| Rooibos Tea (red), infused | 16 | N.A. | N.A. | N.A. | N.A. | 32 | 1 tea cup (200 mL) |
| Iced Tea | 6 | 1 | 18 | 4 | 7 | 18 | 1 bottle (300 mL) |
| Cocoa | |||||||
| Cocoa, powder | 230 | N.A. | N.A. | N.A. | N.A. | 23 | 1 tablespoon (10 g) |
| Chocolate | |||||||
| Milk chocolate | 19 | N.A. | N.A. | N.A. | N.A. | 9 | 1/2 bar (45 g) |
| Semisweet Chocolate | 70 | N.A. | N.A. | N.A. | N.A. | 32 | 1/2 bar (45 g) |
| Dark Chocolate | 114 | N.A. | N.A. | N.A. | N.A. | 51 | 1/2 bar (45 g) |
| Cocoa-based beverages | |||||||
| Cocoa-based beverages * | 3 | 2 | 58 | 2 | 6 | 6 | 1 cup (200 mL) |
| Desserts | |||||||
| Coffee Pudding | 22 | 8 | 36 | N.A. | N.A. | 22 | 1 dessert cup (100 mL) |
| Coffee Cake | 35 | 38 | 110 | 6 | 78 | 21 | 1 slice (60 g) |
| Coffee Brigadeiro | 39 | 16 | 42 | 28 | 57 | 20 | 1/2 dessert cup (50 mL) |
| Coffee Mousse | 67 | 27 | 40 | 48 | 98 | 67 | 1 dessert cup (100 mL) |
| Tiramisu | 9 | 4 | 38 | 7 | 13 | 4 | 1 slice (45 g) |
| Chocolate Pudding | 10 | 3 | 30 | 7 | 13 | 10 | 1 dessert cup (100 mL) |
| Chocolate Mousse | 13 | 12 | 91 | 6 | 27 | 13 | 1 dessert cup (100 mL) |
| Brownie | 18 | 10 | 59 | 11 | 29 | 8 | 1 piece (45 g) |
| Chocolate Cake | 9 | 2 | 29 | 7 | 11 | 5 | 1 slice (60 g) |
| Brigadeiro with Chocolate Powder | 9 | 2 | 22 | 7 | 11 | 5 | 1/2 dessert cup (50 mL) |
| Brigadeiro with Cocoa | 9 | 3 | 35 | 5 | 11 | 5 | 1/2 dessert cup (50 mL) |
| Brigadeiro with Chocolate Milk | 3 | 0 | 4 | 3 | 3 | 2 | 1/2 dessert cup (50 mL) |
| Soft Drinks | |||||||
| Guaraná Soda | 1 | N.A. | N.A. | N.A. | N.A. | 4 | 1 can (350 mL) |
| Cola Soda | 9 | 1 | 9 | 8 | 10 | 32 | 1 can (350 mL) |
| Energy Drink | |||||||
| Energy Drink ** | 30 | 3 | 10 | 24 | 34 | 75 | 1 can (250 mL) |
| Guaraná | |||||||
| Guaraná, powder | 3044 | 1380 | 45 | 2068 | 4020 | 61 | 1 coffee spoon (2 g) |
| Dietary Supplements | |||||||
| Caffeine (anhydrous) ¥ | 200 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Energy Bar with Caffeine *** | 233 | 98 | 42 | 130 | 375 | 82 | 1 bar (35 g) |
| Energy Gel with Caffeine **** | 167 | N.A. | N.A. | N.A. | N.A. | 70 | 1 sachet (30 mL) |
| Protein Supplement with Caffeine, powder | 347 | 87 | 25 | 286 | 409 | 104 | 1 scoop (30 g) |
| Pre-workout Supplement, powder | 3620 | 1012 | 28 | 2800 | 4878 | 181 | 1 teaspoon/doser (5 g) |
| Thermogenic Supplement, powder | 900 | 196 | 22 | 670 | 1200 | 45 | 1 teaspoon/doser (5 g) |
| Thermogenic Supplement (concentrate) ¥ | 420 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Thermogenic Supplement ¥ | 154 | 33 | 21 | 125 | 200 | N.A. | N.A. |
| Medications ¥ | |||||||
| Anti-inflammatory | 50 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Myorelaxant A | 50 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Myorelaxant B | 30 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Analgesic C | 100 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Analgesic D | 65 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Analgesic E | 30 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, P.L.d.A.; Lima, A.L.C.; Saunders, B.; Reis, C.E.G. Development of a Caffeine Content Table for Foods, Drinks, Medications and Supplements Typically Consumed by the Brazilian Population. Nutrients 2022, 14, 4417. https://doi.org/10.3390/nu14204417
Rocha PLdA, Lima ALC, Saunders B, Reis CEG. Development of a Caffeine Content Table for Foods, Drinks, Medications and Supplements Typically Consumed by the Brazilian Population. Nutrients. 2022; 14(20):4417. https://doi.org/10.3390/nu14204417
Chicago/Turabian StyleRocha, Pedro Lucas de Amorim, Anna Luisa Caldeira Lima, Bryan Saunders, and Caio Eduardo Gonçalves Reis. 2022. "Development of a Caffeine Content Table for Foods, Drinks, Medications and Supplements Typically Consumed by the Brazilian Population" Nutrients 14, no. 20: 4417. https://doi.org/10.3390/nu14204417
APA StyleRocha, P. L. d. A., Lima, A. L. C., Saunders, B., & Reis, C. E. G. (2022). Development of a Caffeine Content Table for Foods, Drinks, Medications and Supplements Typically Consumed by the Brazilian Population. Nutrients, 14(20), 4417. https://doi.org/10.3390/nu14204417





