Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. Measurement of Brachial and Aortic Blood Pressure, and Vascular Function
2.4. Venipuncture and Quantification of L-Arginine
2.5. L-CIT Supplementation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chomistek, A.K.; Manson, J.E.; Stefanick, M.L.; Lu, B.; Sands-Lincoln, M.; Going, S.B.; Garcia, L.; Allison, M.A.; Sims, S.T.; LaMonte, M.J. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: Results from the Women’s Health Initiative. J. Am. Coll. Cardiol. 2013, 61, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Ong, K.L.; Tso, A.W.; Lam, K.S.; Cheung, B.M. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension 2008, 51, 1142–1148. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2013, 61, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Chan, N. Endothelial function and nitric oxide: Clinical relevance. Heart 2001, 85, 342–350. [Google Scholar] [CrossRef]
- Faulx, M.D.; Wright, A.T.; Hoit, B.D. Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am. Heart J. 2003, 145, 943–951. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Deanfield, J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994, 24, 471–476. [Google Scholar] [CrossRef]
- Klawitter, J.; Hildreth, K.L.; Christians, U.; Kohrt, W.M.; Moreau, K.L. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017, 5, e13409. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Mattei, P.; Sudano, I.; Bernini, G.; Pinto, S.; Salvetti, A. Menopause is associated with endothelial dysfunction in women. Hypertension 1996, 28, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Donnelly, T.; Lyons, D. Impaired endothelial nitric oxide bioavailability: A common link between aging, hypertension, and atherogenesis? J. Am. Geriatr. Soc. 2009, 57, 140–145. [Google Scholar] [CrossRef]
- Safar, M.E. A reappraisal of clinical research on arterial stiffness in hypertension in France. J. Am. Soc. Hypertens. 2016, 10, 482–488. [Google Scholar] [CrossRef]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; Volino-Souza, M.; Leitão, R.; Pinheiro, V.; Alvares, T.S. Is flow-mediated dilatation associated with near-infrared spectroscopy-derived magnitude of muscle O2 desaturation in healthy young and individuals at risk for cardiovascular disease? Microvasc. Res. 2020, 129, 103967. [Google Scholar] [CrossRef]
- Mackey, R.H.; Sutton-Tyrrell, K.; Vaitkevicius, P.V.; Sakkinen, P.A.; Lyles, M.F.; Spurgeon, H.A.; Lakatta, E.G.; Kuller, L.H. Correlates of aortic stiffness in elderly individuals: A subgroup of the Cardiovascular Health Study. Am. J. Hypertens. 2002, 15, 16–23. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Parise, H.; Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Vita, J.A.; Vasan, R.S.; Levy, D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart Study. Hypertension 2004, 43, 1239–1245. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Vita, J.A.; Larson, M.G.; Parise, H.; Keyes, M.J.; Warner, E.; Vasan, R.S.; Levy, D.; Benjamin, E.J. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: The Framingham Heart Study. Circulation 2005, 112, 3722–3728. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P. Endothelial dysfunction and hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef]
- Dernellis, J.; Panaretou, M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension 2005, 45, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef]
- Laurent, S.p.; Boutouyrie, P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 2007, 49, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Quyyumi, A.A.; Patel, R.S. Endothelial dysfunction and hypertension: Cause or effect? Hypertension 2010, 55, 1092–1094. [Google Scholar] [CrossRef]
- Yannoutsos, A.; Levy, B.I.; Safar, M.E.; Slama, G.; Blacher, J. Pathophysiology of hypertension: Interactions between macro and microvascular alterations through endothelial dysfunction. J. Hypertens. 2014, 32, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, L.; Yang, T.; Sun, K.; Chen, J.; Hui, R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 77–84. [Google Scholar] [CrossRef]
- Lekakis, J.P.; Papathanassiou, S.; Papaioannou, T.G.; Papamichael, C.M.; Zakopoulos, N.; Kotsis, V.; Dagre, A.G.; Stamatelopoulos, K.; Protogerou, A.; Stamatelopoulos, S.F. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. Int. J. Cardiol. 2002, 86, 317–323. [Google Scholar] [CrossRef]
- Blum, A.; Hathaway, L.; Mincemoyer, R.; Schenke, W.H.; Kirby, M.; Csako, G.; Waclawiw, M.A.; Panza, J.A.; Cannon, R.O. Effects of oral L-arginine on endothelium-dependent vasodilation and markers of inflammation in healthy postmenopausal women. J. Am. Coll. Cardiol. 2000, 35, 271–276. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Mügge, A.; Kienke, S.; Brandes, R.; Dwenger, A.; Frölich, J.C. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis 1995, 117, 273–284. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Böger, R.H.; Frölich, J.C. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef]
- Shiraseb, F.; Asbaghi, O.; Bagheri, R.; Wong, A.; Figueroa, A.; Mirzaei, K. Effect of l-Arginine Supplementation on Blood Pressure in Adults: A Systematic Review and Dose–Response Meta-analysis of Randomized Clinical Trials. Adv. Nutr. 2022, 13, 1226–1242. [Google Scholar] [CrossRef] [PubMed]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and nitrogen homeostasis: An overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.; Chapman, T.E.; Yu, Y.-M.; Ajami, A.; Burke, J.F.; Young, V.R. Dietary arginine uptake by the splanchnic region in adult humans. Am. J. Physiol.-Endocrinol. Metab. 1993, 265, E532–E539. [Google Scholar] [CrossRef]
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2016, 115, 399–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Shatanawi, A.; Momani, M.S.; Al-Aqtash, R.; Hamdan, M.H.; Gharaibeh, M.N. L-Citrulline supplementation increases plasma nitric oxide levels and reduces arginase activity in patients with Type 2 Diabetes. Front. Pharmacol. 2020, 11, 584669. [Google Scholar] [CrossRef]
- Xuan, C.; Lun, L.-M.; Zhao, J.-X.; Wang, H.-W.; Wang, J.; Ning, C.-P.; Liu, Z.; Zhang, B.-B.; He, G.-W. L-citrulline for protection of endothelial function from ADMA–induced injury in porcine coronary artery. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Ezaki, H.; Morishita, K.; Miyake, T. Effects of oral L-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immunol. Endocr. Metab. Agents Med. Chem. 2013, 13, 214–220. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects of watermelon supplementation on arterial stiffness and wave reflection amplitude in postmenopausal women. Menopause 2013, 20, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole-body vibration training and l-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2016, 41, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Alvarez-Alvarado, S.; Ormsbee, M.J.; Madzima, T.A.; Campbell, J.C.; Wong, A. Impact of L-citrulline supplementation and whole-body vibration training on arterial stiffness and leg muscle function in obese postmenopausal women with high blood pressure. Exp. Gerontol. 2015, 63, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Cifu, A.S.; Davis, A.M. Prevention, detection, evaluation, and management of high blood pressure in adults. JAMA 2017, 318, 2132–2134. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Ten Have, G.A.; Wolfe, R.R.; Deutz, N.E. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef]
- Masi, S.; Colucci, R.; Duranti, E.; Nannipieri, M.; Anselmino, M.; Ippolito, C.; Tirotta, E.; Georgiopoulos, G.; Garelli, F.; Nericcio, A. Aging modulates the influence of arginase on endothelial dysfunction in obesity. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2474–2483. [Google Scholar] [CrossRef]
- Wierzchowska-McNew, R.; Engelen, M.; Thaden, J.; Have, G.T.; Deutz, N. Obesity-and Sex-Related Disturbances in Arginine and Nitric Oxide Kinetics. Curr. Dev. Nutr. 2022, 6, 1091. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Böger, R.H.; Galland, A.; Tsikas, D.; Frölich, J.C. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharmacol. 1998, 46, 489–497. [Google Scholar] [CrossRef]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The effects of oral l-arginine and l-citrulline supplementation on blood pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-term effects of L-citrulline supplementation on arterial stiffness in middle-aged men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Cotie, L.M.; MacDonald, M.J.; Mitchell, C.J.; Prior, T.; Baker, S.K.; Phillips, S.M. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E71–E83. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.; Wolfe, R.R. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.; Fischer, S.M.; Dillon, K.N.; Kang, Y.; Martinez, M.A.; Figueroa, A. Acute Citrulline Blunts Aortic Systolic Pressure during Exercise and Sympathoactivation in Hypertensive Postmenopausal Women. Med. Sci. Sport. Exerc. 2021, 54, 761–768. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial dysfunction in hypertension: Current concepts and clinical implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- Lu, Y.; Pechlaner, R.; Cai, J.; Yuan, H.; Huang, Z.; Yang, G.; Wang, J.; Chen, Z.; Kiechl, S.; Xu, Q. Trajectories of age-related arterial stiffness in Chinese men and women. J. Am. Coll. Cardiol. 2020, 75, 870–880. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Lee, E.T.; Galloway, J.M.; Ali, T.; Umans, J.G.; Howard, B.V. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The Strong Heart Study. Hypertension 2007, 50, 197–203. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Yang, J.H.; Obokata, M.; Reddy, Y.N.; Redfield, M.M.; Lerman, A.; Borlaug, B.A. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 432–441. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 2018, 39, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Rush, C.J.; Berry, C.; Oldroyd, K.G.; Rocchiccioli, J.P.; Lindsay, M.M.; Touyz, R.M.; Murphy, C.L.; Ford, T.J.; Sidik, N.; McEntegart, M.B. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2021, 6, 1130–1143. [Google Scholar] [CrossRef]
- Sandesara, P.B.; O’Neal, W.T.; Kelli, H.M.; Topel, M.; Samman-Tahhan, A.; Sperling, L.S. Diastolic blood pressure and adverse outcomes in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial. J. Am. Heart Assoc. 2018, 7, e007475. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Cohen, J.; Hebert, P.R.; Taylor, J.O.; Hennekens, C.H. Implications of small reductions in diastolic blood pressure for primary prevention. Arch. Intern. Med. 1995, 155, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Tschakovsky, M.E.; Hughson, R.L. Rapid blunting of sympathetic vasoconstriction in the human forearm at the onset of exercise. J. Appl. Physiol. 2003, 94, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Bertinieri, G.; Turri, C.; Dell’Oro, R.; Stella, M.L.; Mancia, G. Sympathetic and reflex alterations in systo-diastolic and systolic hypertension of the elderly. J. Hypertens. 2000, 18, 587–593. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Kalfon, R. Effects of watermelon supplementation on aortic hemodynamic responses to the cold pressor test in obese hypertensive adults. Am. J. Hypertens. 2014, 27, 899–906. [Google Scholar] [CrossRef]
- Ramchandra, R.; Barrett, C.J.; Malpas, S.C. Chronic blockade of nitric oxide does not produce hypertension in baroreceptor denervated rabbits. Hypertension 2003, 42, 974–977. [Google Scholar] [CrossRef][Green Version]
- Ramchandra, R.; Barrett, C.J.; Malpas, S.C. Nitric oxide and sympathetic nerve activity in the control of blood pressure. Clin. Exp. Pharmacol. Physiol. 2005, 32, 440–446. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Qin, L.-Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral L-arginine supplementation on blood pressure: A meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Safar, M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005, 46, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Melgarejo, J.D.; Yang, W.-Y.; Thijs, L.; Li, Y.; Asayama, K.; Hansen, T.W.; Wei, F.-F.; Kikuya, M.; Ohkubo, T.; Dolan, E. Association of fatal and nonfatal cardiovascular outcomes with 24-hour mean arterial pressure. Hypertension 2021, 77, 39–48. [Google Scholar] [CrossRef]
- Kajikawa, M.; Higashi, Y. Obesity and Endothelial Function. Biomedicines 2022, 10, 1745. [Google Scholar] [CrossRef]
- Maruhashi, T.; Higashi, Y. Pathophysiological association between diabetes mellitus and endothelial dysfunction. Antioxidants 2021, 10, 1306. [Google Scholar] [CrossRef]

| Characteristics | L-CIT (n = 14) | PL (n = 11) | p |
|---|---|---|---|
| Age (years) | 61 ± 6 | 64 ± 6 | 0.23 |
| Height (meters) | 1.58 ± 0.06 | 1.57 ± 0.07 | 0.97 |
| Weight (kg) | 74 ± 10 | 75 ± 15 | 0.78 |
| Body Mass Index (kg/m2) | 29.9 ± 4.1 | 30.9 ± 5.5 | 0.60 |
| Hormone replacement therapy, n | |||
| Estrogen | 5 | 2 | |
| Progesterone | 0 | 1 | |
| Anti-hypertensive medications, n | |||
| Diuretic | 0 | 1 | |
| ACE Inhibitor | 2 | 0 | |
| CA2+ Channel Blocker | 1 | 2 | |
| ANG II Receptor Blocker | 4 | 1 | |
| Unmedicated | 7 | 6 | |
| Measure | L-CIT | PL | |||
|---|---|---|---|---|---|
| 0 Week | 4 Week | 0 Week | 4 Week | p * | |
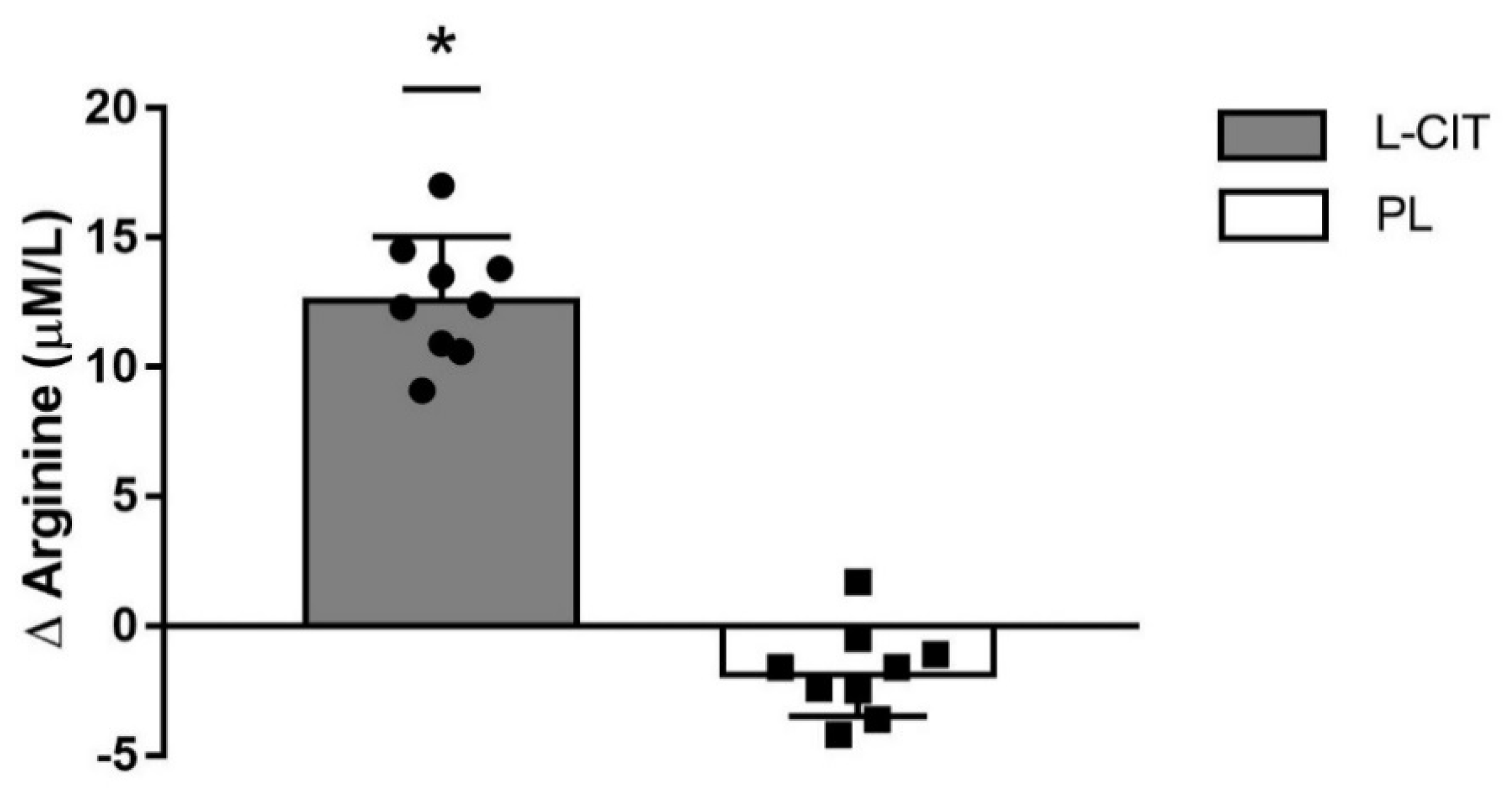
| L-ARG (µmol/L) ¥ | 81 ± 9 | 93 ± 8 †,‡ | 81 ± 3 | 79 ± 3 | 0.01 |
| Baseline brachial diameter (mm) | 3.7 ± 0.5 | 3.7 ± 0.4 | 3.6 ± 0.3 | 3.7 ± 0.4 | 0.41 |
| Peak brachial diameter (mm) | 3.9 ± 0.5 | 3.9 ± 0.4 | 3.8 ± 0.3 | 3.9 ± 0.4 | 0.52 |
| Baseline shear rate (s−1) | 122 ± 37 | 124 ± 44 | 150 ± 56 | 146 ± 53 | 0.80 |
| Peak shear rate (s−1) | 1039 ± 428 | 1082 ± 455 | 1075 ± 267 | 1100 ± 323 | 0.91 |
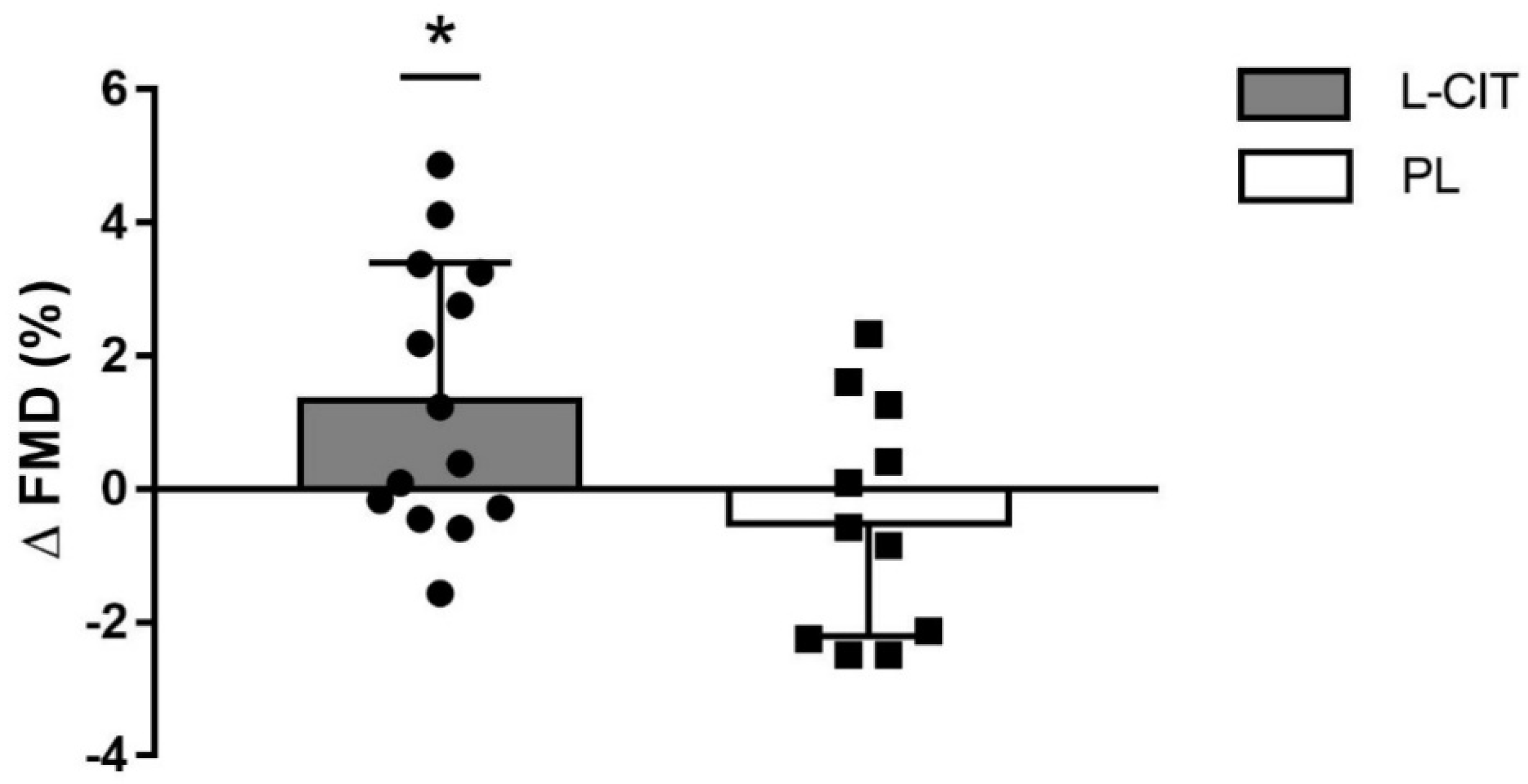
| FMD (%) | 4.8 ± 2.1 | 6.2 ± 2.2 †,* | 4.7 ± 1.8 | 4.3 ± 1.7 | 0.03 |
| cfPWV (m/s) | 9.1 ± 2 | 8.5 ± 1.1 | 9.9 ± 1.2 | 9.3 ± 1.4 | 0.83 |
| Heart Rate (beats/min) | 64 ± 5 | 63 ± 6 | 63 ± 9 | 62 ± 8 | 0.65 |
| Brachial Pressures | |||||
| SBP (mmHg) | 139 ± 17 | 135 ± 17 | 136 ± 14 | 139 ± 14 | 0.30 |
| DBP (mmHg) | 83 ± 9 | 81 ± 8 | 79 ± 11 | 79 ± 14 | 0.47 |
| MAP (mmHg) | 101 ± 11 | 99 ± 10 | 98 ± 10 | 99 ± 13 | 0.60 |
| Aortic Pressures | |||||
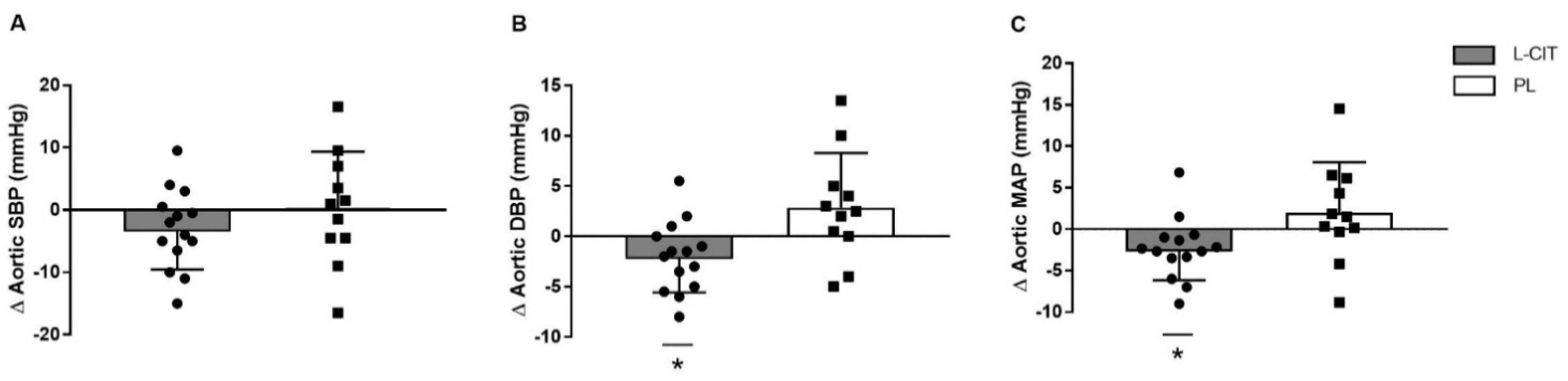
| SBP (mmHg) | 126 ± 15 | 123 ± 11 | 127 ± 12 | 127 ± 14 | 0.29 |
| DBP (mmHg) | 84 ± 8 | 82 ± 8† | 78 ± 11 | 81 ± 13 | 0.01 |
| MAP (mmHg) | 98 ± 9 | 96 ± 8† | 94 ± 10 | 96 ± 13 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharaj, A.; Fischer, S.M.; Dillon, K.N.; Kang, Y.; Martinez, M.A.; Figueroa, A. Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients 2022, 14, 4396. https://doi.org/10.3390/nu14204396
Maharaj A, Fischer SM, Dillon KN, Kang Y, Martinez MA, Figueroa A. Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients. 2022; 14(20):4396. https://doi.org/10.3390/nu14204396
Chicago/Turabian StyleMaharaj, Arun, Stephen M. Fischer, Katherine N. Dillon, Yejin Kang, Mauricio A. Martinez, and Arturo Figueroa. 2022. "Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women" Nutrients 14, no. 20: 4396. https://doi.org/10.3390/nu14204396
APA StyleMaharaj, A., Fischer, S. M., Dillon, K. N., Kang, Y., Martinez, M. A., & Figueroa, A. (2022). Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients, 14(20), 4396. https://doi.org/10.3390/nu14204396







