Current Evidence of Watermelon (Citrullus lanatus) Ingestion on Vascular Health: A Food Science and Technology Perspective
Abstract
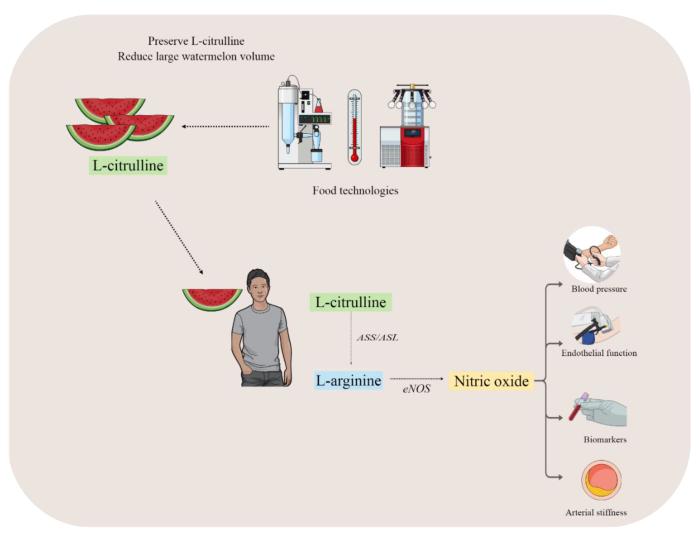
:1. Introduction
2. L-Citrulline Properties
3. Mechanism of Vascular (Dys) Function
4. Evidence of Watermelon Ingestion on Vascular Health
4.1. Endothelial Function
4.2. Arterial Stiffness and Aortic Hemodynamics
4.3. Blood Pressure
4.4. Vascular Biomarkers
5. Food Technology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects of watermelon supplementation on arterial stiffness and wave reflection amplitude in postmenopausal women. Menopause 2013, 20, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Park, E.; Zhang, L.; Edirisinghe, I.; Burton-Freeman, B.; Sandhu, A.K. Pharmacokinetic parameters of watermelon (rind, flesh, and seeds) bioactive components in human plasma: A pilot study to investigate the relationship to endothelial function. J. Agric. Food Chem. 2020, 68, 7393–7403. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Vincellette, C.M.; Losso, J.; Early, K.; Spielmann, G.; Irving, B.A.; Allerton, T.D. Supplemental Watermelon Juice Attenuates Acute Hyperglycemia-Induced Macro-and Microvascular Dysfunction in Healthy Adults. J. Nutr. 2021, 151, 3450–3458. [Google Scholar] [CrossRef]
- Ellis, A.C.; Mehta, T.; Nagabooshanam, V.A.; Dudenbostel, T.; Locher, J.L.; Crowe-White, K.M. Daily 100% watermelon juice consumption and vascular function among postmenopausal women: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2959–2968. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Kalfon, R. Effects of watermelon supplementation on aortic hemodynamic responses to the cold pressor test in obese hypertensive adults. Am. J. Hypertens. 2014, 27, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Massa, N.M.; Silva, A.S.; Toscano, L.T.; Silva, J.D.; Persuhn, D.C.; Gonçalves, M.C. Watermelon extract reduces blood pressure but does not change sympathovagal balance in prehypertensive and hypertensive subjects. Blood Press. 2016, 25, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, P.T.; Gadomski, S.J.; Zavorsky, G.S. The effect of l-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J. Sports Sci. 2015, 33, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and nitrogen homeostasis: An overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.J.; Pendleton, L.C.; Eichler, D.C. Argininosuccinate synthase: At the center of arginine metabolism. Int. J. Biochem. Mol. Biol. 2011, 2, 8–23. [Google Scholar]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Does l-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef] [Green Version]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.L.; Wehner, T.C.; Ma, G.; Perkins-Veazie, P. Citrulline and arginine content of taxa of Cucurbitaceae. Horticulturae 2019, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Akashi, K.; Mifune, Y.; Morita, K.; Ishitsuka, S.; Tsujimoto, H.; Ishihara, T. Spatial accumulation pattern of citrulline and other nutrients in immature and mature watermelon fruits. J. Sci. Food Agric. 2017, 97, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition 2007, 23, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef]
- Volino-Souza, M.; Oliveira, G.V.; Vargas, R.; Tavares, A.C.; Conte-Junior, C.A.; Alvares, T.A. Effect of microencapsulated watermelon (Citrullus lanatus) intake on plasma amino acids and glycemic response in healthy adults. Food Biosci. 2022, 46, 101553. [Google Scholar] [CrossRef]
- Assefa, A.D.; Hur, O.S.; Ro, N.Y.; Lee, J.E.; Hwang, A.J.; Kim, B.S.; Rhee, J.H.; Yi, J.Y.; Kim, J.H.; Lee, H.S.; et al. Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections. Plants 2020, 9, 1054. [Google Scholar] [CrossRef]
- Mone, P.; Pansini, A.; Jankauskas, S.S.; Varzideh, F.; Kansakar, U.; Lombardi, A.; Trimarco, V.; Frullone, S.; Santulli, G. L-Arginine Improves Cognitive Impairment in Hypertensive Frail Older Adults. Front. Cardiovasc. Med. 2022, 9, 868521. [Google Scholar] [CrossRef]
- Mone, P.; Izzo, R.; Marazzi, G.; Manzi, M.V.; Gallo, P.; Campolongo, G.; Cacciotti, L.; Tartaglia, D.; Caminiti, G.; Varzideh, F.; et al. L-Arginine Enhances the Effects of Cardiac Rehabilitation on Physical Performance: New Insights for Managing Cardiovascular Patients During the COVID-19 Pandemic. J. Pharmacol. Exp. Ther. 2022, 381, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.; Varzideh, F.; Wilson, S.; Gambardella, J.; Eacobacci, M.; Jankauskas, S.S.; Donkor, K.; Kansakar, U.; Trimarco, V.; Mone, P.; et al. l-Arginine and COVID-19: An Update. Nutrients 2021, 13, 3951. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Freeman, M.; Zhang, X.; Sandhu, A.; Edirisinghe, I. Watermelon and L-Citrulline in Cardio-Metabolic Health: Review of the Evidence 2000–2020. Curr. Atheroscler. Rep. 2021, 23, 81. [Google Scholar] [CrossRef]
- Tomiyama, H.; Yamashina, A. Non-invasive vascular function tests: Their pathophysiological background and clinical application. Circ. J. 2010, 74, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Félétou, M.; Vanhoutte, P.M. Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture). Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M.; Flammer, A.; Lüscher, T.F. Nitric oxide in hypertension. J. Clin. Hypertens. 2006, 8, 17–29. [Google Scholar] [CrossRef]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef] [Green Version]
- Feron, O.; Dessy, C.; Moniotte, S.; Desager, J.P.; Balligand, J.L. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Invest. 1999, 103, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar]
- Gielis, J.F.; Lin, J.Y.; Wingler, K.; Van Schil, P.E.; Schmidt, H.H.; Moens, A.L. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic. Biol. Med. 2011, 50, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Rafikov, R.; Fonseca, F.V.; Kumar, S.; Pardo, D.; Darragh, C.; Elms, S.; Fulton, D.; Black, S.M. eNOS activation and NO function: Structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J. Endocrinol. 2011, 210, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003, 111, 1201–1209. [Google Scholar] [CrossRef]
- Ghiadoni, L.; Versari, D.; Magagna, A.; Kardasz, I.; Plantinga, Y.; Giannarelli, C.; Taddei, S.; Salvetti, A. Ramipril dose-dependently increases nitric oxide availability in the radial artery of essential hypertension patients. J. Hypertens. 2007, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Jones, H.; Thijssen, D.; Cable, N.T.; Atkinson, G. Flow-mediated dilation and cardiovascular event prediction: Does nitric oxide matter? Hypertension 2011, 57, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 22534–22547. [Google Scholar] [CrossRef]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound assessment of flow-mediated dilation. Hypertension 2010, 55, 1075–1085. [Google Scholar] [CrossRef]
- Koivistoinen, T.; Virtanen, M.; Hutri-Kähönen, N.; Lehtimäki, T.; Jula, A.; Juonala, M.; Moilanen, L.; Aatola, H.; Hyttinen, J.; Viikari, J.S.; et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: The Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis 2012, 220, 387–393. [Google Scholar] [CrossRef]
- Blacher, J.; Asmar, R.; Djane, S.; London, G.M.; Safar, M.E. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999, 33, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Alastruey, J.; Parker, K.H.; Sherwin, S.J. Arterial pulse wave haemodynamics. In Proceedings of the 11th International Conference on Pressure Surges, Lisbon, Portugal, 24–26 October 2012; pp. 401–443. [Google Scholar]
- Munakata, M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 2014, 10, 49–57. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Tropeano, A.I.; Asmar, R.; Gautier, I.; Benetos, A.; Lacolley, P.; Laurent, S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 2006, 39, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, I.B.; Prasad, K.; Hall, I.R.; Thomas, A.; MacCallum, H.; Webb, D.J.; Frenneaux, M.P.; Cockcroft, J.R. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 2002, 39, 1005–1011. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Balasubramanian, V.; Li, J.K.-J. Augmentation index in the assessment of wave reflections and systolic loading. Comput. Biol. Med. 2019, 113, 103418. [Google Scholar] [CrossRef]
- Mynard, J.P.; Kondiboyina, A.; Kowalski, R.; Cheung, M.M.H.; Smolich, J.J. Measurement, Analysis and Interpretation of Pressure/Flow Waves in Blood Vessels. Front. Physiol. 2020, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Gatzka, C.D.; Kingwell, B.A.; Cameron, J.D.; Berry, K.L.; Liang, Y.L.; Dewar, E.M.; Reid, C.M.; Jennings, G.L.; Dart, A.M. ANBO2 investigators. Australian Comparative Outcome Trial of Angiotensin-Converting Enzyme Inhibitor- and Diuretic-Based Treatment of Hypertension in the Elderly. Gender differences in the timing of arterial wave reflection beyond differences in body height. J. Hypertens. 2001, 19, 2197–2203. [Google Scholar]
- Nichols, W.W.; Singh, B.M. Augmentation index as a measure of peripheral vascular disease state. Curr. Opin. Cardiol. 2002, 17, 543–551. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; MacCallum, H.; Rooijmans, D.F.; Murray, G.D.; Cockcroft, J.R.; McKnight, J.A.; Webb, D.J. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM 2000, 93, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; O’Rourke, M.F.; Safar, M.E.; Baou, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 2010, 31, 1865–1871. [Google Scholar] [CrossRef] [Green Version]
- Blum, A.; Hathaway, L.; Mincemoyer, R.; Schenke, W.H.; Kirby, M.; Csako, G.; Waclawiw, M.A.; Panza, J.A.; Cannon, R.O., 3rd. Effects of oral L-arginine on endothelium-dependent vasodilation and markers of inflammation in healthy postmenopausal women. J. Am. Coll. Cardiol. 2000, 35, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Sun, L.; Yang, T.; Sun, K.; Chen, J.; Hui, R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-term effects of L-citrulline supplementation on arterial stiffness in middle-aged men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Jaime, S.J.; Nagel, J.; Maharaj, A.; Fischer, S.M.; Schwab, E.; Martinson, C.; Radtke, K.; Mikat, R.P.; Figueroa, A. L-Citrulline supplementation attenuates aortic pulse pressure and wave reflection responses to cold stress in older adults. Exp. Gerontol. 2022, 159, 111685. [Google Scholar] [CrossRef]
- Lum, T.; Connolly, M.; Marx, A.; Beidler, J.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M.Y. Effects of Fresh Watermelon Consumption on the Acute Satiety Response and Cardiometabolic Risk Factors in Overweight and Obese Adults. Nutrients 2019, 11, 595. [Google Scholar] [CrossRef] [Green Version]
- Szmitko, P.E.; Wang, C.H.; Weisel, R.D.; de Almeida, J.R.; Anderson, T.J.; Verma, S. New markers of inflammation and endothelial cell activation: Part I. Circulation 2003, 108, 1917–1923. [Google Scholar] [CrossRef]
- Ciobanu, D.M.; Mircea, P.A.; Bala, C.; Rusu, A.; Vesa, Ş.; Roman, G. Intercellular adhesion molecule-1 (ICAM-1) associates with 24-hour ambulatory blood pressure variability in type 2 diabetes and controls. Cytokine 2019, 116, 134–138. [Google Scholar] [CrossRef]
- Darabi, Z.; Darand, M.; Yari, Z.; Hedayati, M.; Faghihi, A.; Agah, S.; Hekmatdoost, A. Inflammatory markers response to citrulline supplementation in patients with non-alcoholic fatty liver disease: A randomized, double blind, placebo-controlled, clinical trial. BMC Res. Notes 2019, 12, 89. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi, R.; Mobasseri, M.; Aliasgharzadeh, S.; Abbaszadeh, F.; Ebrahimi-Mameghani, M. The impact of L-citrulline supplementation on glucose homeostasis, lipid profile, and some inflammatory factors in overweight and obese patients with type 2 diabetes: A double-blind randomized placebo-controlled trial. Phytother. Res. 2021, 35, 3157–3166. [Google Scholar] [CrossRef] [PubMed]
- Barón, R.D.; Valle-Vargas, M.F.; Quintero-Gamero, G.; Quintanilla-Carvajal, M.X.; Alean, J. Encapsulation of citrulline extract from watermelon (Citrullus lanatus) by-product using spray drying. Powder Technol. 2021, 385, 455–465. [Google Scholar] [CrossRef]
- Volino-Souza, M.; de Oliveira, G.V.; do Couto Vellozo, O.; Conte-Junior, C.A.; Alvares, T.S. Impact of microencapsulated watermelon (Citrullus lanatus) and beetroot (Beta vulgaris L) on storage stability of l-citrulline and dietary nitrate. J. Food Sci. Technol. 2021, 58, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Singh, S.V. Spray drying of fruit and vegetable juices—A review. Crit. Rev. Food Sci. Nutr. 2014, 55, 701–719. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Silveira, A.C.; Tarazona, M.P. Effects of pasteurization and storage time on watermelon juice quality enriched with L-citrulline. In Proceedings of the V International Symposium on Cucurbits, Cartagena, Murcia, 22–26 June 2015; Volume 1151, pp. 267–272. [Google Scholar]
- Tarazona-Díaz, M.P.; Martínez-Sánchez, A.; Aguayo, E. Preservation of bioactive compounds and quality parameters of watermelon juice enriched with L-Citrulline through short thermal treatment. J. Food Qual. 2017, 2017, 3283054. [Google Scholar] [CrossRef] [Green Version]
- Milczarek, R.R.; Olsen, C.W.; Sedej, I. Quality of watermelon juice concentrated by forward osmosis and conventional processes. Processes 2020, 8, 1568. [Google Scholar] [CrossRef]
- Rastogi, N.K. Opportunities and Challenges in Application of Forward Osmosis in Food Processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 266–291. [Google Scholar] [CrossRef]
- El-Badry, N.; El-Waseif, M.A.; Badr, S.A.; Ali, H.E. Effect of addition watermelon rind powder on the rheological, physiochemical and sensory quality attributes of pan bread. Middle East J. Appl. Sci. 2014, 4, 1046–1051. [Google Scholar]
- Sadji, M.; Perkins-Veazie, P.M.; Ndiaye, N.F.; Ma, G.; Zongo, C.; Traore, Y.; Sall, M.D.; Traore, A. Fermentative Ability of Wheat Flour and Watermelon Puree Citrullus Lanatus (Thunb) Mixture Usable in Bread Making: A Preliminary Study. J. Nutr. Health Food Sci. 2019, 7, 1–6. [Google Scholar] [CrossRef]
| Study | Population | Intervention | Measure | Outcomes |
|---|---|---|---|---|
| Vincellette et al. [8] | n = 17 (6M/11F) Healthy young adults db, r, crossover | 500 mL of watermelon juice (795 mg of L-citrulline) for two weeks | FMD NIRS | ↔ FMD (%) ↑ FMD AUC (%. min) ↑ Blood flow AUC ↑ Peak O2 total AUC |
| Ellis et al. [9] | n = 17 Healthy postmenopausal women db, r, crossover | 360 mL of watermelon juice twice a day for four weeks (1.63 g of L-citrulline) | FMD | ↔ FMD (%) |
| Fan et al. [5] | n = 6 Overweight/obese subjects db, r, crossover | Watermelon rind (19.3 mg of L-citrulline) or watermelon flesh (10 mg of L-citrulline) or watermelon seeds (1.4 g of L-citrulline) 1, 3, 5, and 7h prior the analysis | FMD | ↔ FMD (%) |
| Cutrufello et al. [12] | n = 22 (11M/11F) Healthy adults db, r, crossover | 710 mL of watermelon juice (1 g of L-citrulline) | FMD | ↔ FMD (%) |
| Study | Population | Intervention | Technique | Outcomes |
|---|---|---|---|---|
| Figueroa et al. [64] | n = 9 (4M/5F) Middle-aged individual’s with prehypertension. db, r, crossover | Watermelon powder (2.7 g of L-citrulline) for six weeks | Tonometry | ↓ AIx (%) ↓ AIx75 (%) ↔ cfPWV (m/s) |
| Ellis et al. [9] | n = 17 Postmenopausal women db, r, crossover | 360 mL of watermelon juice (1.63 g of L-citrulline) for four weeks | Mobil-O-Graph system | ↔ calculated aPWV (m/s) |
| Figueroa et al. [2] | n = 12 postmenopausal women r, crossover | Watermelon extract (4 g of L-citrulline) for six weeks | Tonometry | ↓ baPWV (m/s)↔ aAIx (%) ↔ rAIx (%) |
| Figueroa et al. [10] | n = 13 (3M/10F) Middle-aged adults’ with hypertension, obesity, and sedentary db, r, crossover | Watermelon extract (4 g of L-citrulline) for six weeks | Tonometry rest and during cold pressor test | ↔ AIx (%) ↔ AIx75 (%) ↓ ∆AIx75 (%) |
| Study | Population | Intervention | Outcomes |
|---|---|---|---|
| Figueroa et al. [10] | n = 13 (3M/10F) Middle-aged adults with hypertension, obesity, and sedentary db, r, crossover | Watermelon extract (4 g of L-citrulline) for six weeks | ↓ aSBP (mmHg) ↓ aDBP (mmHg) ↓ bSBP (mmHg) ↓ bDBP (mmHg) |
| Figueroa et al. [2] | n = 12 Postmenopausal women r, crossover | 6 g of watermelon extract (4 g of L-citrulline) for six weeks | ↓ aSBP (mmHg) ↓ aDBP (mmHg) |
| Massa et al. [11] | n = 40 Prehypertensive and hypertensive individuals db, r, crossover | 6 g of watermelon extract (4 g of L-citrulline) for six weeks | ↓ bSBP (mmHg) ↓ bDBP (mmHg) |
| Ellis et al. [9] | Postmenopausal women (n = 17) Db, r, crossover | 360 mL of WJ twice a day for four weeks (360 mL WJ = 1.63 g of L-citrulline) | ↔ Office SBP (mmHg) ↔ Office DBP (mmHg) ↔ Office Pulse Pressure (mmHg) ↔ Office Pulse Pressure Amplification ↔ 24-Hour ABPM SBP (mmHg) ↔ 24-Hour ABPM DBP (mmHg) |
| Lum et al. [68] | n = 23 (20M/13F) Overweight and obese adultscrossover | 2 cups of fresh watermelon for four weeks | ↔ bSBP (mmHg) ↔ bDBP (mmHg) (only in men) |
| Figueroa et al. [66] | n = 9 (4M/5F) Middle-aged individuals with prehypertension. db, r, crossover | Watermelon powder (2.7 g of L-citrulline) for six weeks | ↔ bSBP (mmHg) ↔ bDBP (mmHg) ↓ aSBP (mmHg) ↔ aDBP (mmHg) |
| Study | Population | Intervention | Outcomes |
|---|---|---|---|
| Lum et al. [68] | n = 23 (20M/13F) Overweight and obese adults Crossover | 2 cups of fresh watermelon for four weeks | ↔ CRP (mg/L) |
| Ellis et al. [9] | Postmenopausal women (n = 17) db, r, crossover | 360 mL of WJ twice a day for four weeks (360 mL WJ = 1.63 g of L-citrulline) | ↔ ADMA (µM) |
| Shanely et al. [27] | n = 51 Overweight and obese postmenopausal women r, parallel | 710 mL of watermelon puree (1.88 g of L-citrulline) for six weeks | ↔ sVCAM-1 (ng/mL) ↔ sP-Selectin (ng/mL) ↔ hs-CRP (mg/L) ↔ sICAM-1 (ng/mL) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volino-Souza, M.; Oliveira, G.V.d.; Conte-Junior, C.A.; Figueroa, A.; Alvares, T.S. Current Evidence of Watermelon (Citrullus lanatus) Ingestion on Vascular Health: A Food Science and Technology Perspective. Nutrients 2022, 14, 2913. https://doi.org/10.3390/nu14142913
Volino-Souza M, Oliveira GVd, Conte-Junior CA, Figueroa A, Alvares TS. Current Evidence of Watermelon (Citrullus lanatus) Ingestion on Vascular Health: A Food Science and Technology Perspective. Nutrients. 2022; 14(14):2913. https://doi.org/10.3390/nu14142913
Chicago/Turabian StyleVolino-Souza, Mônica, Gustavo Vieira de Oliveira, Carlos Adam Conte-Junior, Arturo Figueroa, and Thiago Silveira Alvares. 2022. "Current Evidence of Watermelon (Citrullus lanatus) Ingestion on Vascular Health: A Food Science and Technology Perspective" Nutrients 14, no. 14: 2913. https://doi.org/10.3390/nu14142913
APA StyleVolino-Souza, M., Oliveira, G. V. d., Conte-Junior, C. A., Figueroa, A., & Alvares, T. S. (2022). Current Evidence of Watermelon (Citrullus lanatus) Ingestion on Vascular Health: A Food Science and Technology Perspective. Nutrients, 14(14), 2913. https://doi.org/10.3390/nu14142913








