The Impact of Higher Protein Intake in Patients with Prolonged Mechanical Ventilation
Abstract
1. Introduction
2. Materials and Methods
3. Data Collection
4. Nutrition
5. Outcomes
6. Ethical Approval
7. Statistical Analysis
8. Results
9. The Optimal Cut-Off-Value for Nutrition Parameters
10. Prognostic Factors of Weaning Success and in-Hospital Mortality
11. Renal Function
12. Discussion
13. Limitations
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cox, C.E.; Carson, S.S.; Lindquist, J.H.; Olsen, M.K.; Govert, J.A.; Chelluri, L. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: A prospective cohort study. Crit. Care 2007, 11, R9. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Carson, S.S.; Govert, J.A.; Chelluri, L.; Sanders, G.D. An economic evaluation of prolonged mechanical ventilation. Crit. Care Med. 2007, 35, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Damuth, E.; Mitchell, J.A.; Bartock, J.L.; Roberts, B.W.; Trzeciak, S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 544–553. [Google Scholar] [CrossRef]
- Dettmer, M.R.; Damuth, E.; Zarbiv, S.; Mitchell, J.A.; Bartock, J.L.; Trzeciak, S. Prognostic Factors for Long-Term Mortality in Critically Ill Patients Treated with Prolonged Mechanical Ventilation: A Systematic Review. Crit. Care Med. 2017, 45, 69–74. [Google Scholar] [CrossRef]
- Peñuelas, O.; Frutos-Vivar, F.; Fernández, C.; Anzueto, A.; Epstein, S.K.; Apezteguía, C.; González, M.; Nin, N.; Raymondos, K.; Tomicic, V.; et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am. J. Respir. Crit. Care Med. 2011, 184, 430–437. [Google Scholar] [CrossRef]
- Ghiani, A.; Paderewska, J.; Sainis, A.; Crispin, A.; Walcher, S.; Neurohr, C. Variables predicting weaning outcome in prolonged mechanically ventilated tracheotomized patients: A retrospective study. J. Intensive Care 2020, 8, 19. [Google Scholar] [CrossRef]
- Baptistella, A.R.; Sarmento, F.J.; da Silva, K.R.; Baptistella, S.F.; Taglietti, M.; Zuquello, R.A.; Nunes Filho, J.R. Predictive factors of weaning from mechanical ventilation and extubation outcome: A systematic review. J. Crit. Care 2018, 48, 56–62. [Google Scholar] [CrossRef]
- Leonov, Y.; Kisil, I.; Perlov, A.; Stoichev, V.; Ginzburg, Y.; Nazarenko, A.; Gimelfarb, Y. Predictors of successful weaning in patients requiring extremely prolonged mechanical ventilation. Adv. Respir. Med. 2020, 88, 477–484. [Google Scholar] [CrossRef]
- Sharma, K.; Mogensen, K.M.; Robinson, M.K. Pathophysiology of Critical Illness and Role of Nutrition. Nutr. Clin. Pract. 2019, 34, 12–22. [Google Scholar] [CrossRef]
- Van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E. Nutrition therapy and critical illness: Practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit. Care 2019, 23, 368. [Google Scholar] [CrossRef]
- Reignier, J.; Boisramé-Helms, J.; Brisard, L.; Lascarrou, J.B.; Ait Hssain, A.; Anguel, N.; Argaud, L.; Asehnoune, K.; Asfar, P.; Bellec, F.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Weijs, P.J.; Looijaard, W.G.; Beishuizen, A.; Girbes, A.R.; Oudemans-van Straaten, H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit. Care 2014, 18, 701. [Google Scholar] [CrossRef]
- Koekkoek, W.; van Setten, C.H.C.; Olthof, L.E.; Kars, J.; van Zanten, A.R.H. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: The PROTINVENT retrospective study. Clin. Nutr. 2019, 38, 883–890. [Google Scholar] [CrossRef]
- Kao, K.C.; Hu, H.C.; Fu, J.Y.; Hsieh, M.J.; Wu, Y.K.; Chen, Y.C.; Chen, Y.H.; Huang, C.C.; Yang, C.T.; Tsai, Y.H. Renal replacement therapy in prolonged mechanical ventilation patients with renal failure in Taiwan. J. Crit. Care 2011, 26, 600–607. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lee, C.S.; Chiu, T.H.; Chen, H.H.; Chan, L.Y.; Chang, C.J.; Chang, S.C.; Hu, H.C.; Kao, K.C.; Chen, N.H.; et al. Clinical outcomes and prognostic factors for prolonged mechanical ventilation in patients with acute stroke and brain trauma. J. Formos. Med. Assoc. 2022, 121 Pt 1, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schutz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Allingstrup, M.J.; Esmailzadeh, N.; Wilkens Knudsen, A.; Espersen, K.; Hartvig Jensen, T.; Wiis, J.; Perner, A.; Kondrup, J. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin. Nutr. 2012, 31, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Nicolo, M.; Heyland, D.K.; Chittams, J.; Sammarco, T.; Compher, C. Clinical Outcomes Related to Protein Delivery in a Critically Ill Population: A Multicenter, Multinational Observation Study. JPEN J. Parenter. Enter. Nutr. 2016, 40, 45–51. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, H.S.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; et al. The influence of protein provision in the early phase of intensive care on clinical outcomes for critically ill patients on mechanical ventilation. Asia Pac. J. Clin. Nutr. 2017, 26, 234–240. [Google Scholar] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Matejovic, M.; Huet, O.; Dams, K.; Elke, G.; Vaquerizo Alonso, C.; Csomos, A.; Krzych, Ł.J.; Tetamo, R.; Puthucheary, Z.; Rooyackers, O.; et al. Medical nutrition therapy and clinical outcomes in critically ill adults: A European multinational, prospective observational cohort study (EuroPN). Crit. Care 2022, 26, 143. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Supinski, G.S.; Morris, P.E.; Dhar, S.; Callahan, L.A. Diaphragm Dysfunction in Critical Illness. Chest 2018, 153, 1040–1051. [Google Scholar] [CrossRef]
- Braunschweig, C.A.; Sheean, P.M.; Peterson, S.J.; Gomez Perez, S.; Freels, S.; Lateef, O.; Gurka, D.; Fantuzzi, G. Intensive nutrition in acute lung injury: A clinical trial (INTACT). JPEN J. Parenter. Enter. Nutr. 2015, 39, 13–20. [Google Scholar] [CrossRef]
- Heyland, D.K.; Cahill, N.; Day, A.G. Optimal amount of calories for critically ill patients: Depends on how you slice the cake! Crit. Care Med. 2011, 39, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Crosara, I.C.; Mélot, C.; Preiser, J.C. A J-shaped relationship between caloric intake and survival in critically ill patients. Ann. Intensive Care 2015, 5, 37. [Google Scholar] [CrossRef][Green Version]
- Marik, P.E.; Hooper, M.H. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 316–323. [Google Scholar] [CrossRef]
- Peterson, S.J.; Lateef, O.B.; Freels, S.; McKeever, L.; Fantuzzi, G.; Braunschweig, C.A. Early Exposure to Recommended Calorie Delivery in the Intensive Care Unit Is Associated with Increased Mortality in Patients with Acute Respiratory Distress Syndrome. JPEN J. Parenter. Enter. Nutr. 2018, 42, 739–747. [Google Scholar] [CrossRef]
- Hartl, W.H.; Bender, A.; Scheipl, F.; Kuppinger, D.; Day, A.G.; Küchenhoff, H. Calorie intake and short-term survival of critically ill patients. Clin. Nutr. 2019, 38, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Abrahamse, E.; Ludwig, T.; Boirie, Y.; Verlaan, S. Protein type and caloric density of protein supplements modulate postprandial amino acid profile through changes in gastrointestinal behaviour: A randomized trial. Clin. Nutr. 2016, 35, 48–58. [Google Scholar] [CrossRef][Green Version]
- Brenner, B.M.; Lawler, E.V.; Mackenzie, H.S. The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int. 1996, 49, 1774–1777. [Google Scholar] [CrossRef]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Sabatino, A.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; De Waele, E.; Jonckheer, J.; Singer, P.; Cuerda, C. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 2021, 40, 1644–1668. [Google Scholar] [CrossRef]


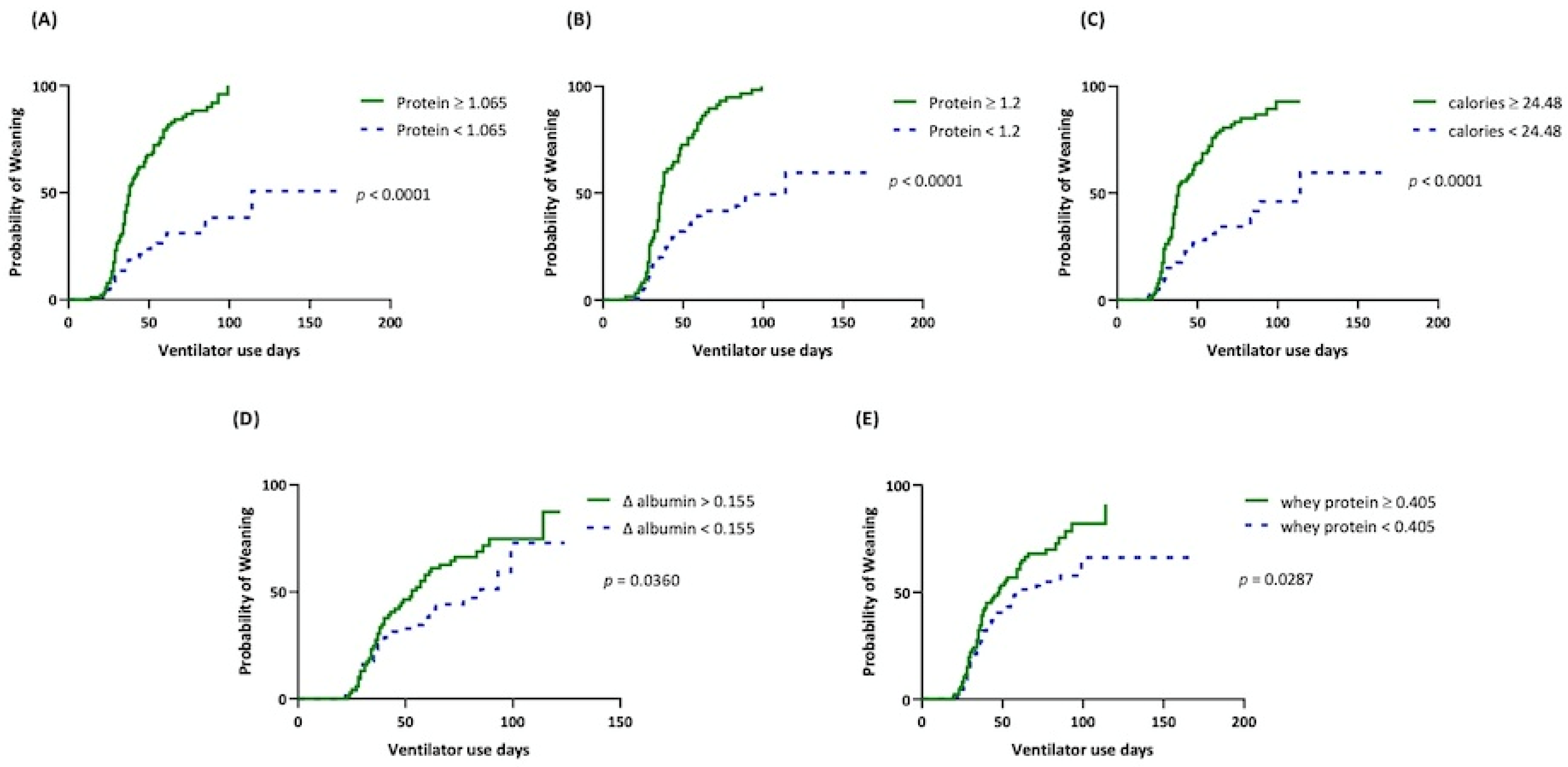


| Characteristic | Successful, n = 109 | Fail, n = 63 | Total, n = 172 | p-Value |
|---|---|---|---|---|
| Male, n (%) | 52 (47.7%) | 32 (50.8%) | 84 (49%) | 0.7525 |
| Age, years, mean ± SD | 74.02 ± 12.71 | 74.63 ± 11.79 | 74.24 ± 12.35 | 0.7535 |
| BMI, kg/m2, means ± SD | 22.96 ± 4.211 | 24.40 ± 4.485 | 23.49 ± 4.356 | 0.0366 |
| APACHE II score, mean ± SD | 16.25 ± 4.516 | 17.65 ± 4.78 | 16.76 ± 4.65 | 0.0563 |
| ICU length of stay, mean ± SD | 27.63 ± 10.26 | 31.71 ± 13.14 | 29.13 ± 11.53 | 0.0249 |
| ICU indication | ||||
| Pneumonia with respiratory failure | 53 (48.6%) | 32 (50.8%) | 83 (%) | 0.8744 |
| Septic shock | 4 (3.7%) | 3 (4.8%) | 7 (%) | 0.7078 |
| Myocardial infarction | 6 (5.5%) | 1 (1.6%) | 7 (%) | 0.4249 |
| Cardiac arrest | 7 (6.42%) | 9 (14.3%) | 16 (%) | 0.1052 |
| Acute decompensate heart failure | 4 (3.7%) | 2 (3.2%) | 6 (%) | >0.9999 |
| Intracranial hemorrhage | 8 (7.3%) | 1 (1.6%) | 9 (%) | 0.1573 |
| Massive GI bleeding | 2 (1.8%) | 0 (0%) | 2 (%) | 0.5333 |
| Ischemic stroke | 13 (11.9%) | 5 (7.9%) | 18 (%) | 0.4529 |
| Status epilepsy | 5 (4.6%) | 2 (3.2%) | 7 (%) | >0.9999 |
| Cardiac surgery | 1 (0.9%) | 4 (6.4%) | 5 (%) | 0.0608 |
| Abdominal surgery | 4 (3.7%) | 1 (1.6%) | 5 (%) | 0.6534 |
| Head and neck surgery | 1 (0.92%) | 0 (0%) | 1 (%) | >0.9999 |
| Thoracic surgery | 1 (0.92%) | 0 (0%) | 1 (%) | >0.9999 |
| Spinal cord injury | 0 (0%) | 3 (4.8%) | 3 (%) | 0.0477 |
| Length of mechanical ventilation before RCC admission, mean ± SD | 27.18 ± 9.81 | 31.59 ± 13.11 | 28.8 ±11.29 | 0.0133 |
| Tracheostomy, n (%) | 36 (33%) | 41 (66.1%) | 77 (44.8%) | <0.0001 |
| Underlying disease | ||||
| Hypertension, n (%) | 62 (56.9%) | 27 (42.9%) | 89 (51.8%) | 0.0836 |
| Diabetes mellitus, n (%) | 50 (45.9%) | 25 (39.7%) | 75 (43.6%) | 0.5235 |
| Cardiovascular disease, n (%) | 54 (49.5%) | 32 (50.8%) | 86 (50%) | >0.9999 |
| Chronic lung disease, n (%) | 18 (16.5%) | 13 (20.6%) | 31 (18%) | 0.5399 |
| Chronic kidney disease, n (%) | 35 (32.1%) | 20 (31.8%) | 55 (32%) | >0.9999 |
| Liver cirrhosis, n (%) | 6 (5.5%) | 3 (4.8%) | 9 (5.2%) | >0.9999 |
| Cerebrovascular disease, n (%) | 51 (46.8%) | 16 (25.4%) | 67 (39%) | 0.0060 |
| Malignancy, n (%) | 24 (22%) | 20 (31.8%) | 44 (40.4%) | 0.2040 |
| Death, n (%) | 20 (18.4%) | 19 (30.2%) | 39 (22.7%) | 0.0898 |
| Death at ward, n (%) | 20 (18.4%) | 0 (0%) | ||
| Death at RCC, n (%) | 0 (0%) | 19 (30.2%) | ||
| Nutritional parameters | ||||
| Daily protein intake, g/kg/day, mean ± SD | 1.29 ± 0.29 | 0.84 ± 0.23 | 1.13 ± 0.34 | <0.0001 |
| Daily calories intake, kcal/kg/day, mean ± SD | 28.04 ± 6.18 | 19.9 ± 5.83 | 25.06 ± 7.21 | <0.0001 |
| Δ albumin, g/dL, mean ± SD | 0.25 ± 0.35 | 0.002 ± 0.48 | 0.15 ± 0.43 | 0.0004 |
| Daily whey protein intake, g/kg/day, mean ± SD | 0.58 ± 0.54 | 0.37 ± 0.36 | 0.5 ± 0.49 | 0.0075 |
| Factor | Univariate Regression Analysis | Multivariate Regression Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Daily protein intake | ||||||
| ≥1.065 g/kg/day | 18.22 | 7.95–41.74 | <0.001 | 4.97 | 1.14–21.79 | 0.033 |
| ≥1.2 g/kg/day | 75.92 | 10.16–567.41 | <0.001 | 89.07 | 6.8–1166.41 | 0.001 |
| Daily caloric intake | ||||||
| ≥24.48 kcal/kg/day | 10.13 | 4.83–21.25 | <0.001 | 3.78 | 0.88–16.21 | 0.073 |
| Δ albumin | ||||||
| ≥0.155 g/dL | 2.88 | 1.44–5.76 | 0.003 | 3.68 | 1.16–11.69 | 0.027 |
| Daily whey protein intake | ||||||
| ≥0.405 g/kg/day | 2.23 | 1.18–4.20 | 0.014 | 2.66 | 0.82–8.7 | 0.105 |
| BMI | 0.93 | 0.86–1.00 | 0.039 | 1.235 | 1.04–1.46 | 0.014 |
| Cerebrovascular disease | 2.58 | 1.31–5.10 | 0.006 | 2.43 | 0.73–8.06 | 0.146 |
| ICU length of stay | 0.97 | 0.94–1 | 0.03 | 1.042 | 0.81–1.34 | 0.75 |
| Length of mechanical ventilation before RCC admission | 0.97 | 0.94–0.99 | 0.018 | 1 | 0.77–1.31 | 0.984 |
| Tracheostomy | 0.27 | 0.14–0.51 | 0.000 | 0.08 | 0.02–0.29 | 0.000 |
| Factor | Univariate Regression Analysis | Multivariate Regression Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Daily protein intake | ||||||
| ≥1.065 g/kg/day | 0.62 | 0.3–1.27 | 0.187 | 0.87 | 0.24–3.24 | 0.839 |
| ≥1.2 g/kg/day | 0.65 | 0.3–1.42 | 0.283 | 0.27 | 0.07–1.15 | 0.077 |
| Daily caloric intake | ||||||
| ≥24.48 kcal/kg/day | 0.68 | 0.33–1.40 | 0.300 | 0.69 | 0.19–2.48 | 0.573 |
| Δ albumin | ||||||
| ≥0.155 g/dL | 0.86 | 0.40–1.89 | 0.714 | 1.08 | 0.45–2.61 | 0.857 |
| Daily whey protein intake | ||||||
| ≥0.405 g/kg/day | 0.70 | 0.34–1.43 | 0.322 | 0.93 | 0.39–2.2 | 0.862 |
| BMI | 0.94 | 0.86–1.02 | 0.13 | 0.85 | 0.74–0.97 | 0.016 |
| Cerebrovascular disease | 1.28 | 0.62–2.65 | 0.5 | 1.42 | 0.58–3.5 | 0.444 |
| ICU length of stay | 1 | 0.97–1.03 | 0.949 | 1.07 | 0.9–1.27 | 0.469 |
| Length of mechanical ventilation before RCC admission | 1 | 0.97–1.03 | 0.999 | 0.936 | 0.78–1.13 | 0.481 |
| Tracheostomy | 0.54 | 0.26–1.14 | 0.105 | 0.34 | 0.14–0.84 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-W.; Lin, H.-C.; Chou, Y.-F.; Lin, T.-Y.; Lo, C.-Y.; Huang, H.-Y.; Fang, Y.-F.; Hsieh, M.-H.; Lin, S.-M.; Lo, Y.-L.; et al. The Impact of Higher Protein Intake in Patients with Prolonged Mechanical Ventilation. Nutrients 2022, 14, 4395. https://doi.org/10.3390/nu14204395
Huang S-W, Lin H-C, Chou Y-F, Lin T-Y, Lo C-Y, Huang H-Y, Fang Y-F, Hsieh M-H, Lin S-M, Lo Y-L, et al. The Impact of Higher Protein Intake in Patients with Prolonged Mechanical Ventilation. Nutrients. 2022; 14(20):4395. https://doi.org/10.3390/nu14204395
Chicago/Turabian StyleHuang, Shih-Wei, Horng-Chyuan Lin, Yu-Feng Chou, Ting-Yu Lin, Chun-Yu Lo, Hung-Yu Huang, Yueh-Fu Fang, Meng-Heng Hsieh, Shu-Min Lin, Yu-Lun Lo, and et al. 2022. "The Impact of Higher Protein Intake in Patients with Prolonged Mechanical Ventilation" Nutrients 14, no. 20: 4395. https://doi.org/10.3390/nu14204395
APA StyleHuang, S.-W., Lin, H.-C., Chou, Y.-F., Lin, T.-Y., Lo, C.-Y., Huang, H.-Y., Fang, Y.-F., Hsieh, M.-H., Lin, S.-M., Lo, Y.-L., Hsieh, M.-J., Kao, K.-C., Lin, C.-Y., & Huang, C.-C. (2022). The Impact of Higher Protein Intake in Patients with Prolonged Mechanical Ventilation. Nutrients, 14(20), 4395. https://doi.org/10.3390/nu14204395






