Comparison of the Effectiveness of Low Carbohydrate Versus Low Fat Diets, in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Outcomes Evaluated
2.4. Data Extraction and Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Identification and Selection
3.2. Study Characteristics and Risk of Bias
3.3. Measures of Glycaemia
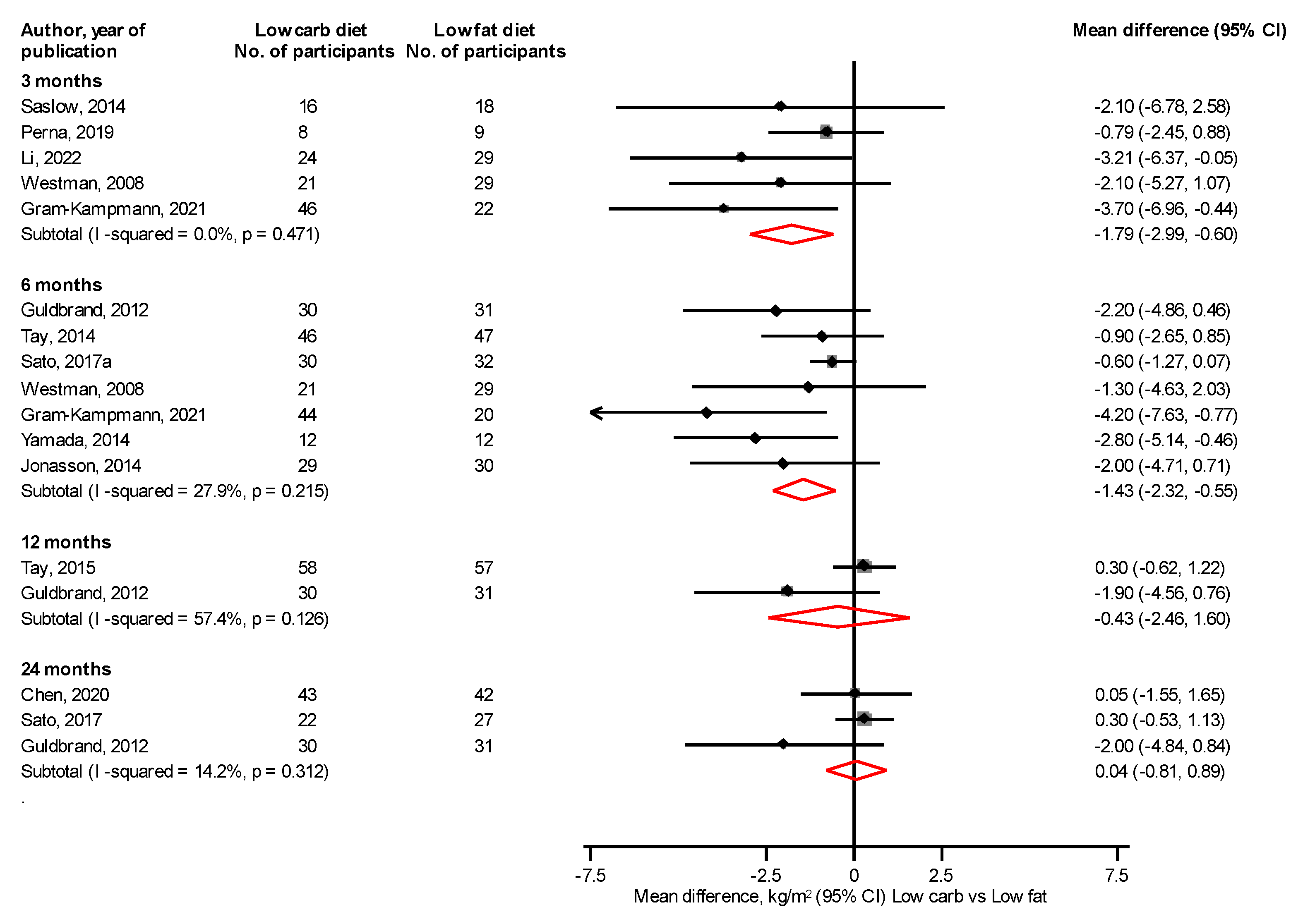
3.4. Body Composition
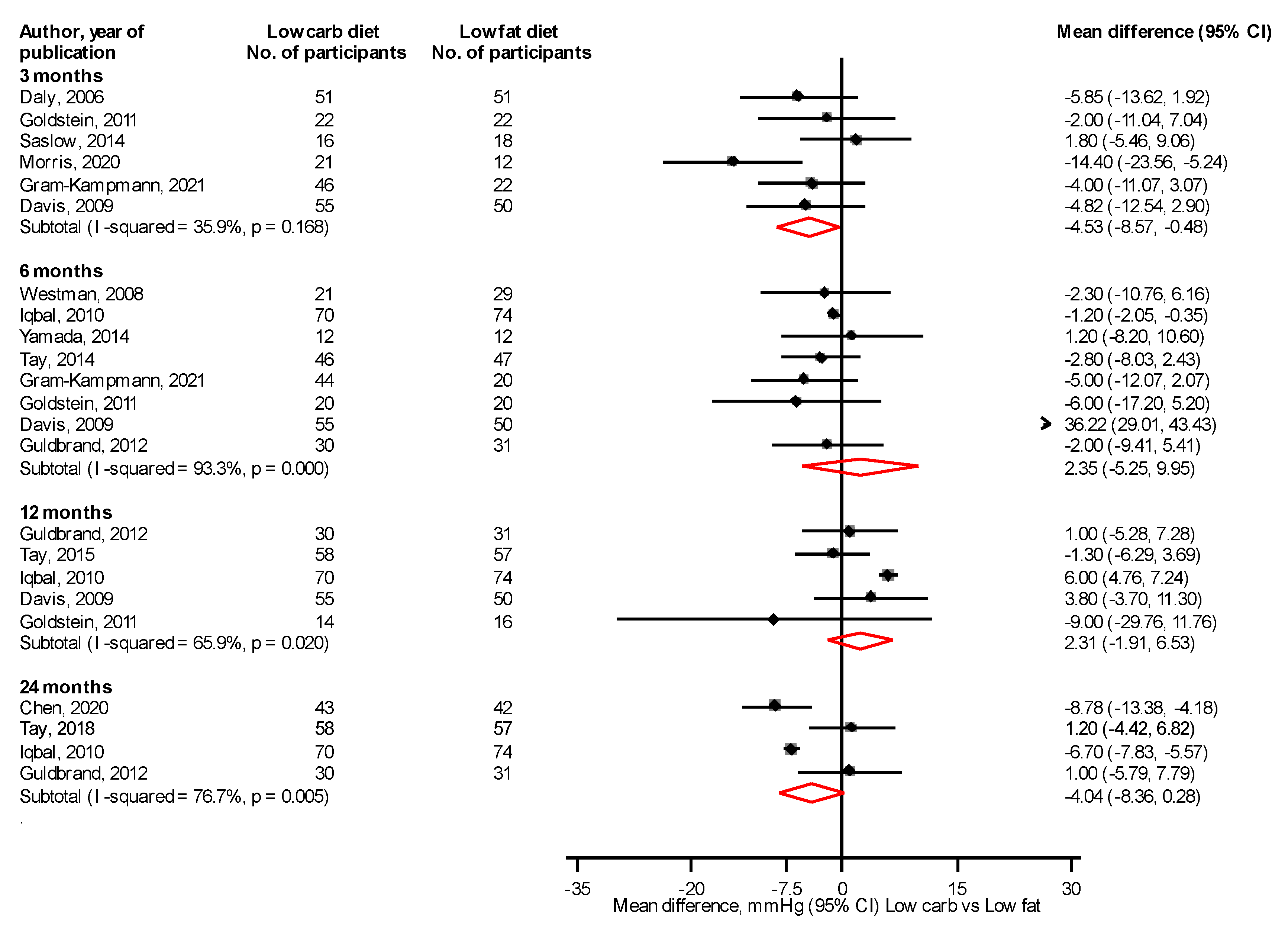
3.5. Blood Pressure
3.6. Lipids
3.7. Measures of Inflammation
3.8. Other Cardiovascular Risk Markers
3.9. Liver Function
3.10. Renal Function
3.11. Medication Changes
3.12. Adverse Events
3.13. GRADE Summary of Findings
4. Discussion
4.1. Key Findings
4.2. Comparison with Previous Studies
4.3. Explanations for Findings
4.4. Implications of Findings
4.5. Strengths and Limitations
5. Conclusions
6. Key Points
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, G.; Wu, W.; Yi, X. Type 2 Diabetes Mellitus- Disease, Diagnosis and Treatment. J. Diabetes Metab. 2015, 6, 1–6. [Google Scholar]
- World Health Organisation. Diabetes. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes. (accessed on 12 December 2021).
- Joy, S.M.; Little, E.; Maruthur, N.M.; Purnell, T.S.; Bridges, J.F. Patient preferences for the treatment of type 2 diabetes: A scoping review. Pharmacoeconomics 2013, 31, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.O.; Hwang, J.K.; Rhee, S.Y.; Chon, S.; Hwang, Y.C.; Oh, S.; Ahn, K.J.; Chung, H.Y.; Woo, J.T.; Kim, S.W.; et al. Risk factors for the progression of intima-media thickness of carotid arteries: A 2-year follow-up study in patients with newly diagnosed type 2 diabetes. Diabetes Metab. J. 2013, 37, 365–374. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas 10th Edition. Available online: https://diabetesatlas.org/ (accessed on 2 December 2021).
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Diabetes UK. Diabetes Remission. What is Diabetes Remission and How Does It Work? 2021. Available online: https://www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes (accessed on 12 December 2021).
- Bi, Y.; Wang, T.; Xu, M.; Xu, Y.; Li, M.; Lu, J.; Zhu, X.; Ning, G. Advanced research on risk factors of type 2 diabetes. Diabetes Metab. Res. Rev. 2012, 28 (Suppl. S2), 32–39. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Hamalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pei, J.H.; Kuang, J.; Chen, H.M.; Chen, Z.; Li, Z.W.; Yang, H.Z. Effect of lifestyle intervention in patients with type 2 diabetes: A meta-analysis. Metabolism 2015, 64, 338–347. [Google Scholar] [CrossRef]
- Zeng, Y.; Ali, M.K.; Du, J.; Li, X.; Yang, X.; Yang, J.; Pu, X.; Yang, L.E.; Hong, J.; Mou, B.; et al. Resistant Starch in Rice: Its Biosynthesis and Mechanism of Action Against Diabetes-Related Diseases. Food Rev. Int. 2022, 12, 1–24. [Google Scholar] [CrossRef]
- Zeng, Y.; Pu, X.; Du, J.; Yang, X.; Li, X.; Mandal, M.S.N.; Yang, T.; Yang, J. Molecular Mechanism of Functional Ingredients in Barley to Combat Human Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 3836172. [Google Scholar] [CrossRef]
- Diabetes Canada Position Statement on Low-Carbohydrate Diets for Adults With Diabetes: A Rapid Review. Can. J. Diabetes 2020, 44, 295–299. [CrossRef]
- NICE. Type 2 Diabetes in Adults: Management [NG28]. 2021. Available online: www.nice.org.uk/Guidance/NG28 (accessed on 21 December 2021).
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- SACN Report: Lower Carbohydrate Diets for Type 2 Diabetes. 2021. Available online: https://www.gov.uk/government/publications/sacn-report-lower-carbohydrate-diets-for-type-2-diabetes (accessed on 2 December 2021).
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jonsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in Epidemiology. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Thompson, S.G.; Sharp, S.J. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat. Med. 1999, 18, 2693–2708. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Asle Mohammadi Zadeh, M.; Kargarfard, M.; Marandi, S.M.; Habibi, A. Diets along with interval training regimes improves inflammatory & anti-inflammatory condition in obesity with type 2 diabetes subjects. J. Diabetes Metab. Disord. 2018, 17, 253–267. [Google Scholar] [CrossRef]
- Chen, C.Y.; Huang, W.S.; Chen, H.C.; Chang, C.H.; Lee, L.T.; Chen, H.S.; Kang, Y.D.; Chie, W.C.; Jan, C.F.; Wang, W.D.; et al. Effect of a 90 g/day low-carbohydrate diet on glycaemic control, small, dense low-density lipoprotein and carotid intima-media thickness in type 2 diabetic patients: An 18-month randomised controlled trial. PLoS ONE 2020, 15, e0240158. [Google Scholar] [CrossRef]
- Daly, M.E.; Paisey, R.; Paisey, R.; Millward, B.A.; Eccles, C.; Williams, K.; Hammersley, S.; MacLeod, K.M.; Gale, T.J. Short-term effects of severe dietary carbohydrate-restriction advice in Type 2 diabetes--a randomized controlled trial. Diabet Med. 2006, 23, 15–20. [Google Scholar] [CrossRef]
- Davis, N.J.; Tomuta, N.; Schechter, C.; Isasi, C.R.; Segal-Isaacson, C.J.; Stein, D.; Zonszein, J.; Wylie-Rosett, J. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care 2009, 32, 1147–1152. [Google Scholar] [CrossRef]
- Goldstein, T.; Kark, J.D.; Berry, E.M.; Adler, B.; Ziv, E.; Raz, I. The effect of a low carbohydrate energy-unrestricted diet on weight loss in obese type 2 diabetes patients—A randomized controlled trial. e-SPEN Eur. E-J. Clin. Nutr. Metab. 2011, 6, e178–e186. [Google Scholar] [CrossRef]
- Guldbrand, H.; Dizdar, B.; Bunjaku, B.; Lindstrom, T.; Bachrach-Lindstrom, M.; Fredrikson, M.; Ostgren, C.J.; Nystrom, F.H. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia 2012, 55, 2118–2127. [Google Scholar] [CrossRef]
- Iqbal, N.; Vetter, M.L.; Moore, R.H.; Chittams, J.L.; Dalton-Bakes, C.V.; Dowd, M.; Williams-Smith, C.; Cardillo, S.; Wadden, T.A. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity 2010, 18, 1733–1738. [Google Scholar] [CrossRef]
- Jonasson, L.; Guldbrand, H.; Lundberg, A.K.; Nystrom, F.H. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann. Med. 2014, 46, 182–187. [Google Scholar] [CrossRef]
- Khoo, J.; Piantadosi, C.; Duncan, R.; Worthley, S.G.; Jenkins, A.; Noakes, M.; Worthley, M.I.; Lange, K.; Wittert, G.A. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J. Sex. Med. 2011, 8, 2868–2875. [Google Scholar] [CrossRef]
- Morris, E.; Aveyard, P.; Dyson, P.; Noreik, M.; Bailey, C.; Fox, R.; Jerome, D.; Tan, G.D.; Jebb, S.A. A food-based, low-energy, low-carbohydrate diet for people with type 2 diabetes in primary care: A randomized controlled feasibility trial. Diabetes Obes. Metab. 2020, 22, 512–520. [Google Scholar] [CrossRef]
- Nishimori, E.; Tetsu, O.; Takasugi, K.; Yoda, T.; Oi, S.; Sekiguchi, K.; Kudo, K.; Yoda, A.; Minami, S.; Naka, M. Carbohydrate-restricted diet is a non-alcoholic fatty liver disease associated with type 2 diabetes Improve calorie-restricted diet. Diabetes 2018, 61, 297–306. [Google Scholar]
- Perna, S.; Alalwan, T.A.; Gozzer, C.; Infantino, V.; Peroni, G.; Gasparri, C.; Spadaccini, D.; Riva, A.; Rondanelli, M. Effectiveness of a hypocaloric and low-carbohydrate diet on visceral adipose tissue and glycemic control in overweight and obese patients with type 2 diabetes. Bahrain Med. Bull. 2019, 41, 159–164. [Google Scholar]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stern, L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef]
- Saslow, L.R.; Kim, S.; Daubenmier, J.J.; Moskowitz, J.T.; Phinney, S.D.; Goldman, V.; Murphy, E.J.; Cox, R.M.; Moran, P.; Hecht, F.M. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE 2014, 9, e91027. [Google Scholar] [CrossRef]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An Online Intervention Comparing a Very Low-Carbohydrate Ketogenic Diet and Lifestyle Recommendations Versus a Plate Method Diet in Overweight Individuals With Type 2 Diabetes: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e36. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Hatae, C.; Makita, S.; Komiya, K.; Shimizu, T.; Ikeda, F.; Tamura, Y.; Ogihara, T.; Mita, T.; et al. One year follow-up after a randomized controlled trial of a 130 g/day low-carbohydrate diet in patients with type 2 diabetes mellitus and poor glycemic control. PLoS ONE 2017, 12, e0188892. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Makita, S.; Hatae, C.; Komiya, K.; Shimizu, T.; Ikeda, F.; Tamura, Y.; Ogihara, T.; Mita, T.; et al. A randomized controlled trial of 130 g/day low-carbohydrate diet in type 2 diabetes with poor glycemic control. Clin. Nutr. 2017, 36, 992–1000. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. A very low-carbohydrate, low-saturated fat diet for type 2 diabetes management: A randomized trial. Diabetes Care 2014, 37, 2909–2918. [Google Scholar] [CrossRef]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S., Jr.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef]
- Yamada, Y.; Uchida, J.; Izumi, H.; Tsukamoto, Y.; Inoue, G.; Watanabe, Y.; Irie, J.; Yamada, S. A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Intern. Med. 2014, 53, 13–19. [Google Scholar] [CrossRef]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Gram-Kampmann, E.M.; Hansen, C.D.; Hugger, M.B.; Jensen, J.M.; Brond, J.C.; Hermann, A.P.; Krag, A.; Olsen, M.H.; Beck-Nielsen, H.; Hojlund, K. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes. Metab. 2022, 24, 693–703. [Google Scholar] [CrossRef]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- Sainsbury, E.; Kizirian, N.V.; Partridge, S.R.; Gill, T.; Colagiuri, S.; Gibson, A.A. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 239–252. [Google Scholar] [CrossRef]
- Van Zuuren, E.J.; Fedorowicz, Z.; Kuijpers, T.; Pijl, H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: A systematic review including GRADE assessments. Am. J. Clin. Nutr. 2018, 108, 300–331. [Google Scholar] [CrossRef] [PubMed]
- Korsmo-Haugen, H.K.; Brurberg, K.G.; Mann, J.; Aas, A.M. Carbohydrate quantity in the dietary management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Huntriss, R.; Campbell, M.; Bedwell, C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Nutr. 2018, 72, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Astbury, N.M.; Aveyard, P.; Nickless, A.; Hood, K.; Corfield, K.; Lowe, R.; Jebb, S.A. Doctor Referral of Overweight People to Low Energy total diet replacement Treatment (DROPLET): Pragmatic randomised controlled trial. BMJ 2018, 362, k3760. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Response to comment on Tay et al. A very low-carbohydrate, low-saturated fat diet for type 2 diabetes management: A randomized trial. Diabetes Care 2014, 37, 2909–2918. Diabetes Care 2015, 38, e65–e66. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef]
- Hackel, J.M. ‘Patient-centered care’ for complex patients with type 2 diabetes mellitus-analysis of two cases. Clin. Med. Insights Endocrinol. Diabetes 2013, 6, 47–61. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 21 September 2022).





| Author, Year of Publication | Country | Population | Baseline Year | Mean Age, years | Male % | Mean BMI, kg/m2 | Mean HbA1c, % | Diabetes Duration, years | Trial Duration, years | No. Randomized | No. In Intervention | No. In Comparator | Overall Risk of Bias * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samaha, 2003 [42] | USA | Obese with diabetes | 2001 | NR | NR | NR | NR | NR | 6.0 | 79 | 41 | 38 | High |
| Daly, 2006 [32] | UK | Obese with poorly controlled T2DM | NR | 58.7 | 48.0 | 36.1 | 9.1 | NR | 3.0 | 102 | 51 | 51 | High |
| Westman, 2008 [50] | USA | Obesity and T2DM | NR | 51.8 | 21.4 | 38.1 | NR | NR | 6.0 | 84 | 38 | 46 | High |
| Shai, 2008 [47] | Israel | Obese with T2DM | 2005–2007 | NR | NR | NR | NR | NR | 24.0 | 31 | 19 | 12 | High |
| Davis, 2009 [33] | USA | T2DM with BMI ≥ 25 kg/m2, and A1C between 6 and 11% | 2004–2006 | 53.5 | 21.9 | 36.0 | 7.5 | NR | 12.0 | 105 | 55 | 50 | High |
| Iqbal, 2010 [36] | USA | Obese with T2DM | 2004–2008 | 60.0 | 89.6 | 37.5 | NR | NR | 24.0 | 144 | 70 | 74 | High |
| Goldstein, 2011 [34] | Israel | Obese T2DM | 2001–2004 | 56.0 | 48.1 | 33.2 | 8.9 | 8.0 | 12.0 | 52 | 26 | 26 | High |
| Khoo, 2011 [38] | Australia | Obese with T2DM | 2007–2008 | 59.7 | 35.3 | 4.5 | 2.0 | 31 | 12 | 19 | High | ||
| Guldbrand, 2012 [35]; Jonasson, 2014 [37] | Sweden | Diagnosis of T2DM treated with diet with or without additional oral glucose-lowering medication, incretin-based therapy or insulin | 2008–2009 | 62.0 | 44.3 | 32.7 | 7.3 | 9.3 | 24.0 | 61 | 30 | 31 | High |
| Tay, 2014 [48]; Tay 2015 [54] | Australia | Obese adults with T2DM; taking antiglycaemic medication | 2012–2013 | 58.0 | 57.4 | 34.6 | 7.3 | 8.0 | 12.0 | 115 | 58 | 57 | High |
| Yamada, 2014 [51] | Japan | Poorly controlled T2DM | 2011–2012 | 63.3 | 50.0 | 25.8 | 7.7 | 9.2 | 6.0 | 24 | 12 | 12 | High |
| Saslow, 2014 [43] | USA | Overweight or obese adults with T2DM or prediabetes | 2012 | 59.7 | 26.5 | 36.8 | 6.8 | 7.1 | 3.0 | 34 | 16 | 18 | High |
| Sato, 2017 [45]; Sato 2017a [46] | Japan | T2DM with poor glycaemic control | 2013–2014 | 59.4 | 75.8 | 26.6 | 8.2 | 13.5 | 18.0 | 66 | 33 | 33 | High |
| Saslow, 2017 [44] | USA | Overweight with T2DM | 2013 | 55.7 | 40.0 | 7.2 | 5.5 | 7.4 | 25 | 12 | 13 | High | |
| Nishimori, 2018 [40] | Japan | T2DM and NFLD | NR | 49.5 | 64.0 | NR | NR | 3.0 | 28 | 14 | 14 | High | |
| Zadeh, 2018 [30] | Iran | Obese and T2DM | NR | 48.2 | 34.1 | 7.0 | 6.5 | 6.0 | 22 | 11 | 11 | High | |
| Tay, 2018 [49] | Australia | T2DM under the care of a GP/endocrinologist | 2012–2014 | 58.0 | 57.4 | 34.5 | 7.3 | 7.0 | 24.0 | 115 | 58 | 57 | High |
| Perna, 2019 [41] | Italy | Obese and overweight with T2DM; only treated with metformin | NR | 67.0 | 35.3 | 31.4 | 6.0 | NR | 3.0 | 17 | 8 | 9 | High |
| Chen, 2020 [31] | Taiwan | Poorly controlled T2DM | 2016 | 63.6 | 38.8 | NR | NR | 9.9 | 18.0 | 92 | 47 | 45 | High |
| Morris, 2020 [39] | UK | T2D and BMI of at least 30 kg/m2 | 2018 | 67.0 | 45.0 | 35.4 | NR | 9.2 | 3.0 | 33 | 21 | 12 | High |
| Gram-Kampmann, 2022 [53] | Denmark | T2DM with HbAIc > 48 mmol/mol | 2016–2018 | 56.6 | 43.7 | NR | NR | 5.1 | 0.5 | 71 | 49 | 22 | High |
| Li, 2022 [52] | China | Overweight or obese with T2DM | 2018–2020 | 36.8 | NR | 29.4 | 8.7 | 0.3 | 0.25 | 60 | 30 | 30 | High |
| Author, Year of Publication | Intervention | Description of Intervention | Comparator | Description of Comparator | Exercise Recommendations |
|---|---|---|---|---|---|
| Samaha, 2003 [42] | Low carb diet | 30 g/day or less | Low fat diet | Caloric restriction sufficient to create a deficit of 500 calories per day, with 30 percent or less of total calories derived from fat. | None recommended |
| Daly, 2006 [32] | Low carb diet | 70 g/day | Low fat diet | Both groups | |
| Westman, 2008 [50] | Low carb ketogenic diet | <20 g of carbohydrate daily | Low-glycemic, reduced-calorie diet | 500 kcal/day deficit from weight maintenance diet | Both groups |
| Shai, 2008 [47] | Low-carb, non–restricted-calorie diet | 20 g/day for the 2 month induction phase | Low-fat, restricted-calorie diet | Energy intake of 1500 kcal per day for women and 1800 kcal per day for men, with 30% of calories from fat, 10% of calories from saturated fat, and an intake of 300 mg of cholesterol per day | Not specifically recommended |
| Davis, 2009 [33] | Low carb diet | Initial 2-week phase of carbohydrate restriction of 20–25 g daily depending on baseline weight; increased intake at 5-g increments each week as participants lost weight | Low fat diet | Fat gram goal was 25% of energy needs, based on baseline weight; 53 energy percent | General recommendations made |
| Iqbal, 2010 [36] | Low carb diet | <30 g/day | Low fat diet | ≤30% of calories from fat with a deficit of 500 kcal/day | Not specifically recommended |
| Goldstein, 2011 [34] | Modified Atkins diet (very low carb diet) | Containing up to 25 g of carbs daily for the first 6 weeks after randomization, thereafter increasing to a ceiling of 40 g daily | Standard recommended ADA calorie-restricted diet | Containing 10−20% of the daily energy intake from protein and the other 80% divided between fats [which provided 18–20% of calories as MUFA, 8–10% as polyunsaturated fatty acids (PUFA) and 9–10% as SFA], carbohydrates and 35 g of fibre | Both groups |
| Khoo, 2011 [38] | Low-fat, high-protein, reduced-carb diet | Reduction in daily energy intake by ~600 kcal | Low-calorie diet | 1000 kcal/day | Not specifically recommended |
| Guldbrand, 2012 [35]; Jonasson, 2014 [37] | Low carb diet | 20 energy percent from carb | Low fat diet | 55–60 energy percent | Not specifically recommended |
| Tay, 2014 [48]; Tay 2015 [54] | Low-carbohydrate, high unsaturated/low saturated fat diet | 14% carbohydrate [<50 g/day], 28% protein, and 58% fat [<10% saturated fat] plus structured exercise | High unrefined carbohydrate, low fat diet | 53% carbohydrate, 17% protein, and 30% fat [<10% saturated fat] plus structured exercise | Both groups |
| Yamada, 2014 [51] | Low carb diet | <130 g/day | Calorie restricted diet | Carbohydrates = 50–60%, protein = 1.0–1.2 g/kg (<20%) and fat = <25% | Not reported |
| Saslow, 2014 [43] | Low carb ketogenic diet | Reduce carbohydrate intake over 7–10 days to between 20–50 g of carbohydrates a day with the goal of achieving nutritional ketosis | Moderate carb, calorie-restricted diet | 45% to 50% of calories derived from carbohydrates | Both groups |
| Sato, 2017 [45]; Sato 2017a [46] | Low carb diet | 130 g/day | Calorie restricted diet | The percentage of carbohydrate per total calorie was 50–60%, and that of proteinwas 1.0–1.2 g/kg | |
| Saslow, 2017 [44] | Very low-carb ketogenic diet with lifestyle factors; “intervention” | Reduce carb intake to between 20–50 g of nonfiber carbohydrates a day | Online diet program based around a plate method diet | A low-fat diet that emphasizes green vegetables, lean protein sources, and somewhat limited starchy and sweet foods | Intervention group received exercise recommendations |
| Nishimori, 2018 [40] | Low carb diet | 70–130 g/day | Calorie restricted diet | 25 kcal/kg of ideal body weight per day | Not reported |
| Zadeh, 2018 [30] | Low carb diet plus high intensity interval training | 45% energy (E %) from fat, 20 E% from carbohydrate and 35 E% from protein | Low fat diet plus high intensity interval training | 30 E% from fat (less than 10 E% from saturated fat), 50 E% from carbohydrate and 20 E% from protein | Both groups |
| Tay, 2018 [49] | Low-carb, high-unsaturated/low-saturated fat diet | 14% energy as carb, 28% as protein, 58% as fat (<10% saturated fat) | High-carbohydrate, low-fat diet | 53% as CHO, 17% as protein, 30% as fat (<10% saturated fat) | Both groups |
| Perna, 2019 [41] | Low carb diet and metformin | CHO <125 g/ day, 1600 kcal/day for females and 1800 kcal/day for males | Balanced standard diet | Carbohydrates 55–60%, lipids 25–30%, proteins 15–20% | Not reported |
| Chen, 2020 [31] | Low carb diet | 90 g/day | Traditional diabetic diet | Macronutrient percentage was 50–60% for CHO, 1.0–1.2 g/kg for protein and ≤30% for fat | Both groups |
| Morris, 2020 [39] | Low-energy, low-carb diet | 800–1000 kcal/day, with <26% of daily energy intake from carb and a minimum of 60 g protein/day | Usual care dietary advice | Healthy balanced eating | Not reported |
| Gram-Kampmann, 2022 [53] | Low carb diet | Maximum of 20 E% of carbohydrates (mainly complex and water-soluble), 50–60 E% fat, and 25–30 E% protein. | Control diet | 50–60 E% carbohydrates mainly from fruit, vegetables, and whole-grain sources, 20–30 E% fat | Both groups |
| Li, 2022 [52] | Ketogenic diet | carbohydrate 30–50 g, protein 60 g, fat 130 g, and total calories (1500 ± 50) Kcal | Routine diet for diabetes | Carbohydrate 250–280 g, protein 60 g, fat 20 g, total calories (1500 ± 50) Kcal | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apekey, T.A.; Maynard, M.J.; Kittana, M.; Kunutsor, S.K. Comparison of the Effectiveness of Low Carbohydrate Versus Low Fat Diets, in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4391. https://doi.org/10.3390/nu14204391
Apekey TA, Maynard MJ, Kittana M, Kunutsor SK. Comparison of the Effectiveness of Low Carbohydrate Versus Low Fat Diets, in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(20):4391. https://doi.org/10.3390/nu14204391
Chicago/Turabian StyleApekey, Tanefa A., Maria J. Maynard, Monia Kittana, and Setor K. Kunutsor. 2022. "Comparison of the Effectiveness of Low Carbohydrate Versus Low Fat Diets, in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 20: 4391. https://doi.org/10.3390/nu14204391
APA StyleApekey, T. A., Maynard, M. J., Kittana, M., & Kunutsor, S. K. (2022). Comparison of the Effectiveness of Low Carbohydrate Versus Low Fat Diets, in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 14(20), 4391. https://doi.org/10.3390/nu14204391





