Reply to Chao et al. Comment on “Nahok et al. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 2021, 13, 1865”
Author Contributions
Funding
Conflicts of Interest
References
- Chao, H.; Yoshida, S.; Kohmura, M. Comment on Nahok et al. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 2021, 13, 1865. Nutrients 2022, 14, 4386. [Google Scholar] [CrossRef]
- Nahok, K.; Phetcharaburanin, J.; Li, J.V.; Silsirivanit, A.; Thanan, R.; Boonnate, P.; Joonhuathon, J.; Sharma, A.; Anutrakulchai, S.; Selmi, C.; et al. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 2021, 13, 1865. [Google Scholar] [CrossRef] [PubMed]
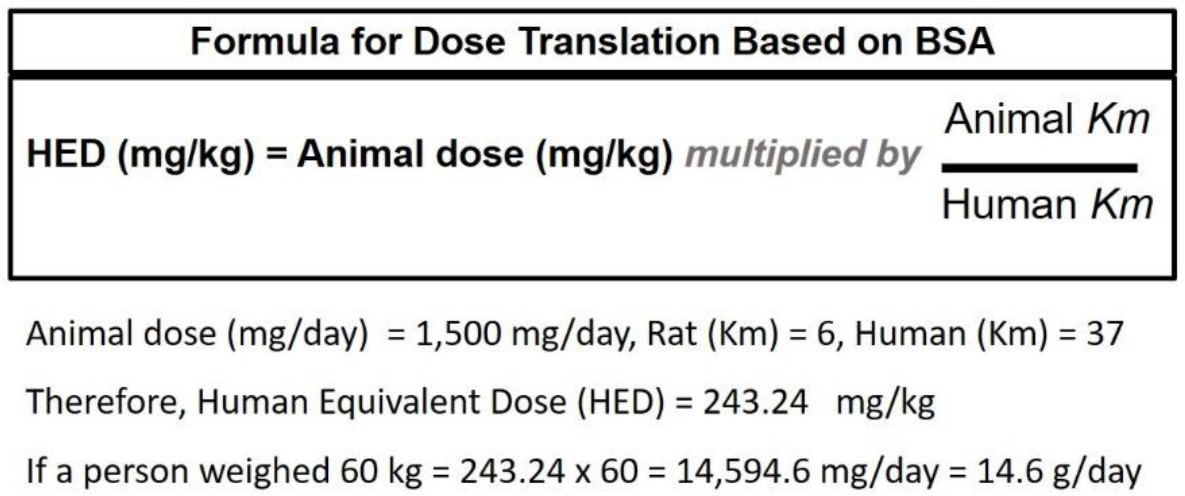
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Chevassus, H.; Renard, E.; Bertrand, G.; Mourand, I.; Puech, R.; Molinier, N.; Bockaert, J.; Petit, P.; Bringer, J. Effects of oral monosodium (L)-glutamate on insulin secretion and glucose tolerance in healthy volunteers. Br. J. Clin. Pharmacol. 2002, 53, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Insawang, T.; Selmi, C.; Cha'on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Boonsiri, P.; Khampitak, T.; Tangrassameeprasert, R.; Pinitsoontorn, C.; et al. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. Lond. 2012, 9, 50. [Google Scholar] [PubMed]
- Peng, Q.N.; Huo, D.X.; Ma, C.C.; Jiang, S.M.; Wang, L.S.; Zhang, J.C. Monosodium glutamate induces limited modulation in gut microbiota. J. Funct. Foods 2018, 49, 493–500. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Bielinska, K.; Radkowski, M.; Grochowska, M.; Perlejewski, K.; Huc, T.; Jaworska, K.; Motooka, D.; Nakamura, S.; Ufnal, M. High salt intake increases plasma trimethylamine N-oxide (TMAO) concentration and produces gut dysbiosis in rats. Nutrition 2018, 54, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Tang, M.Q.; Liu, Y.N.; Xu, J.H.; Xu, X.X. Safety assessment of monosodium glutamate based on intestinal function and flora in mice. Food Sci. Hum. Wellness 2022, 11, 155–164. [Google Scholar] [CrossRef]
- Prosky, L.; Odell, R.G. Effect of Dietary Monosodium L-Glutamate on Some Brain and Liver Metabolites in Rats. Proc. Soc. Exp. Biol. Med. 1971, 138, 517. [Google Scholar] [CrossRef] [PubMed]
- Stegink, L.D.; Brummel, M.C.; Boaz, D.P.; Filer, L.J. Monosodium Glutamate Metabolism in Neonatal Pig-Conversion of Administered Glutamate into Other Metabolites in-Vivo. J. Nutr. 1973, 103, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Owen, G.; Cherry, C.P.; Prentice, D.E.; Worden, A.N. Feeding of Diets Containing up to 4-Percent Monosodium Glutamate to Rats for 2 Years. Toxicol. Lett. 1978, 1, 221–226. [Google Scholar] [CrossRef]
- Owen, G.; Cherry, C.P.; Prentice, D.E.; Worden, A.N. Feeding of Diets Containing up to 10-Percent Monosodium Glutamate to Beagle Dogs for 2 Years. Toxicol. Lett. 1978, 1, 217–219. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahok, K.; Selmi, C.; Sukmak, M.; Phetcharaburanin, J.; Li, J.V.; Silsirivanit, A.; Thanan, R.; Sharma, A.; Anutrakulchai, S.; Hammock, B.D.; et al. Reply to Chao et al. Comment on “Nahok et al. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 2021, 13, 1865”. Nutrients 2022, 14, 4387. https://doi.org/10.3390/nu14204387
Nahok K, Selmi C, Sukmak M, Phetcharaburanin J, Li JV, Silsirivanit A, Thanan R, Sharma A, Anutrakulchai S, Hammock BD, et al. Reply to Chao et al. Comment on “Nahok et al. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 2021, 13, 1865”. Nutrients. 2022; 14(20):4387. https://doi.org/10.3390/nu14204387
Chicago/Turabian StyleNahok, Kanokwan, Carlo Selmi, Manatsaphon Sukmak, Jutarop Phetcharaburanin, Jia V. Li, Atit Silsirivanit, Raynoo Thanan, Amod Sharma, Sirirat Anutrakulchai, Bruce D. Hammock, and et al. 2022. "Reply to Chao et al. Comment on “Nahok et al. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 2021, 13, 1865”" Nutrients 14, no. 20: 4387. https://doi.org/10.3390/nu14204387
APA StyleNahok, K., Selmi, C., Sukmak, M., Phetcharaburanin, J., Li, J. V., Silsirivanit, A., Thanan, R., Sharma, A., Anutrakulchai, S., Hammock, B. D., & Cha’on, U. (2022). Reply to Chao et al. Comment on “Nahok et al. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 2021, 13, 1865”. Nutrients, 14(20), 4387. https://doi.org/10.3390/nu14204387





