Association between Dietary Fatty Acid Patterns and Colorectal Cancer Risk: A Large-Scale Case-Control Study in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Inclusion and Exclusion Criteria for Cases
2.1.2. Inclusion and Exclusion Criteria for Controls
2.2. Data Collection
2.3. Dietary Assessments
2.4. Factor Analysis of Fatty Acid Pattern
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Dietary Fatty Acid Patterns
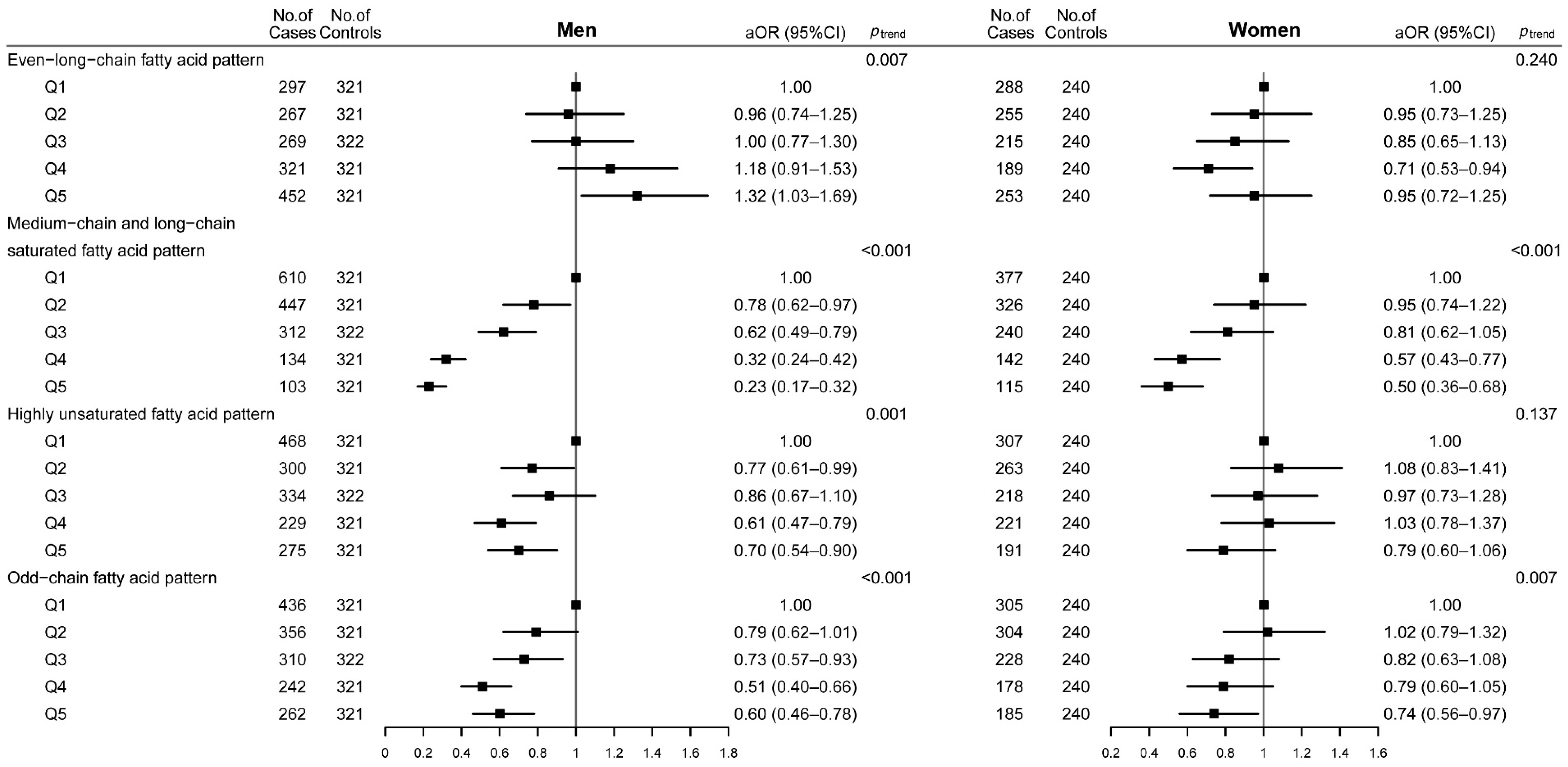
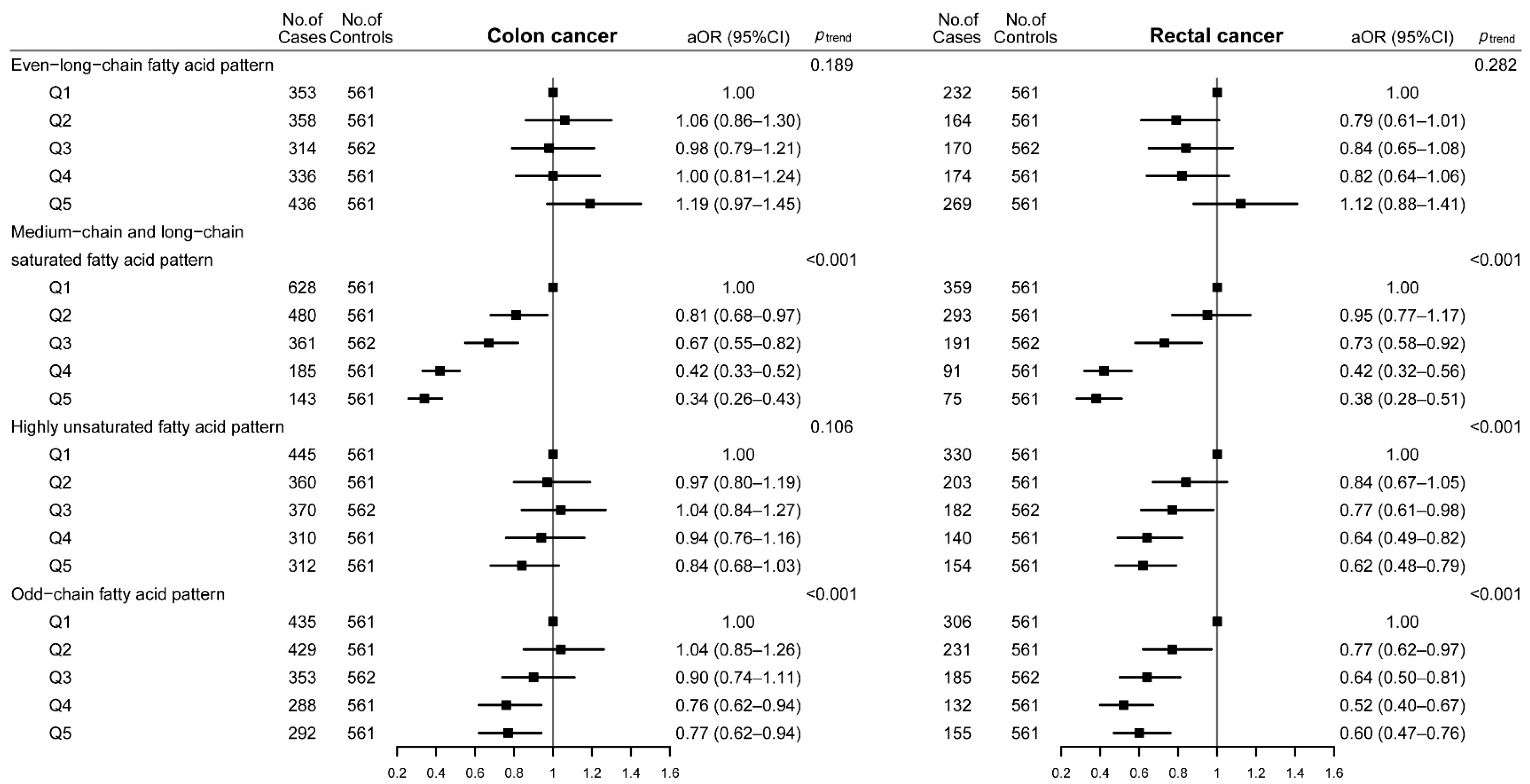
3.3. Dietary Fatty Acid Patterns and CRC Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Binefa, G.; Rodríguez-Moranta, F.; Teule, A.; Medina-Hayas, M. Colorectal cancer: From prevention to personalized medicine. World J. Gastroenterol. 2014, 20, 6786–6808. [Google Scholar] [CrossRef]
- Czene, K.; Lichtenstein, P.; Hemminki, K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int. J. Cancer 2002, 99, 260–266. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Puzzono, M.; Mannucci, A.; Grannò, S.; Zuppardo, R.A.; Galli, A.; Danese, S.; Cavestro, G.M. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 5933. [Google Scholar] [CrossRef]
- Huang, C.Y.; Fang, Y.J.; Abulimiti, A.; Yang, X.; Li, L.; Liu, K.Y.; Zhang, X.; Feng, X.L.; Chen, Y.M.; Zhang, C.X. Dietary Polyamines Intake and Risk of Colorectal Cancer: A Case-Control Study. Nutrients 2020, 12, 3575. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Sledzinski, T.; Mika, A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef]
- Wang, K.; Shi, T.Q.; Lin, L.; Wei, P.; Ledesma-Amaro, R.; Ji, X.J. Engineering Yarrowia lipolytica to Produce Tailored Chain-Length Fatty Acids and Their Derivatives. ACS Synth. Biol. 2022, 11, 2564–2577. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, D.; Li, X.; Liu, D.; Li, C.; Zheng, Y.; Yue, X.; Shao, J.-H. The effect of fatty acid chain length and saturation on the emulsification properties of pork myofibrillar proteins. LWT 2021, 139, 110242. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. 4—The Lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 181–257. [Google Scholar]
- Kim, M.; Park, K. Dietary Fat Intake and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2018, 10, 1963. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Li, H.; Yu, D.; Cai, H.; Gao, J.; Gao, Y.; Luu, H.N.; Tran, H.; Xiang, Y.B.; Zheng, W.; et al. Dietary fatty acids and colorectal cancer risk in men: A report from the Shanghai Men’s Health Study and a meta-analysis. Int. J. Cancer 2021, 148, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.J.; Sohn, S.K.; Song, H.K.; Lee, S.M.; Youn, Y.H.; Lee, S.; Park, H. Associations of colorectal cancer incidence with nutrient and food group intakes in korean adults: A case-control study. Clin. Nutr. Res. 2015, 4, 110–123. [Google Scholar] [CrossRef]
- Hodge, A.M.; Williamson, E.J.; Bassett, J.K.; MacInnis, R.J.; Giles, G.G.; English, D.R. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int. J. Cancer 2015, 137, 1224–1234. [Google Scholar] [CrossRef]
- Karazurna, N.A.; Porter, C.M.; Aytur, S.; Scott, T.; Mattei, J.; Noel, S.E.; Gonzalez, H.M.; Mossavar-Rahmani, Y.; Sotres-Alvarez, D.; Gallo, L.C.; et al. Associations between dietary fatty acid patterns and cognitive function in the Hispanic Community Health Study/Study of Latinos. Br. J. Nutr. 2021, in press. [CrossRef]
- McCullough, M.L. Colorectal cancer: What do studies of diet patterns tell us? Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 567–568. [Google Scholar] [CrossRef]
- Imamura, F.; Lemaitre, R.N.; King, I.B.; Song, X.; Lichtenstein, A.H.; Matthan, N.R.; Herrington, D.M.; Siscovick, D.S.; Mozaffarian, D. Novel circulating fatty acid patterns and risk of cardiovascular disease: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2012, 96, 1252–1261. [Google Scholar] [CrossRef]
- Yang, M.; Ayuningtyas, A.; Kenfield, S.A.; Sesso, H.D.; Campos, H.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Blood fatty acid patterns are associated with prostate cancer risk in a prospective nested case-control study. Cancer Causes Control 2016, 27, 1153–1161. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Gorst-Rasmussen, A.; Nyström, P.W.; Christensen, J.H.; Schmidt, E.B.; Dethlefsen, C.; Tjønneland, A.; Overvad, K.; Dahm, C.C. Baseline patterns of adipose tissue fatty acids and long-term risk of breast cancer: A case-cohort study in the Danish cohort Diet, Cancer and Health. Eur. J. Clin. Nutr. 2014, 68, 1088–1094. [Google Scholar] [CrossRef]
- Fan, Y.; Qiu, Y.; Wang, J.; Chen, Q.; Wang, S.; Wang, Y.; Li, Y.; Weng, Y.; Qian, J.; Chen, F.; et al. Association Between Dietary Fatty Acid Pattern and Risk of Oral Cancer. Front. Nutr. 2022, 9, 864098. [Google Scholar] [CrossRef]
- Zhong, X.; Fang, Y.J.; Pan, Z.Z.; Li, B.; Wang, L.; Zheng, M.C.; Chen, Y.M.; Zhang, C.X. Dietary fat, fatty acid intakes and colorectal cancer risk in Chinese adults: A case-control study. Eur. J. Cancer Prev. 2013, 22, 438–447. [Google Scholar] [CrossRef]
- Abulimiti, A.; Zhang, X.; Shivappa, N.; Hébert, J.R.; Fang, Y.J.; Huang, C.Y.; Feng, X.L.; Chen, Y.M.; Zhang, C.X. The Dietary Inflammatory Index Is Positively Associated with Colorectal Cancer Risk in a Chinese Case-Control Study. Nutrients 2020, 12, 232. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Zhang, C.X.; Ho, S.C. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac. J. Clin. Nutr. 2009, 18, 240–250. [Google Scholar]
- Luo, H.; Fang, Y.J.; Lu, M.S.; Pan, Z.Z.; Huang, J.; Chen, Y.M.; Zhang, C.X. Dietary and serum vitamins A and E and colorectal cancer risk in Chinese population: A case-control study. Eur. J. Cancer. Pre. 2019, 28, 268–277. [Google Scholar] [CrossRef]
- Huang, C.Y.; Abulimiti, A.; Zhang, X.; Feng, X.L.; Luo, H.; Chen, Y.M.; Fang, Y.J.; Zhang, C.X. Dietary B vitamin and methionine intakes and risk for colorectal cancer: A case-control study in China. Br. J. Nutr. 2020, 123, 1277–1289. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Y.J.; Feng, X.L.; Abulimiti, A.; Huang, C.Y.; Luo, H.; Zhang, N.Q.; Chen, Y.M.; Zhang, C.X. Higher intakes of dietary vitamin D, calcium and dairy products are inversely associated with the risk of colorectal cancer: A case-control study in China. Br. J. Nutr. 2020, 123, 699–711. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. China Food Composition 2002; Peking University Medical Press: Beijing, China, 2002; p. 393. [Google Scholar]
- Yang, Y.X. China Food Composition Tables Standard Edition; Peking University Medical Press: Beijing, China, 2019; p. 429. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Linseisen, J.; Grundmann, N.; Zoller, D.; Kühn, T.; Jansen, E.; Chajès, V.; Fedirko, V.; Weiderpass, E.; Dahm, C.C.; Overvad, K.; et al. Red Blood Cell Fatty Acids and Risk of Colorectal Cancer in The European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol. Biomark. Prev. 2021, 30, 874–885. [Google Scholar] [CrossRef]
- Aglago, E.K.; Murphy, N.; Huybrechts, I.; Nicolas, G.; Casagrande, C.; Fedirko, V.; Weiderpass, E.; Rothwell, J.A.; Dahm, C.C.; Olsen, A.; et al. Dietary intake and plasma phospholipid concentrations of saturated, monounsaturated and trans fatty acids and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Int. J. Cancer 2021, 149, 865–882. [Google Scholar] [CrossRef]
- May-Wilson, S.; Sud, A.; Law, P.J.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hänninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Pro-inflammatory fatty acid profile and colorectal cancer risk: A Mendelian randomisation analysis. Eur. J. Cancer 2017, 84, 228–238. [Google Scholar] [CrossRef]
- Kuriki, K.; Wakai, K.; Hirose, K.; Matsuo, K.; Ito, H.; Suzuki, T.; Saito, T.; Kanemitsu, Y.; Hirai, T.; Kato, T.; et al. Risk of colorectal cancer is linked to erythrocyte compositions of fatty acids as biomarkers for dietary intakes of fish, fat, and fatty acids. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1791–1798. [Google Scholar] [CrossRef]
- Slattery, M.L.; Potter, J.D.; Duncan, D.M.; Berry, T.D. Dietary fats and colon cancer: Assessment of risk associated with specific fatty acids. Int. J. Cancer 1997, 73, 670–677. [Google Scholar] [CrossRef]
- Turchini, G.M.; Nichols, P.D.; Barrow, C.; Sinclair, A.J. Jumping on the omega-3 bandwagon: Distinguishing the role of long-chain and short-chain omega-3 fatty acids. Crit. Rev. Food Sci. Nutr. 2012, 52, 795–803. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Hou, J.; Sun, J.; Guo, N.; Wang, Z. Dietary Intake of N-3 and N-6 Polyunsaturated Fatty Acids and Risk of Cancer: Meta-Analysis of Data from 32 Studies. Nutr. Cancer 2021, 73, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.B.; Ren, X.L.; Xue, Y.L.; Tian, Y.; He, B.B.; Xu, C.L.; Yang, B. Association of Dietary Intake and Biomarker of α-Linolenic Acid With Incident Colorectal Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2022, 9, 948604. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Yuan, J.M.; Huang, J.Y.; Su, J.; Wang, R.; Koh, W.P.; Ong, C.N. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese Health Study. NPJ Precis. Oncol. 2017, 1, 38. [Google Scholar] [CrossRef]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.; Venkitanarayanan, K. Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef] [PubMed]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Okumura, N.; Usui, S.; Sajiki, H.; Hirota, K.; Hirano, K. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate 2001, 47, 59–65. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Calder, P.C. A review of the functional effects of pine nut oil, pinolenic acid and its derivative eicosatrienoic acid and their potential health benefits. Prog. Lipid Res. 2021, 82, 101097. [Google Scholar] [CrossRef]
- Yiannakou, I.; Barber, L.E.; Li, S.; Adams-Campbell, L.L.; Palmer, J.R.; Rosenberg, L.; Petrick, J.L. A Prospective Analysis of Red and Processed Meat Intake in Relation to Colorectal Cancer in the Black Women’s Health Study. J. Nutr. 2022, 152, 1254–1262. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Intake or Blood Levels of n-3 Polyunsaturated Fatty Acids and Risk of Colorectal Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2020, 29, 288–299. [Google Scholar] [CrossRef]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.C.; Fournier, A.; et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated With Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. Hepatol. 2020, 18, 654–666.e656. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Fuchs, C.S.; Ogino, S.; Hu, F.B.; Mozaffarian, D.; Ma, J.; Willett, W.C.; Giovannucci, E.L.; Wu, K. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int. J. Cancer 2014, 135, 2413–2423. [Google Scholar] [CrossRef]
- Sasazuki, S.; Inoue, M.; Iwasaki, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Takachi, R.; Tsugane, S. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int. J. Cancer 2011, 129, 1718–1729. [Google Scholar] [CrossRef]
- Whelan, J.; McEntee, M.F. Dietary (n-6) PUFA and intestinal tumorigenesis. J. Nutr. 2004, 134, 3421s–3426s. [Google Scholar] [CrossRef]
- Shin, A.; Cho, S.; Sandin, S.; Lof, M.; Oh, M.Y.; Weiderpass, E. Omega-3 and -6 Fatty Acid Intake and Colorectal Cancer Risk in Swedish Women’s Lifestyle and Health Cohort. Cancer Res. Treat. 2020, 52, 848–854. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, S.Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014, 37, 112–119. [Google Scholar]
- Wang, S.; Xie, J.; Li, H.; Yang, K. Differences of polyunsaturated fatty acid in patients with colorectal cancer and healthy people. J. Cancer Res. Ther. 2015, 11, 459–463. [Google Scholar] [PubMed]
- Yang, K.; Li, H.; Dong, J.; Dong, Y.; Wang, C.Z. Expression profile of polyunsaturated fatty acids in colorectal cancer. World J. Gastroenterol. 2015, 21, 2405–2412. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Ye, X.; Chen, L.; Zhang, L.; Gao, Y.; Kang, J.X.; Cai, C. Characteristics of fatty acid distribution is associated with colorectal cancer prognosis. Prostaglandins Leukot Essent Fat. Acids 2013, 88, 355–360. [Google Scholar] [CrossRef]
- Daniel, C.R.; McCullough, M.L.; Patel, R.C.; Jacobs, E.J.; Flanders, W.D.; Thun, M.J.; Calle, E.E. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 516–525. [Google Scholar] [CrossRef]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef]
- Jenkins, B.; West, J.A.; Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef]
- Prada, M.; Wittenbecher, C.; Eichelmann, F.; Wernitz, A.; Drouin-Chartier, J.P.; Schulze, M.B. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: A targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin. Nutr. 2021, 40, 4988–4999. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Murphy, N.; Bešević, J.; Kliemann, N.; Jenab, M.; Ferrari, P.; Achaintre, D.; Gicquiau, A.; Vozar, B.; Scalbert, A.; et al. Metabolic Signatures of Healthy Lifestyle Patterns and Colorectal Cancer Risk in a European Cohort. Clin. Gastroenterol. Hepatol. 2022, 20, e1061–e1082. [Google Scholar] [CrossRef]
- Fukuzawa, M.; Yamaguchi, R.; Hide, I.; Chen, Z.; Hirai, Y.; Sugimoto, A.; Yasuhara, T.; Nakata, Y. Possible involvement of long chain fatty acids in the spores of Ganoderma lucidum (Reishi Houshi) to its anti-tumor activity. Biol. Pharm. Bull. 2008, 31, 1933–1937. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed. Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Shen, X.J.; Zhou, J.D.; Dong, J.Y.; Ding, W.Q.; Wu, J.C. Dietary intake of n-3 fatty acids and colorectal cancer risk: A meta-analysis of data from 489,000 individuals. Br. J. Nutr. 2012, 108, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.R.N.; Avci, Ç.B.; Ahmadi, M.; Pouyafar, A.; Bagheri, H.S.; Fathi, F.; Heidarzadeh, M.; Rezaie, J.; Mirhosseini, Y.; Saberianpour, S.; et al. Estradiol modulated colorectal cancer stem cells bioactivity and interaction with endothelial cells. Life Sci. 2020, 257, 118078. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Leong, R.W. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J. Gastroenterol. Hepatol. 2010, 25, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Larsson, S.C.; Spyrou, N.; Mantzoros, C.S. Body fatness associations with cancer: Evidence from recent epidemiological studies and future directions. Metabolism 2022, 137, 155326. [Google Scholar] [CrossRef]
- Korn, A.R.; Reedy, J.; Brockton, N.T.; Kahle, L.L.; Mitrou, P.; Shams-White, M.M. The 2018 World Cancer Research Fund/American Institute for Cancer Research Score and Cancer Risk: A Longitudinal Analysis in the NIH-AARP Diet and Health Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1983–1992. [Google Scholar] [CrossRef]
- Mayén, A.L.; Viallon, V.; Botteri, E.; Proust-Lima, C.; Bagnardi, V.; Batista, V.; Cross, A.J.; Laouali, N.; MacDonald, C.J.; Severi, G.; et al. A longitudinal evaluation of alcohol intake throughout adulthood and colorectal cancer risk. Eur. J. Epidemiol. 2022, 37, 915–929. [Google Scholar] [CrossRef]
- Hermelink, R.; Leitzmann, M.F.; Markozannes, G.; Tsilidis, K.; Pukrop, T.; Berger, F.; Baurecht, H.; Jochem, C. Sedentary behavior and cancer-an umbrella review and meta-analysis. Eur. J. Epidemiol. 2022, 37, 447–460. [Google Scholar] [CrossRef]
- Abolhassani, M.; Asadikaram, G.; Paydar, P.; Fallah, H.; Aghaee-Afshar, M.; Moazed, V.; Akbari, H.; Moghaddam, S.D.; Moradi, A. Organochlorine and organophosphorous pesticides may induce colorectal cancer; A case-control study. Ecotoxicol. Environ. Saf. 2019, 178, 168–177. [Google Scholar] [CrossRef]
- Xu, A.G.; Jiang, B.; Zhong, X.H.; Yu, Z.J.; Liu, J.H. The trend of clinical characteristics of colorectal cancer during the past 20 years in Guangdong province. Chin. J. Prev. Med. 2006, 86, 272–275. [Google Scholar]
- Dai, Z.; Zheng, R.S.; Zou, X.N.; Zhang, S.W.; Zeng, H.M.; Li, N.; Chen, W.Q. Analysis and prediction of colorectal cancer incidence trend in China. Chin. J. Prev. Med. 2012, 46, 598–603. [Google Scholar]
- Taira, T.; Yamaguchi, S.; Takahashi, A.; Okazaki, Y.; Yamaguchi, A.; Sakaguchi, H.; Chiji, H. Dietary polyphenols increase fecal mucin and immunoglobulin A and ameliorate the disturbance in gut microbiota caused by a high fat diet. J. Clin. Biochem. Nutr. 2015, 57, 212–216. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Ushiroda, C.; Ohnogi, H.; Kudo, Y.; Yasui, M.; Inui, S.; et al. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G367–G375. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, C.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]

| Characteristics/Dietary Intakes | Cases (n = 2806) | Controls (n = 2806) | p a |
|---|---|---|---|
| Age (years), mean (SD) | 57.11 (10.28) | 57.06 (9.90) | 0.747 |
| Men, n (%) | 1606 (57.23) | 1606 (57.23) | >0.99 |
| Married, n (%) | 2667 (95.05) | 2551 (90.91) | <0.001 |
| Rural, n (%) | 1001 (35.67) | 632 (22.52) | <0.001 |
| Education, n (%) | <0.001 | ||
| Unknown | 1 (0.04) | 3 (0.11) | |
| Primary school or below | 871 (31.04) | 631 (22.49) | |
| Middle school | 784 (27.94) | 704 (25.09) | |
| High school/technical school | 684 (24.38) | 754 (26.87) | |
| College or above | 466 (16.61) | 714 (25.45) | |
| Occupation, n (%) | 0.004 | ||
| Administrator/other white-collar | 398 (14.18) | 488 (17.39) | |
| Blue-collar worker | 624 (22.24) | 607 (21.63) | |
| Farmer/other | 1784 (63.58) | 1711 (60.98) | |
| Income (Yuan/month), n (%) | <0.001 | ||
| <2000 | 381 (13.58) | 359 (12.79) | |
| 2001–5000 | 938 (33.43) | 1086 (38.70) | |
| 5001–8000 | 831 (29.62) | 855 (30.47) | |
| >8001 | 656 (23.38) | 506 (18.03) | |
| Postmenopausal b | 869 (72.42) | 885 (73.75) | 0.462 |
| Menarche age (years), mean (SD) b | 14.81 (2.56) | 14.61 (3.03) | 0.910 |
| Occupational activity, n (%) | <0.001 | ||
| Nonworking | 334 (11.90) | 960 (34.21) | |
| Sedentary | 800 (28.51) | 580 (20.67) | |
| Light | 774 (27.58) | 666 (23.73) | |
| Moderate | 420 (14.97) | 268 (9.55) | |
| Heavy | 478 (17.03) | 332 (11.83) | |
| MET (h/week), median (IQR) | 27.66 (8.50–52.50) | 34.31 (15.75–56.00) | <0.001 |
| BMI (kg/m2), mean (SD) | 23.34 (3.29) | 23.60 (3.13) | 0.002 |
| Ever smokers, n (%) | 1106 (39.42) | 857 (30.54) | <0.001 |
| Passive smoking, n (%) | 795 (28.33) | 812 (28.94) | 0.616 |
| Regular drinkers, n (%) | 506 (18.03) | 395 (14.08) | <0.001 |
| History of cancer in first-degree relatives, n (%) | 416 (14.83) | 235 (8.37) | <0.001 |
| Dietary intakes, median (IQR) c | |||
| Energy (kcal/d) | 1584.66 (1294.21–1940.52) | 1646.06 (1366.02–2018.51) | <0.001 |
| Vegetables (g/d) | 384.13 (281.16–511.12) | 410.75 (304.95–527.37) | <0.001 |
| Fruits (g/d) | 86.31 (42.69–144.88) | 121.92 (67.60–187.00) | <0.001 |
| Total fat (g/d) | 30.42 (22.53–39.23) | 30.42 (23.82–37.55) | 0.909 |
| SFA (g/d) | 11.59 (8.38–15.24) | 11.61 (8.94–14.76) | 0.532 |
| MUFA (g/d) | 13.55 (9.84–17.98) | 13.11 (10.13–16.65) | 0.009 |
| PUFA (g/d) | 4.65 (3.67–5.83) | 4.93 (3.93–6.08) | <0.001 |
| n-3 LC-PUFAs (mg/d) d | 38.29 (18.89–76.04) | 44.40 (23.95–79.77) | <0.001 |
| Fatty Acid | Common Name | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|---|
| Even-Long-Chain Fatty Acid Pattern | Medium-Chain and Long-Chain Saturated Fatty Acid Pattern | Highly Unsaturated Fatty Acid Pattern | Odd-Chain Fatty Acid Pattern | ||
| C18:1 | oleic | 0.962 * | 0.053 | 0.032 | 0.096 |
| C16:0 | palmitic | 0.929 * | 0.185 | 0.041 | 0.152 |
| C18:0 | stearic | 0.921 * | 0.090 | −0.017 | 0.146 |
| C16:1 | palmitoleic | 0.901 * | 0.017 | 0.161 | 0.169 |
| C20:0 | arachidic | 0.874 * | 0.161 | 0.145 | 0.052 |
| C16:2 | hexadecadienoic | 0.857 * | −0.100 | −0.117 | 0.088 |
| C18:3 | ALA | 0.787 * | 0.041 | 0.262 | −0.041 |
| C20:2 | eicosadienoic | 0.774 * | −0.129 | −0.167 | 0.348 |
| C18:2 | LA | 0.593 * | 0.236 | 0.216 | −0.003 |
| C20:1 | gadoleic | 0.531 * | 0.116 | 0.222 | 0.136 |
| C10:0 | capric | 0.025 | 0.968 * | −0.053 | 0.116 |
| C6:0 | caproic | −0.012 | 0.930 * | −0.053 | 0.115 |
| C14:1 | myristoleic | 0.023 | 0.912 * | 0.048 | 0.178 |
| C13:0 | tridecanoic | 0.020 | 0.840 * | −0.025 | 0.379 |
| C14:0 | myristic | 0.405 | 0.797 * | 0.012 | 0.291 |
| C20:3 | eicosatrienoic | 0.003 | 0.785 * | 0.284 | 0.129 |
| C8:0 | caprylic | 0.033 | 0.759 * | −0.061 | −0.017 |
| C12:0 | lauric | 0.173 | 0.658 * | −0.093 | 0.016 |
| C20:5 | EPA | 0.074 | 0.000 | 0.928 * | 0.121 |
| C22:4 | docosatetraenoic | 0.068 | 0.055 | 0.866 * | 0.127 |
| C22:6 | DHA | 0.081 | −0.054 | 0.844 * | 0.116 |
| C22:5 | DPA | 0.127 | −0.045 | 0.843 * | 0.098 |
| C22:3 | docosatrienoic | 0.065 | −0.015 | 0.714 * | −0.031 |
| C20:4 | AA | 0.540 * | 0.004 | 0.558 * | 0.535 * |
| C17:1 | heptadecenoic | 0.103 | 0.282 | 0.153 | 0.905 * |
| C15:1 | pentadecenoic | 0.063 | 0.170 | 0.311 | 0.852 * |
| C17:0 | heptadecanoic | 0.504 * | 0.155 | −0.054 | 0.800 * |
| C11:0 | undecanoic | 0.093 | 0.105 | 0.052 | 0.763 * |
| C15:0 | pentadecanoic | 0.161 | 0.619 * | 0.101 | 0.662 * |
| C19:0 | nonadecanoic | 0.192 | 0.238 | 0.412 | 0.365 |
| C22:0 | behenic | 0.228 | 0.425 | 0.303 | 0.011 |
| C22:1 | erucic | 0.120 | 0.051 | 0.072 | 0.001 |
| Dietary Fatty Acid Patterns | Q1 | Q2 | Q3 | Q4 | Q5 | ptrendb |
|---|---|---|---|---|---|---|
| Even-long-chain fatty acid pattern | ||||||
| No. of cases/controls | 585/561 | 522/561 | 484/562 | 510/561 | 705/561 | |
| cOR (95%CI) | 1.00 | 0.89 (0.76–1.05) | 0.83 (0.70–0.98) | 0.87 (0.74–1.03) | 1.21 (1.03–1.42) | 0.036 |
| aOR (95%CI) a | 1.00 | 0.94 (0.79–1.13) | 0.92 (0.76–1.11) | 0.93 (0.77–1.12) | 1.16 (0.97–1.39) | 0.129 |
| Medium-chain and long-chain saturated fatty acid pattern | ||||||
| No. of cases/controls | 987/561 | 773/561 | 552/562 | 276/561 | 218/561 | |
| cOR (95%CI) | 1.00 | 0.78 (0.67–0.91) | 0.56 (0.48–0.65) | 0.28 (0.23–0.33) | 0.22 (0.18–0.27) | <0.001 |
| aOR (95%CI) a | 1.00 | 0.85 (0.72–1.00) | 0.67 (0.57–0.80) | 0.41 (0.33–0.50) | 0.34 (0.27–0.42) | <0.001 |
| Highly unsaturated fatty acid pattern | ||||||
| No. of cases/controls | 775/561 | 563/561 | 552/562 | 450/561 | 466/561 | |
| cOR (95%CI) | 1.00 | 0.73 (0.62–0.85) | 0.71 (0.61–0.83) | 0.58 (0.49–0.69) | 0.60 (0.51–0.71) | <0.001 |
| aOR (95%CI) a | 1.00 | 0.89 (0.75–1.07) | 0.89 (0.75–1.07) | 0.79 (0.65–0.95) | 0.73 (0.60–0.88) | <0.001 |
| Odd-chain fatty acid pattern | ||||||
| No. of cases/controls | 741/561 | 660/561 | 538/562 | 420/561 | 447/561 | |
| cOR (95%CI) | 1.00 | 0.89 (0.76–1.04) | 0.73 (0.62–0.85) | 0.57 (0.48–0.67) | 0.60 (0.51–0.71) | <0.001 |
| aOR (95%CI) a | 1.00 | 0.92 (0.77–1.09) | 0.78 (0.66–0.94) | 0.66 (0.55–0.79) | 0.69 (0.57–0.83) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, K.; Ma, T.; Zhou, R.; Xu, L.; Fang, Y.; Zhang, C. Association between Dietary Fatty Acid Patterns and Colorectal Cancer Risk: A Large-Scale Case-Control Study in China. Nutrients 2022, 14, 4375. https://doi.org/10.3390/nu14204375
Tu K, Ma T, Zhou R, Xu L, Fang Y, Zhang C. Association between Dietary Fatty Acid Patterns and Colorectal Cancer Risk: A Large-Scale Case-Control Study in China. Nutrients. 2022; 14(20):4375. https://doi.org/10.3390/nu14204375
Chicago/Turabian StyleTu, Kexin, Ting Ma, Ruolin Zhou, Lei Xu, Yujing Fang, and Caixia Zhang. 2022. "Association between Dietary Fatty Acid Patterns and Colorectal Cancer Risk: A Large-Scale Case-Control Study in China" Nutrients 14, no. 20: 4375. https://doi.org/10.3390/nu14204375
APA StyleTu, K., Ma, T., Zhou, R., Xu, L., Fang, Y., & Zhang, C. (2022). Association between Dietary Fatty Acid Patterns and Colorectal Cancer Risk: A Large-Scale Case-Control Study in China. Nutrients, 14(20), 4375. https://doi.org/10.3390/nu14204375







