The Immune System Response to 15-kDa Barley Protein: A Mouse Model Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Barley Grains
2.2. Beer
2.3. Protein Extraction and Purification
Protein Purification
2.4. Determination of Protein Concentration
2.5. Sodium Dodecyl Sulfate−Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Identification of IgE Reactive Barley Fraction
Indirect IgE ELISA
2.7. Biological Experiment
Sample Collection
2.8. Mouse Humoral Response Determination
Indirect ELISA
2.9. Lymphocyte Isolation
2.10. Lymphocyte Culture
2.10.1. Proliferation Assay
2.10.2. B-Cell ELISpot
2.11. In Silico Analysis of Immunoreactivity
2.12. Statistical Analysis
3. Results and Discussion
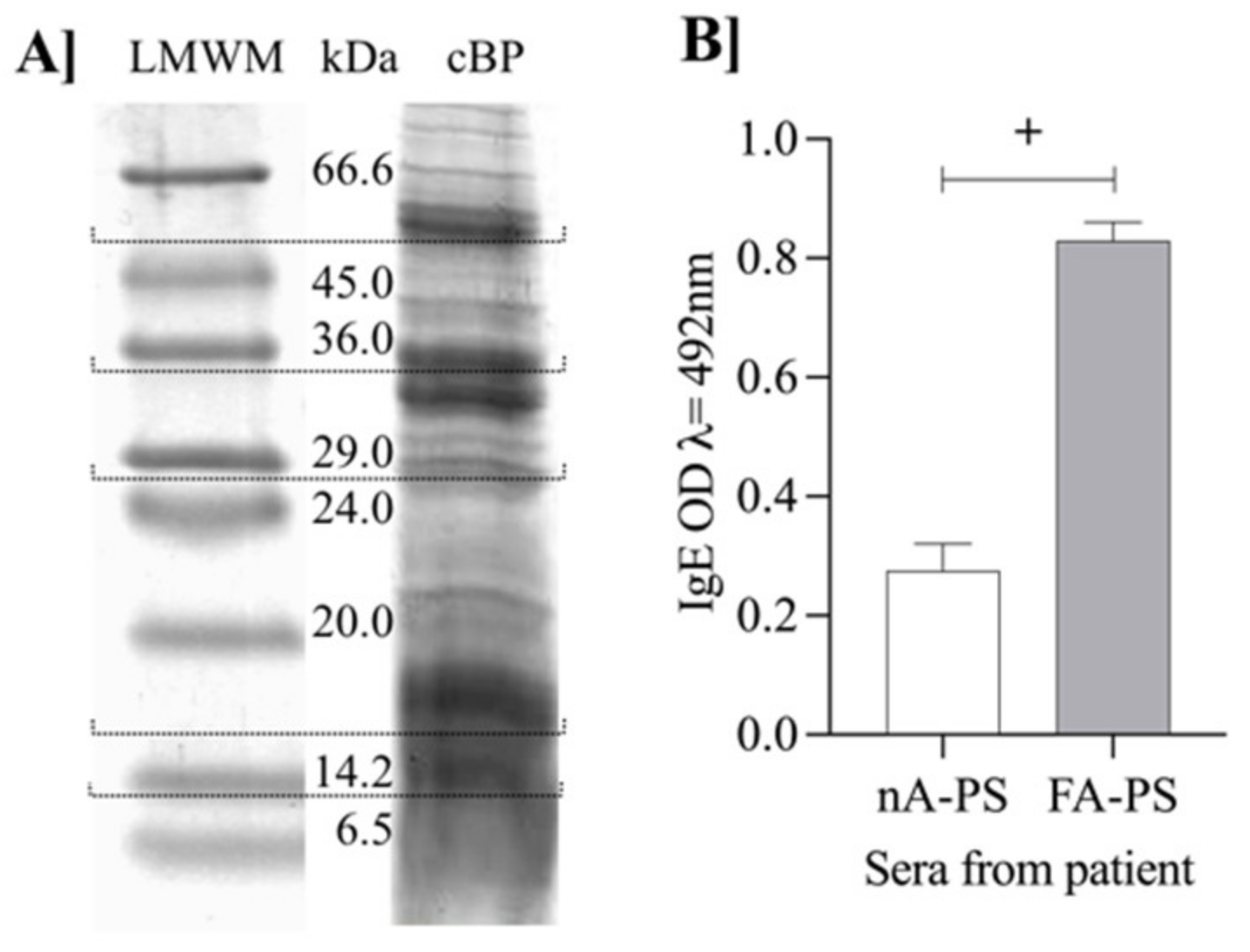
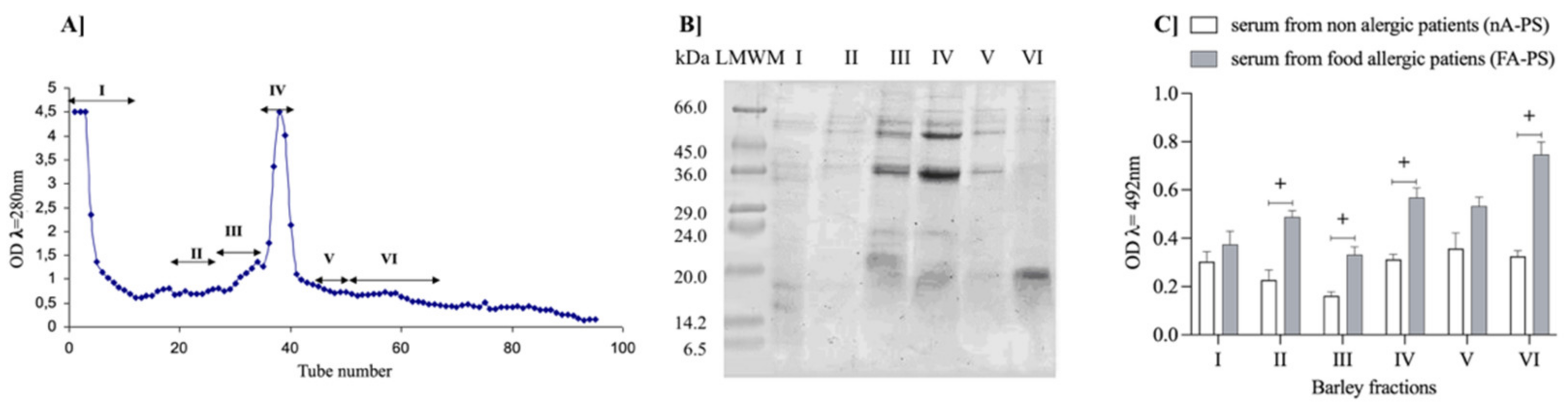
3.1. Characteristic Barley Protein
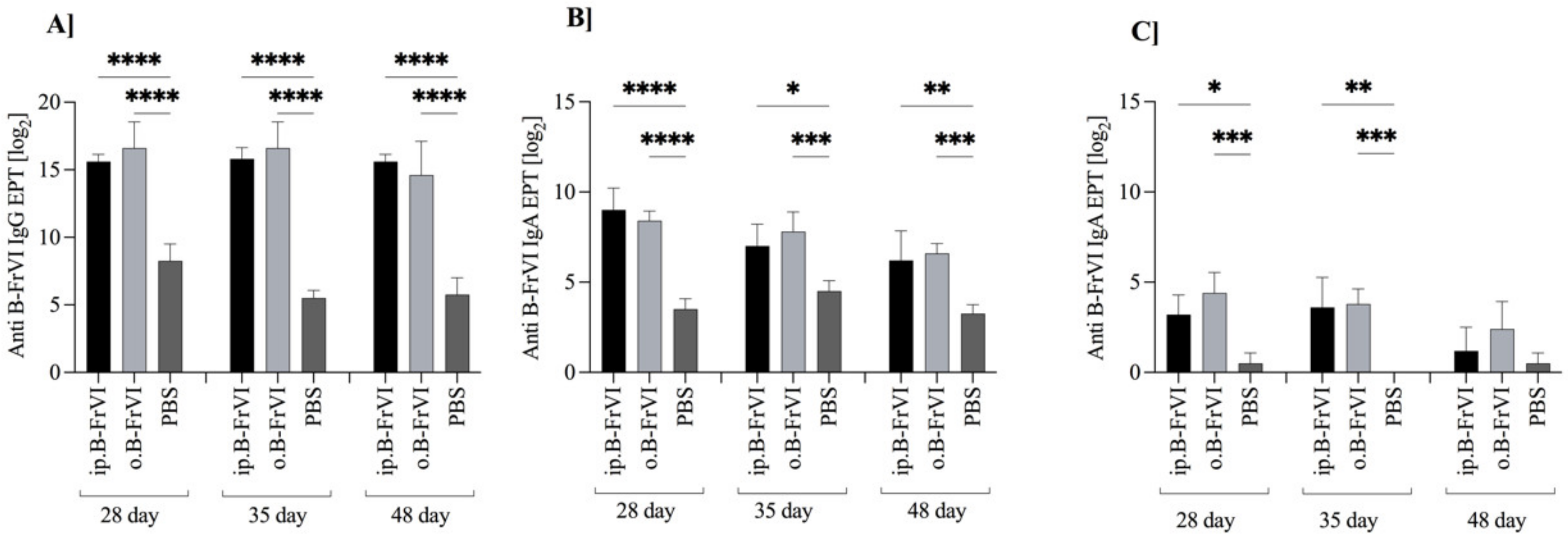
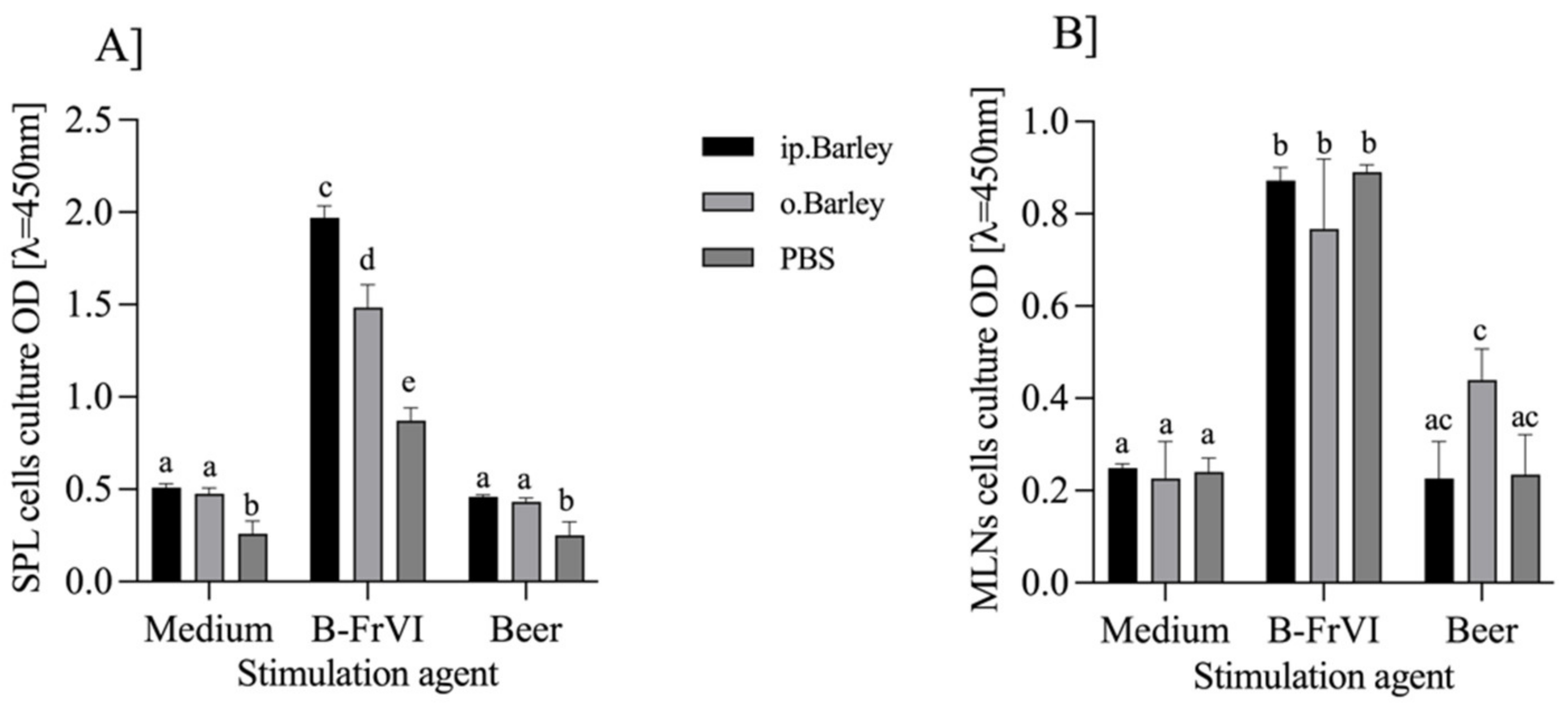
3.2. Immune Response to Barley
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hematian Sourki, A.; Koocheki, A.; Elahi, M. Ultrasound-assisted extraction of β-d-glucan from hull-less barley: Assessment of physicochemical and functional properties. Int. J. Biol. Macromol. 2017, 95, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhong, S.; Tang, Y.; Chen, L. Understanding the nutritional functions of thermally-processed whole grain highland barley in vitro and in vivo. Food Chem. 2020, 310, 125979. [Google Scholar] [CrossRef]
- Punia, S. Barley starch modifications: Physical, chemical and enzymatic—A review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- Sudharson, S.; Kalic, T.; Hafner, C.; Breiteneder, H. Newly defined allergens in the WHO/IUIS Allergen Nomenclature Database during 01/2019-03/2021. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 3359–3373. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Ullrich, S.E. The Barley Crop: Origin and Taxonomy, Production, and End Uses. In Barley: Chemistry and Technology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 1–9. ISBN 9780128123690. [Google Scholar]
- Quirce, S.; Diaz-Perales, A. Management of Grain-Induced Asthma pathogenesis and risk factors. Allergy Asthma Immunol. Res. 2013, 5, 348–356. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. Barley. In Cereal Grains for the Food and Beverage Industries; Arendt, E.K., Zannini, E., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 155–200+201e. [Google Scholar] [CrossRef]
- Farag, M.A.; Xiao, J.; Abdallah, H.M. Nutritional value of barley cereal and better opportunities for its processing as a value-added food: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 62, 1092–1104. [Google Scholar] [CrossRef]
- Barber, D.; Sanchez-Monge, R.; Gomez, L.; Carpizo, J.; Armentia, A.; Lopez-Otin, C.; Juan, F.; Salcedo, G. A barley flour inhibitor of insect alpha-amylase is a major allergen associated with baker’s asthma disease. FEBS Lett. 1989, 248, 119–122. [Google Scholar] [CrossRef]
- Armenita, A.; Sanchez-Monge, R.; Gomez, L.; Barber, D.; Salcedo, G. In vivo allergenic activities of eleven purified members of a major allergen family from wheat and barley flour. Clin. Exp. Allergy 1993, 23, 410–415. [Google Scholar] [CrossRef]
- García-Casado, G.; Crespo, J.F.; Rodríguez, J.; Salcedo, G. Isolation and characterization of barley lipid transfer protein and protein Z as beer allergens. J. Allergy Clin. Immunol. 2001, 108, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jeong, K.; Lee, J.; Jeon, S.A.; Park, B.; Lee, H.; Lee, S. Clinical and laboratory findings of barley allergy in Korean children: A single hospital based retrospective study. J. Korean Med. Sci. 2020, 35, e23. [Google Scholar] [CrossRef] [PubMed]
- Chudzik-Kozłowska, J.; Wasilewska, E.; Złotkowska, D. Evaluation of Immunoreactivity of Pea (Pisum sativum) Albumins in BALB/c and C57BL/6 Mice. J. Agric. Food Chem. 2020, 68, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, B.; Szyc, A.M.; Markiewicz, L.H.; Zakrzewska, M.; Romaszko, E. Increased prevalence of eating disorders as a biopsychosocial implication of food allergy. PLoS ONE 2018, 13, e0198607. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Z.; Hou, Y.; Hu, J.; He, Y.; Chen, J.; Ji, K. Enhanced sensitivity of capture IgE ELISA based on a recombinant Der f 1/2 fusion protein for the detection of IgE antibodies targeting house dust mite allergens. Mol. Med. Rep. 2019, 49, 3497–3504. [Google Scholar] [CrossRef]
- Funda, D.P.; Kaas, A.; Bock, T.; Tlaskalová-Hogenová, H.; Buschard, K. Gluten-free diet prevents diabetes in NOD mice. Diabetes. Metab. Res. Rev. 1999, 15, 323–327. [Google Scholar] [CrossRef]
- Wasilewska, E.; Zlotkowska, D.; Wróblewska, B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis. J. Dairy Sci. 2019, 102, 37–53. [Google Scholar] [CrossRef]
- Złotkowska, D.; Stachurska, E.; Fuc, E.; Wróblewska, B.; Mikołajczyk, A.; Wasilewska, E. Differences in regulatory mechanisms induced by β-lactoglobulin and κ-casein in cow’s milk allergy mouse model-in vivo and ex vivo studies. Nutrients 2021, 13, 349. [Google Scholar] [CrossRef]
- Fuc, E.; Złotkowska, D.; Wasilewska, E.; Wróblewska, B. Ova-experienced cd4+ t cell transfer and chicken protein challenge affect the immune response to ova in a murine model. Int. J. Mol. Sci. 2021, 22, 6573. [Google Scholar] [CrossRef]
- Ogrodowczyk, A.M.; Dimitrov, I.; Wróblewska, B. Two Faces of Milk Proteins Peptides with Both Allergenic and Multidimensional Health Beneficial Impact—Integrated In Vitro/In Silico Approach. Foods 2021, 10, 163. [Google Scholar] [CrossRef]
- Kadiyska, T.; Mladenova, M.; Dimitrov, I.; Doytchinova, I. Milk allergy in HLA-drb1∗14:19/14:21 pediatric patients: A bioinformatics approach. Pharmacia 2018, 65, 23–27. [Google Scholar]
- Gilissen, L.J.W.J.; Van der Meer, I.M.; Smulders, M.J.M. Reducing the incidence of allergy and intolerance to cereals. J. Cereal Sci. 2014, 59, 337–353. [Google Scholar] [CrossRef]
- Varjonen, E.; Savolainen, J.; Mattila, L.; Kalimo, K. IgE-binding components of wheat, rye, barley and oats recognized by immunoblotting analysis with sera from adult atopic dermatitis patients. Clin. Exp. Allergy 1994, 24, 481–489. [Google Scholar] [CrossRef]
- Chmelík, J.; Řehulka, P.; Střelcová, M.; Kubáň, V.; Mayrhofer, C.; Allmaier, G. Proteomic analysis of different extracts from barley grains. Rostl. Vyroba 2002, 48, 261–264. [Google Scholar] [CrossRef]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley Protein Properties, Extraction and Applications, with a Focus on Brewers’ Spent Grain Protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.; Sanchez-Monge, R.; Gomez, L.; Salcedo, G.; Carbonero, P. A major barley allergen associated with baker’s asthma disease is a glycosylated monomeric inhibitor of insect alpha-amylase: cDNA cloning and chromosomal location of the gene. Plant Mol. Biol. 1992, 20, 451–458. [Google Scholar] [CrossRef]
- Weiberg, D.; Basic, M.; Smoczek, M.; Bode, U.; Bornemann, M.; Buettnerid, M.; Buettner, M. Participation of the spleen in the IgA immune response in the gut. PLoS ONE 2018, 13, e0205247. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, S.; Wakamatsu, E.; Ishida, Y.; Obata, Y.; Kurashima, Y.; Kiyono, H.; Abe, R. The spleen is the site where mast cells are induced in the development of food allergy. Int. Immunol. 2017, 29, 31–45. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, B.; Kubicka, E.; Semenowicz, E.; Ogrodowczyk, A.; Mikołajczyk, A.; Złotkowska, D. The Immune System Response to 15-kDa Barley Protein: A Mouse Model Study. Nutrients 2022, 14, 4371. https://doi.org/10.3390/nu14204371
Wróblewska B, Kubicka E, Semenowicz E, Ogrodowczyk A, Mikołajczyk A, Złotkowska D. The Immune System Response to 15-kDa Barley Protein: A Mouse Model Study. Nutrients. 2022; 14(20):4371. https://doi.org/10.3390/nu14204371
Chicago/Turabian StyleWróblewska, Barbara, Ewa Kubicka, Ewelina Semenowicz, Anna Ogrodowczyk, Anita Mikołajczyk, and Dagmara Złotkowska. 2022. "The Immune System Response to 15-kDa Barley Protein: A Mouse Model Study" Nutrients 14, no. 20: 4371. https://doi.org/10.3390/nu14204371
APA StyleWróblewska, B., Kubicka, E., Semenowicz, E., Ogrodowczyk, A., Mikołajczyk, A., & Złotkowska, D. (2022). The Immune System Response to 15-kDa Barley Protein: A Mouse Model Study. Nutrients, 14(20), 4371. https://doi.org/10.3390/nu14204371







