Effects of Polyphenol Consumption on Recovery in Team Sport Athletes of Both Sexes: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
3. Results
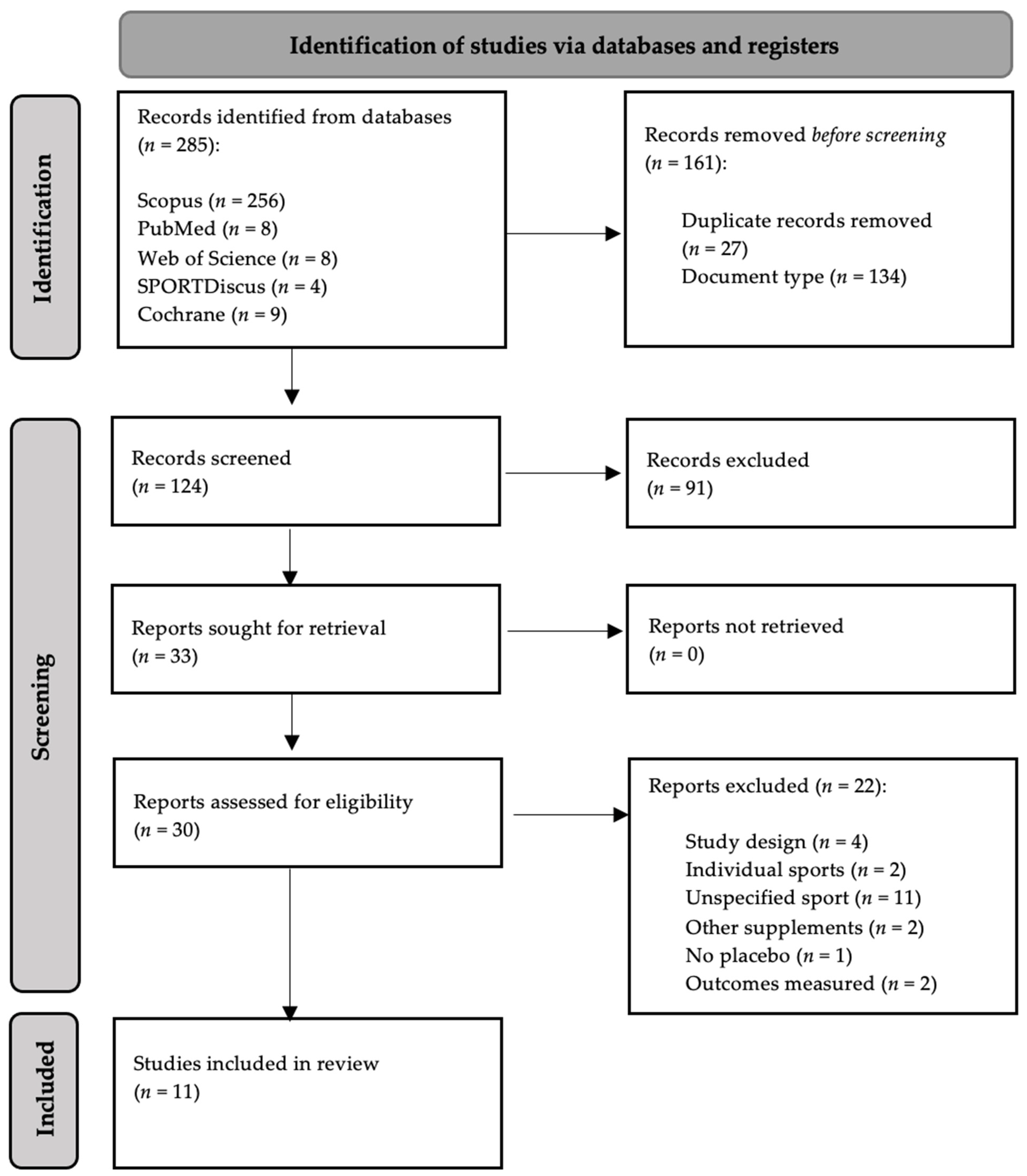
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Methods
3.2.2. Participants
3.2.3. Intervention
3.3. Outcomes
3.3.1. Physical Performance
3.3.2. Muscle Damage Biomarkers
3.3.3. Oxidative Stress Biomarkers
3.4. Risk of Bias
3.5. Synthesis of Results
3.5.1. Physical Performance Tests Findings in This Review
3.5.2. Effect of Polyphenols on Muscle Damage Biomarkers
3.5.3. Oxidative Stress Biomarkers Findings in This Review
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, L.; Rollo, I.; Stein, K.; Jeukendrup, A. Acute Effects of Carbohydrate Supplementation on Intermittent Sports Performance. Nutrients 2015, 7, 5733–5763. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.; Stevenson, E.; Davison, G.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.C.; Lucey, A.; Doyle, L. Flavonoid Containing Polyphenol Consumption and Recovery from Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1293–1316. [Google Scholar] [CrossRef]
- Jamurtas, A. Exercise-Induced Muscle Damage and Oxidative Stress. Antioxidants 2018, 7, 50. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sport. Med.—Open 2019, 5. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6323061/#CR88 (accessed on 18 June 2022). [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2018, 19, 71–85. [Google Scholar] [CrossRef]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1589–1599. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5925142/ (accessed on 10 September 2021). [CrossRef]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.C.; Hueglin, S.; Broad, E. Tart Cherry Juice in Athletes. Curr. Sport. Med. Rep. 2017, 16, 230–239. [Google Scholar] [CrossRef]
- Connolly, D.A.J. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sport. Med. 2006, 40, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Trombold, J.R.; Reinfeld, A.S.; Casler, J.R.; Coyle, E.F. The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. J. Strength Cond. Res. 2011, 25, 1782–1788. Available online: https://pubmed.ncbi.nlm.nih.gov/21659887/ (accessed on 12 June 2020). [CrossRef] [PubMed]
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. J. Int. Soc. Sport. Nutr. 2012, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Berntzen, B.; Davison, G.; West, D.; Howatson, G.; Stevenson, E. Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.; Wollman, P.; Jackman, S.; Bowtell, J. Flavanol-Rich Cacao Mucilage Juice Enhances Recovery of Power but Not Strength from Intensive Exercise in Healthy, Young Men. Sports 2018, 6, 159. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Flieller, E.B.; Dillon, K.J.; Leverett, B.D. Black Currant Nectar Reduces Muscle Damage and Inflammation Following a Bout of High-Intensity Eccentric Contractions. J. Diet. Suppl. 2014, 13, 1–15. [Google Scholar] [CrossRef]
- Doma, K.; Gahreman, D.; Connor, J. Fruit supplementation reduces indices of exercise-induced muscle damage: A systematic review and meta-analysis. Eur. J. Sport Sci. 2020, 21, 1–36. [Google Scholar] [CrossRef]
- Rickards, L.; Lynn, A.; Harrop, D.; Barker, M.E.; Russell, M.; Ranchordas, M.K. Effect of Polyphenol-Rich Foods, Juices, and Concentrates on Recovery from Exercise Induced Muscle Damage: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2988. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Gaspar, D.; Flores-Félix, J.D.; Falcão, A.; Alves, G.; Silva, L.R. Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review. Int. J. Mol. Sci. 2022, 23, 4652. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Casado, A.; Domínguez, R.; Fernandes da Silva, S.; Bailey, S.J. Influence of Sex and Acute Beetroot Juice Supplementation on 2 KM Running Performance. Appl. Sci. 2021, 11, 977. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- O’Connor, D.A.; Green, S.E.; Higgins, J.P.T. Defining the review question and developing criteria for including studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: West Sussex, UK, 2008; pp. 83–94. Available online: https://research.monash.edu/en/publications/defining-the-review-question-and-developing-criteria-for-includin (accessed on 29 July 2022).
- RevMan 5. Available online: https://training.cochrane.org/online-learning/core-software/revman/revman-5-download (accessed on 13 January 2022).
- PEDro Scale (English). PEDro. 2019. Available online: https://www.pedro.org.au/english/downloads/pedro-scale/ (accessed on 27 August 2022).
- Jówko, E.; Sacharuk, J.; Bałasinska, B.; Wilczak, J.; Charmas, M.; Ostaszewski, P.; Charmas, R. Effect of a Single Dose of Green Tea Polyphenols on the Blood Markers of Exercise-Induced Oxidative Stress in Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Pourmasoumi, M.; Kafeshani, M.; Karimian, J.; Maracy, M.R.; Entezari, M.H. The Effect of Green Tea and Sour Tea (Hibiscus sabdariffa L.) Supplementation on Oxidative Stress and Muscle Damage in Athletes. J. Diet. Suppl. 2016, 14, 346–357. [Google Scholar] [CrossRef]
- Assunção Carvalho, L.C.S.; de Freitas, M.C.; Silva, A.S.; Biasoto, A.C.T.; Martins, M.D.C.C.E.; de Moura, R.C.; Brito, A.K.D.S.; Silva, A.S.V.E.; Ribeiro, S.L.G.; Rossi, F.E.; et al. Syzygium cumini NectarSupplementation Reduced Biomarkers of Oxidative Stress, Muscle Damage, and Improved Psychological Response in Highly Trained Young Handball Players. Front. Physiol. 2018, 9, 1508. [Google Scholar] [CrossRef]
- Kupusarevic, J.; McShane, K.; Clifford, T. Cherry Gel Supplementation Does Not Attenuate Subjective Muscle Soreness or Alter Wellbeing Following a Match in a Team of Professional Rugby Union players: A Pilot Study. Sports 2019, 7, 84. [Google Scholar] [CrossRef]
- Abbott, W.; Brashill, C.; Brett, A.; Clifford, T. Tart Cherry Juice: No Effect on Muscle Function Loss or Muscle Soreness in Professional Soccer Players After a Match. Int. J. Sport. Physiol. Perform. 2019, 15, 1–21. [Google Scholar] [CrossRef]
- Quinlan, R.; Hill, J.A. The Efficacy of Tart Cherry Juice in Aiding Recovery After Intermittent Exercise. Int. J. Sport. Physiol. Perform. 2019, 15, 368–374. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31614329 (accessed on 7 December 2021). [CrossRef]
- Morehen, J.C.; Clarke, J.; Batsford, J.; Barrow, S.; Brown, A.D.; Stewart, C.E.; Morton, J.P.; Close, G.L. Montmorency tart cherry juice does not reduce markers of muscle soreness, function and inflammation following professional male rugby League match-play. Eur. J. Sport Sci. 2020, 21, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.C.; Dorneles, G.P.; Blembeel, A.S.; Marinho, J.P.; Proença, I.C.T.; da Cunha Goulart, M.J.V.; Moller, G.B.; Marques, E.P.; Pochmann, D.; Salvador, M.; et al. Effects of grape juice consumption on oxidative stress and inflammation in male volleyball players: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2020, 54, C102570. Available online: https://pubmed.ncbi.nlm.nih.gov/33183673/ (accessed on 5 August 2021). [CrossRef] [PubMed]
- Stankiewicz, B.; Cieślicka, M.; Kujawski, S.; Piskorska, E.; Kowalik, T.; Korycka, J.; Skarpańska-Stejnborn, A. Effects of antioxidant supplementation on oxidative stress balance in young footballers—A randomized double-blind trial. J. Int. Soc. Sport. Nutr. 2021, 18, 44. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 414–423. Available online: https://pubmed.ncbi.nlm.nih.gov/25794236/ (accessed on 24 January 2021). [CrossRef] [PubMed]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC) and Phenolic and Anthocyanin Concentrations in Fruit and Leaf Tissues of Highbush Blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Clifford, T.; Allerton, D.; Brown, M.; Harper, L.; Horsburgh, S.; Keane, K.; Stevenson, E.J.; Howatson, G. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Et Metab. 2017, 42, 263–270. Available online: https://pubmed.ncbi.nlm.nih.gov/28165768/ (accessed on 6 May 2022). [CrossRef]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur. J. Sport Sci. 2018, 19, 95–102. [Google Scholar] [CrossRef]
- de Assumpção, D.; Domene, S.M.Á.; Fisberg, R.M.; Canesqui, A.M.; de Azevedo Barros, M.B. Diferenças entre homens e mulheres na qualidade da dieta: Estudo de base populacional em Campinas, São Paulo. Ciência Saúde Coletiva 2017, 22, 347–358. [Google Scholar] [CrossRef]
- Le, G.; Novotny, S.A.; Mader, T.L.; Greising, S.M.; Chan, S.S.K.; Kyba, M.; Lowe, D.A.; Warren, G.L. A moderate oestradiol level enhances neutrophil number and activity in muscle after traumatic injury but strength recovery is accelerated. J. Physiol. 2018, 596, 4665–4680. [Google Scholar] [CrossRef]
- Sánchez-Martínez, L.; Periago, M.-J.; García-Alonso, J.; García-Conesa, M.-T.; González-Barrio, R. A Systematic Review of the Cardiometabolic Benefits of Plant Products Containing Mixed Phenolics and Polyphenols in Postmenopausal Women: Insufficient Evidence for Recommendations to This Specific Population. Nutrients 2021, 13, 4276. [Google Scholar] [CrossRef]
| Study Id | Population | Exposure to Polyphenols | Exercise | Outcomes Analyzed | ||||
|---|---|---|---|---|---|---|---|---|
| Author/s-Year | Study Design | Characteristics (Sample Size) Age | Kind Dose, Timing | Polyphenolic Content | EIMD | Physical Performance Test | Muscle Damage Biomarkers | Oxidative Stress Biomarkers |
| Jówko et al., 2012 [28] | RCT Double-blind Parallel | Local club soccer players (n = 16) 22.4 ± 3.4 | Green tea polyphenols (GTP) 640 mg per 1 day | * 1 capsule: Total of 320 mg polyphenols, including about 250 mg catechins | Muscle-endurance test | n/a | Pre, 5 min and 24 h post: ↔ CK | Pre, 5 min and 24 h post: ↔ TBARS ↔ UA ↔ Total catechins ↔ TAS ↔ SOD |
| Bell et al., 2016 [2] | RCT Double-blind Parallel | Semi-professional male soccer players (n = 16) 25 ± 4 years | Montmorency tart cherry concentrate (MC) 2 × 30 mL per day for 8 days | * 1000 mL: Total flavonoids: 73.5 mg cyanidin-3-glucoside Total phenols: 178.8 mg of GAE | 12 × 20 m sprint followed by LIST (6 × 15 min sections). | 24, 48, 72 h post: ↑ MVIC ↑ CMJ ↓ DOMS (VAS) ↑ 20 m sprint ↑ 5-0-5 Agility | 0, 1, 3, 5, 24, 48, 72 h post: ↔ CK ↔ hsCRP | 0, 1, 3, 5, 24, 48, 72 h post: ↔ LOOH |
| Clifford et al., 2016 [16] | RCT Double-blind Parallel | Collegiate male team sports players (n = 20) (Soccer (n = 10), rugby (n = 5), basketball (n = 2) hockey (n = 2) or handball (n = 1)) 21–23 years | Beetroot juice (BTJ) 2 × 250 mL per day for 3 days | - | 2 RST: RST1 (20 × 30 m) and RST2 (72 h later). | Pre, post, 24, 48 and 72 h after RST1 and post and 24 h after RST2: ↔ MVIC ↑ CMJ ↑ RI ↔ PPT | Pre, post, 2.5, 24, 48 and 72 h after RST1 and post, 2.5 and 24 h after RST2: ↔ CK ↔ hsCRP | Pre, post, 2.5, 24, 48 and 72 h after RST1 and post, 2.5 and 24 h after RST2: ↔ PC ↔ LOOH ↔ A•− |
| Hadi et al., 2016 [29] | RCT Double-blind Parallel | University male soccer players (n = 49) 18–25 years | Green tea extract (GTE) Sour tea extract (STE) 450 mg per day for 6 weeks | - | No specifications given | n/a | Pre and post (6 weeks): ↔ CK ↔ AST ↔ LDH | Pre and post (6 weeks): ↑ TAC (STE) |
| Assunção et al., 2018 [30] | RCT Double-blind Parallel | Elite high school male handball players (n = 25) 18 ± 2.4 years | Syzygium cumini (SC)/ jamelon nectar 10 mL per kilogram per day for 28 days | - | 4 w of periodization of medium-intensity endurance training, maximal power and speed, sport-specific strength and power and techno-tactical skills. | Pre and post (4 weeks): ↔ Vertical jump height ↔ 20 m shuttle run test ↔ Running anaerobic sprint test (RAST) | Pre and post (4 weeks): ↔ CK ↔ LDH | Pre and post (4 weeks): ↔ TAC |
| Kupusarevic et al., 2019 [31] | RCT Double-blind Crossover | Rugby union (RU) elite male players (n = 10) 28 ± 4 years | Tart cherry juice (TCJ) 2 × 30 mL per day for 5 days (2 days before, the day of the match and 2 days after). | - | RU 80 min match | 24, 48, 72 h post: ↔ DOMS ↔ Subjective wellbeing | n/a | n/a |
| Abbott et al., 2020 [32] | RCT Double-blind Crossover | Professional male soccer players (n = 10) 19 ± 1 years | Tart cherry juice (TCJ) 2 × 30 mL per day per 3 days | - | 90-min soccer match | 12, 36, 60 h post: ↔ CMJ ↔ DOMS ↔ RSI ↔ Subjective wellbeing | n/a | n/a |
| Quinlan et al., 2019 [33] | RCT Single-blind Parallel | Team sports male (n = 8) and female (n = 12) players (Soccer, hockey, or netball) 26 ± 4 years | Tart cherry juice (TCJ) 2 × 30 mL per day for 8 days | - | LIST (6 × 15 min sections) followed by 12 × 20 m sprint. | Pre and 1, 24, 48 h post: ↑ MVIC ↑ Agility ↑ CMJ ↓ DOMS ↑ 20 m sprint | Pre and 1, 24, 48 h post: ↔CK ↔ CRP | n/a |
| Morehen et al., 2021 [34] | RCT Single-blind Crossover | Professional Rugby male players (n = 11) 18 ± 1 years | Montmorency cherry juice (MC) 2 × 30 mL per day for 7 days (5 days before and 2 after the match) | * 30 mL: 320 mg of anthocyanins | RU match | 24 pre, 24 and 48 h post: ↔ CMJ ↔ DOMS | n/a | n/a |
| Martins et al., 2020 [35] | RCT Double-blind Crossover | National competitors’ male volleyball players (n = 12) 16 ± 0.6 years | Grape juice (GJ) 400 mL per day for 14 days | * 2.08 ± 0.02 g EAG/L Total flavonoids: 0.258 ± 0.00 g EQ/L | 3 volleyball match simulations | Pre and post each match: ↔ Vertical jump height ↔ Handgrip strength (HG) | Pre and post each match: ↔ CK | Pre and post each match: ↓ TBARS ↔ Carbonyls ↓ DNA damage |
| Stankiewicz et al., 2021 [36] | RCT Double-blind Parallel | Semi-professional male soccer players (n = 20) 15.8 ± 0.7 years | Chokeberry juice 2 × 100 mL per day for 7 weeks | * 165.3 mg/100 mL of anthocyanins | Regular physical training program (microcycle) during the 7 w of supplementation. “The beep test”: Pre and post the 7-w supplementation period, maximal multistage 20-m shuttle run test | Before and after 7 weeks: ↔ 20 m sprint | n/a | 0, 3, 24 h post the beep test. ↔ TAC ↔ TBARS ↔ 8-OHdG |
| Jówko et al., 2012 [28] | Bell et al., 2016 [2] | Clifford et al., 2016 [16] | Hadi et al., 2016 [29] | Assunção et al., 2018 [30] | Kupusarevic et al., 2019 [31] | Abbott et al., 2020 [32] | Quinlan et al., 2019 [33] | Morehen et al., 2021 [34] | Martins et al., 2020 [35] | Stankiewicz et al., 2021 [36] | Studies Meeting Criterion n (%) | |
| 1.Eligibility criteria | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 6 (55%) |
| 2.Randomized allocation | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 11 (100%) |
| 3.Concealed allocation | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 7 64%) |
| 4.Comparable at baseline | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 9 (82%) |
| 5.Blinded subjects | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 11 (100%) |
| 6.Blinded therapists | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 9 (82%) |
| 7.Blinded assessors | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 9 (82%) |
| 8.Adequate follow-up | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 9 (82%) |
| 9.Intention to treat analysis | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 9 (82%) |
| 10.Between-group comparisons | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 11 (100%) |
| 11.Point estimates and variability | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 11 (100%) |
| Total points | 10 (100%) | 6 (60%) | 9 (90%) | 10 (100%) | 10 (100%) | 9 (90%) | 10 (100%) | 7 (70%) | 8 (80%) | 10 (100%) | 7 (70%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Díaz, M.; Martín-Castellanos, A.; Fernández-Elías, V.E.; López Torres, O.; Lorenzo Calvo, J. Effects of Polyphenol Consumption on Recovery in Team Sport Athletes of Both Sexes: A Systematic Review. Nutrients 2022, 14, 4085. https://doi.org/10.3390/nu14194085
Sánchez Díaz M, Martín-Castellanos A, Fernández-Elías VE, López Torres O, Lorenzo Calvo J. Effects of Polyphenol Consumption on Recovery in Team Sport Athletes of Both Sexes: A Systematic Review. Nutrients. 2022; 14(19):4085. https://doi.org/10.3390/nu14194085
Chicago/Turabian StyleSánchez Díaz, Mariana, Adrián Martín-Castellanos, Valentín E. Fernández-Elías, Olga López Torres, and Jorge Lorenzo Calvo. 2022. "Effects of Polyphenol Consumption on Recovery in Team Sport Athletes of Both Sexes: A Systematic Review" Nutrients 14, no. 19: 4085. https://doi.org/10.3390/nu14194085
APA StyleSánchez Díaz, M., Martín-Castellanos, A., Fernández-Elías, V. E., López Torres, O., & Lorenzo Calvo, J. (2022). Effects of Polyphenol Consumption on Recovery in Team Sport Athletes of Both Sexes: A Systematic Review. Nutrients, 14(19), 4085. https://doi.org/10.3390/nu14194085









