Lotus (Nelumbo nucifera Gaertn.) Leaf-Fermentation Supernatant Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains and Culture Conditions
2.2. Preparation of Enterococcus Fermented Lotus Leaf Supernatant (FLLS)
2.3. Cell Culture and Differentiation
2.4. Cell Viability Assay
2.5. Oil Red O Staining
2.6. Animals and Experimental Design
2.7. Biochemical Analysis
2.8. Hepatic TC and TG
2.9. Histopathology Examination
2.10. RT-qPCR
2.11. Statistical Analysis
3. Results
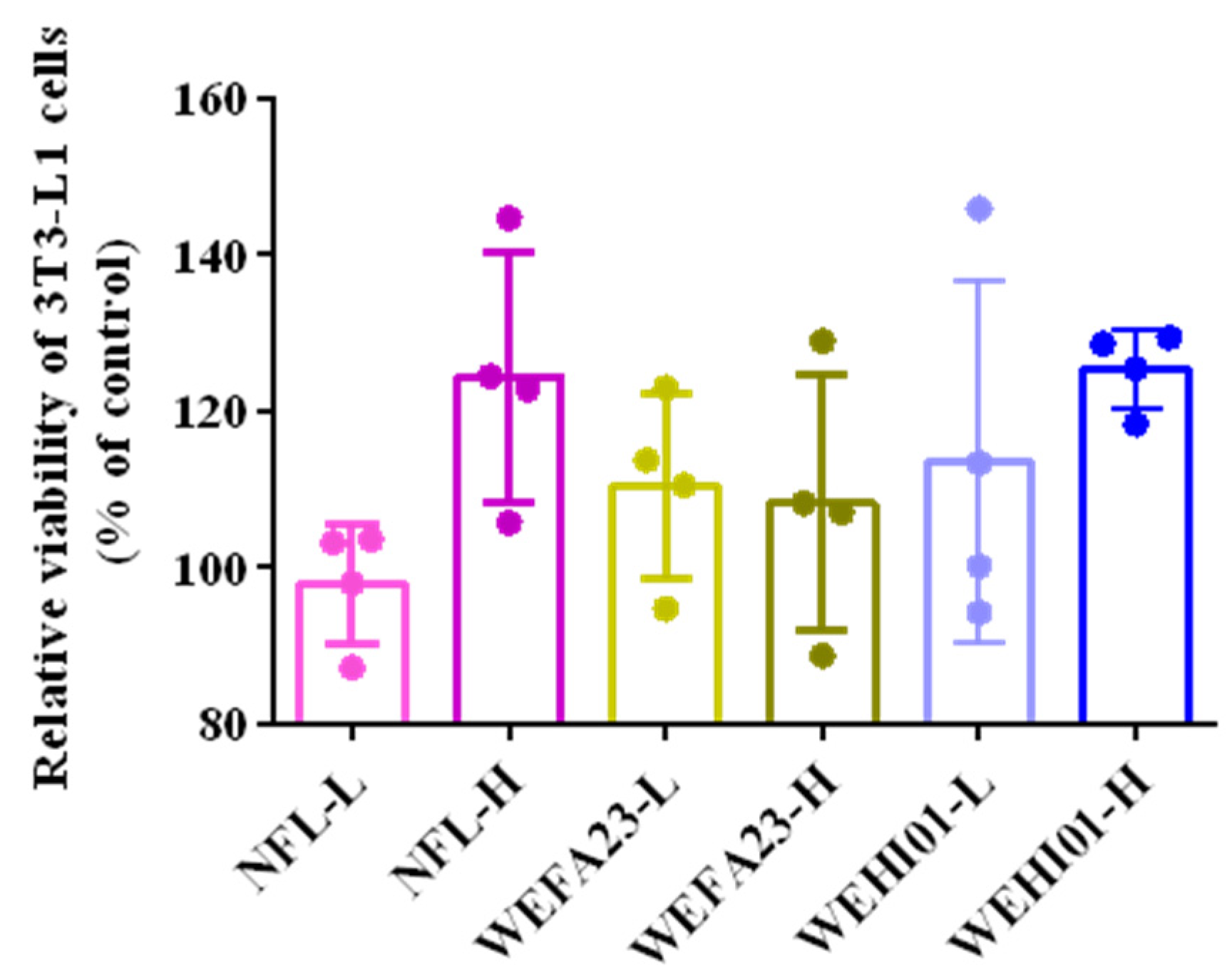
3.1. Effect of FLLS on the Viability of 3T3-L1 Preadipocytes
3.2. Effect of FLLS on Intracellular Lipid Accumulation in 3T3-L1 Preadipocytes
3.3. Effects of FLLS on the Adipogenesis of 3T3-L1 Preadipocytes
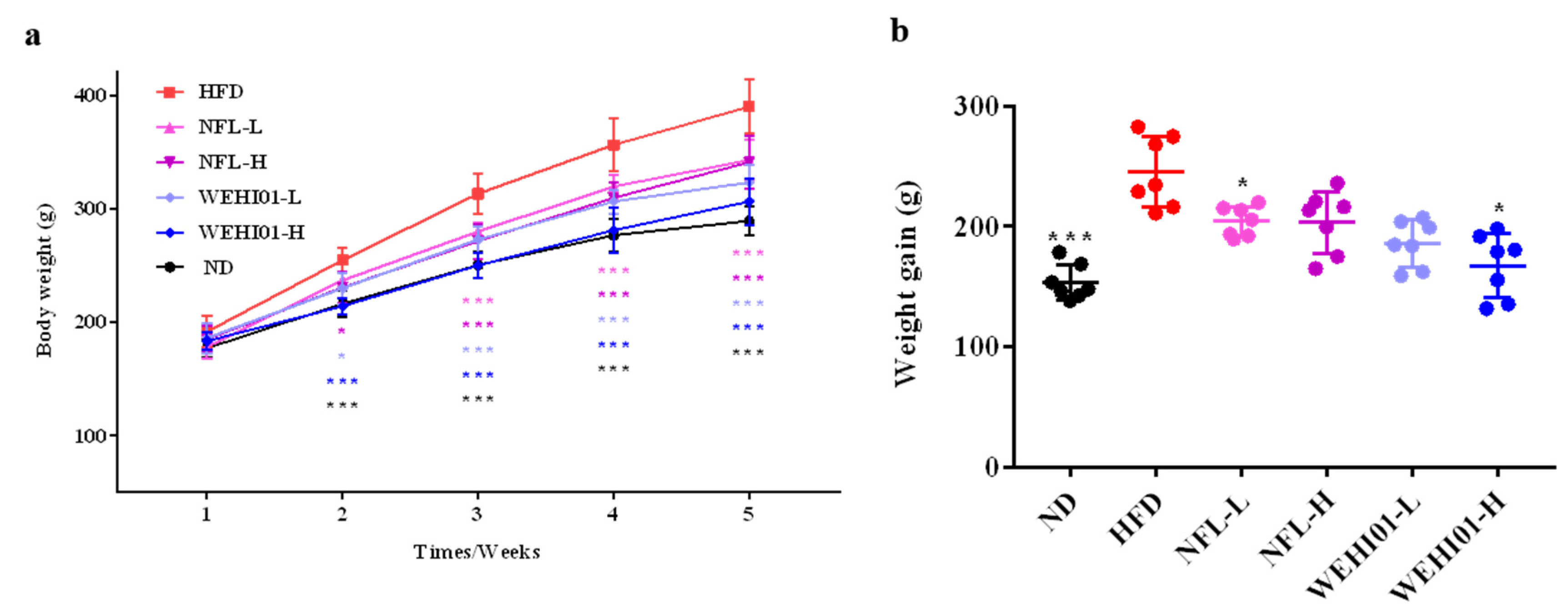
3.4. Effect of FLLS-WEHI01 on Body Weight in Obese Rats
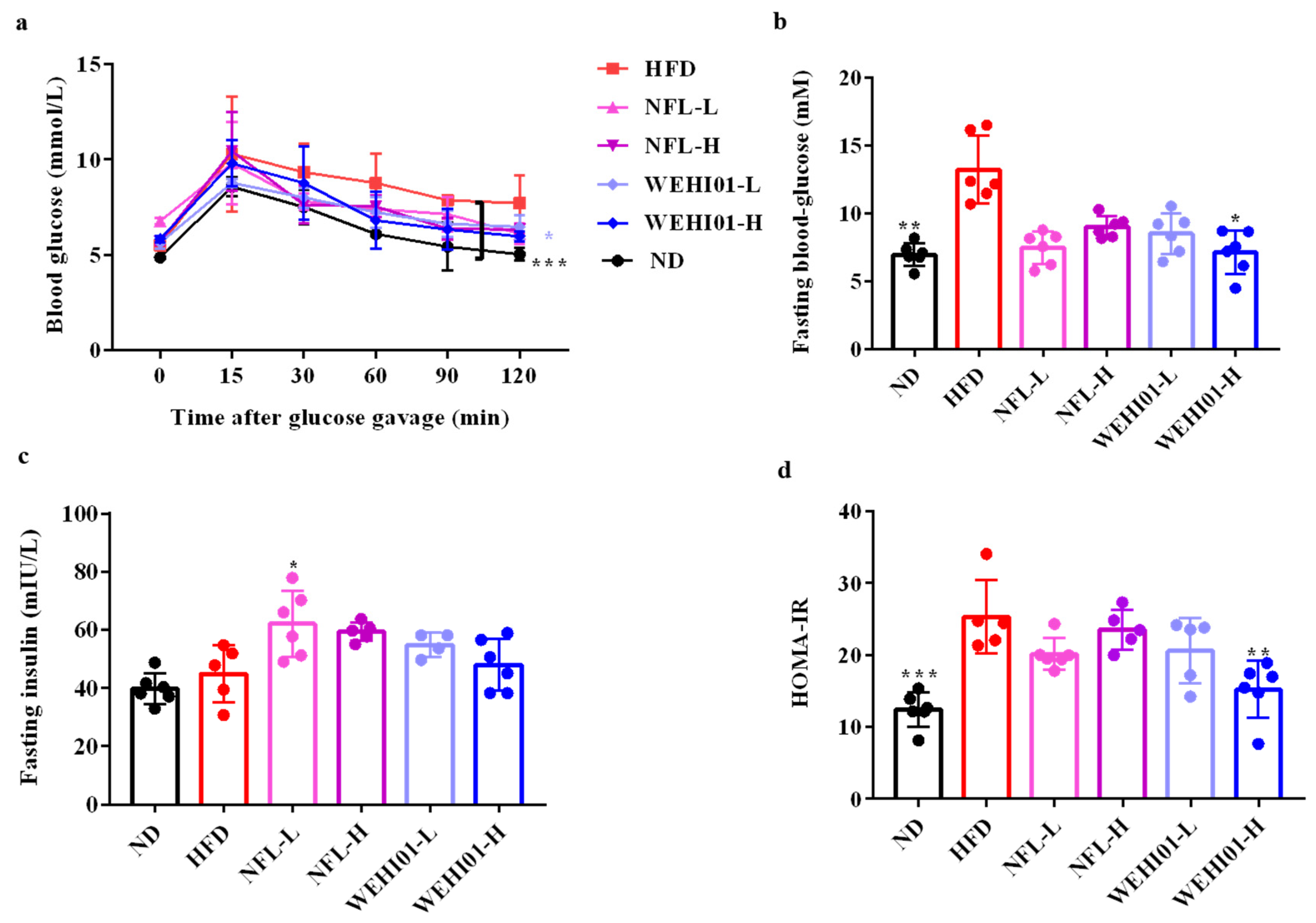
3.5. Effect of FLLS-WEHI01 on the Glucose and Insulin Level in Obese Rats
3.6. Effects of FLLS-WEHI01 on the Lipid Levels of the Serum and Liver in Obese Rats
3.7. Effects of FLLS-WEHI01 on the Histopathology of Liver and Epididymis Tissue in Obese Rats
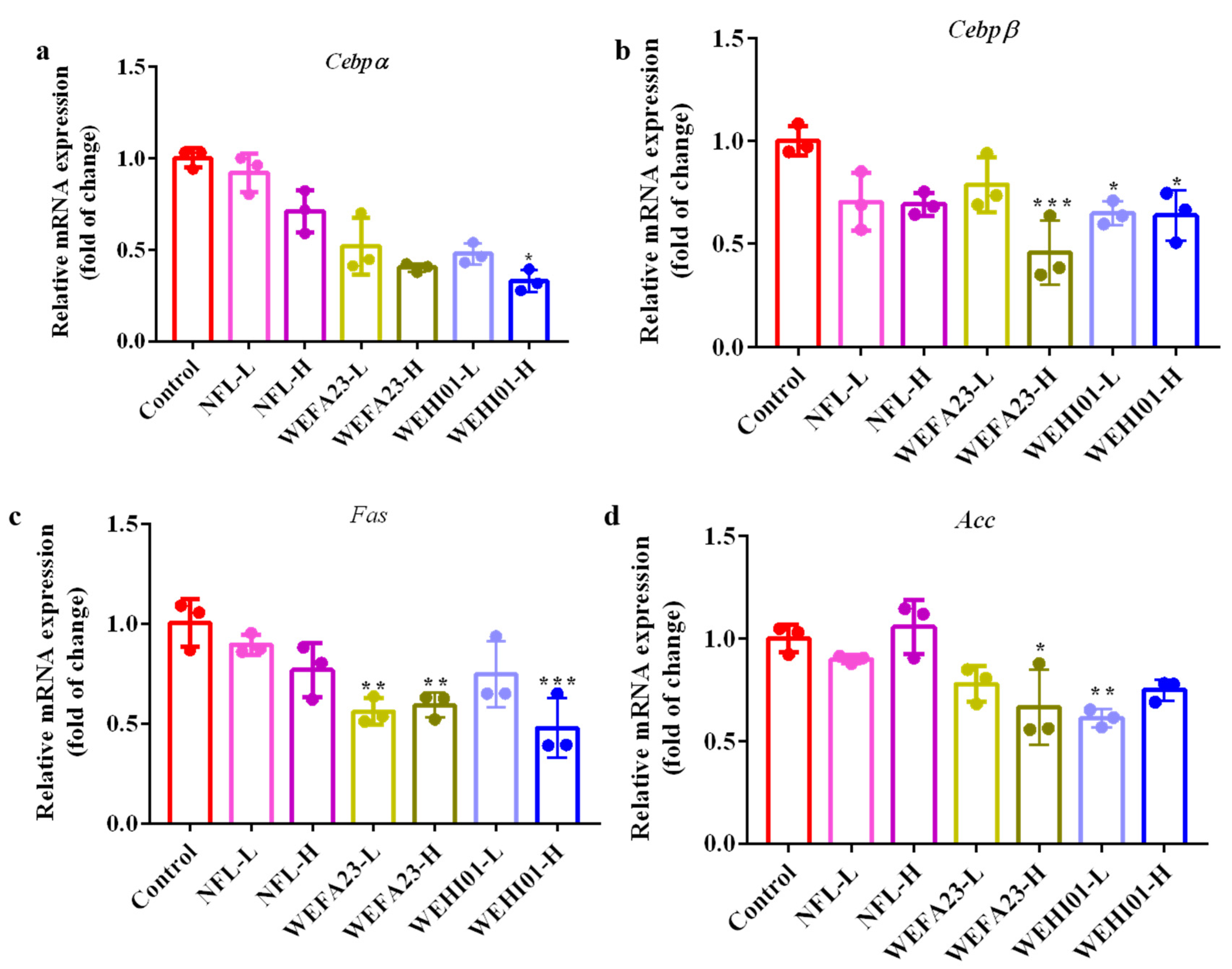
3.8. Effect of FLLS-WEHI01 on the Expression of Adipogenic Genes in Epididymal Adipose Tissue
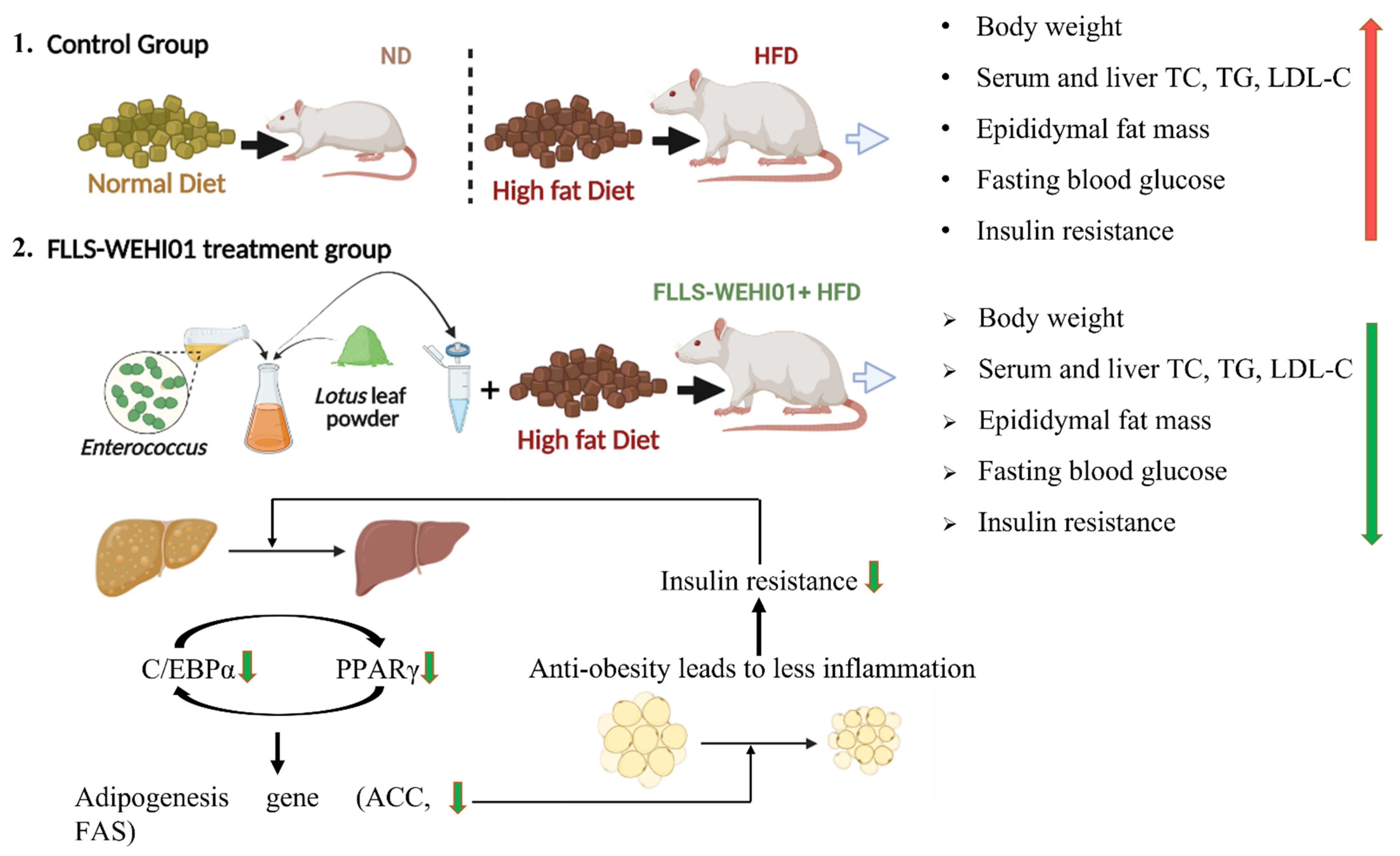
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Switzer, N.J.; Mangat, H.S.; Karmali, S. Current trends in obesity: Body composition assessment, weight regulation, and emerging techniques in managing severe obesity. J. Interv. Gastroenterol. 2013, 1, 34–36. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell 1976, 7, 105–113. [Google Scholar] [CrossRef]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Devlopment 2000, 14, 1293–1307. [Google Scholar] [CrossRef]
- Seaton, D.; Duncan, L. Treatment of “refractory obesity” with “formula diet”. Br. Med. J. 1963, 2, 219–221. [Google Scholar] [CrossRef]
- Wirth, A.; Wabitsch, M.; Hauner, H. The prevention and treatment of obesity. Dtsch. Ärzteblatt Int. 2014, 111, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Schweinlin, A. Obesity therapy. Clin. Nutr. ESPEN 2020, 38, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, C. Nutrition Concepts for the Treatment of Obesity in Adults. Nutrients 2021, 14, 169. [Google Scholar] [CrossRef]
- Wu, J.; Jia, R.B.; Luo, D.; Li, Z.R.; Lin, L.; Zheng, Q.; Zhao, M. Sargassum fusiforme polysaccharide is a potential auxiliary substance for metformin in the management of diabetes. Food Funct. 2022, 13, 3023–3025. [Google Scholar] [CrossRef]
- Eb, A.; Asb, C.; Hmh, D.; Es, E.; Aek, F. The therapeutic role of Lactobacillus and montelukast in combination with metformin in diabetes mellitus complications through modulation of gut microbiota and suppression of oxidative stress. Int. Immunopharmacol. 2021, 96, 107757. [Google Scholar] [CrossRef]
- Kashyap, H.; Gupta, S.; Bist, R. Impact of active antihyperglycemic components as herbal therapy for preventive health care management of diabetes. Curr. Mol. Med. 2019, 19, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Kang, A.N.; Kang, S.Y.; Park, Y.-K.; Song, M.Y. The root extract of Pueraria lobata and its main compound, puerarin, prevent obesity by increasing the energy metabolism in skeletal muscle. Nutrients 2017, 9, 33. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lim, W.-C.; Lee, S.-J.; Lee, S.-H.; Lee, J.-H.; Cho, H.-Y. Antiobesity effect of garlic extract fermented by Lactobacillus plantarum BL2 in diet-induced obese mice. J. Med. Food 2016, 19, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Ma, D.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Christian, M.; He, Z. Alkaloids from lotus (Nelumbo nucifera): Recent advances in biosynthesis, pharmacokinetics, bioactivity, safety, and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 1, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Patel, P.A.; Sharma, P.; Thoutireddy, S.; Das, N. Lotus (Nelumbo nucifera Gaertn.) and its bioactive phytocompounds: A tribute to cancer prevention and intervention. Cancers 2022, 14, 529. [Google Scholar] [CrossRef]
- Liu, E.; Tsuboi, H.; Ikegami, S.; Kamiyama, T.; Asami, Y.; Ye, L.; Oda, M.; Ji, Z.S. Effects of Nelumbo nucifera leaf extract on obesity. Plant Foods Hum. Nutr. 2021, 76, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bharathi Priya, L.; Huang, C.Y.; Hu, R.M.; Balasubramanian, B.; Baskaran, R. An updated review on pharmacological properties of neferine-A bisbenzylisoquinoline alkaloid from Nelumbo nucifera. J. Food Biochem. 2021, 45, e13986. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Pinthong, D.; Hano, C. Flavonoids from Nelumbo nucifera Gaertn., a medicinal plant: Uses in traditional medicine, phytochemistry and pharmacological activities. Medicines 2018, 5, 127. [Google Scholar] [CrossRef]
- Marthandam Asokan, S.; Mariappan, R.; Muthusamy, S.; Velmurugan, B.K. Pharmacological benefits of neferine-a comprehensive review. Life Sci. 2018, 199, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Y.; Yang, Y.; Gou, Y.; Li, S.; Wang, R.; Zeng, S.; Zhao, X. Antioxidant and inflammatory effects of Nelumbo nucifera Gaertn. leaves. Oxidative Med. Cell. Longev. 2021, 2021, 8375961. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; You, J.S.; Zhao, X.; Park, J.Y.; Kim, S.H.; Chang, K.J. Antiobesity and hypolipidemic effects of lotus leaf hot water extract with taurine supplementation in rats fed a high fat diet. J. Biomed. Sci. 2010, 17 (Suppl. S1), S42. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, E.; Miyazaki, H.; Shindo, K.; Watanabe, H.; Yoshida, A.; Yajima, H. Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice. Planta Med. 2007, 73, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus flavonoids and phenolic acids: Health promotion and safe consumption dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)-phytochemical and therapeutic profile. Folia Pharmacol. Jpn. 2009, 61, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, J.; Chu, H.; Zhang, X.; Wang, Z.; Wang, H.; Li, G. Purification and characterization of aporphine alkaloids from leaves of Nelumbo nucifera Gaertn and their effects on glucose consumption in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2014, 15, 3481–3494. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yang, M.Y.; Chan, K.C.; Chung, P.J.; Ou, T.T.; Wang, C.J. Improvement in high-fat diet-induced obesity and body fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract in mice. J. Agric. Food Chem. 2010, 58, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, W.; Li, B.; Qian, S.; Wei, B.; Gong, S.; Wang, J.; Liu, M.; Wei, M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020, 52, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Charchoghlyan, H.; Lee, J.S.; Kim, M. Bioactive compounds and antioxidant activities of the Korean lotus leaf (Nelumbo nucifera) condiment: Volatile and nonvolatile metabolite profiling during fermentation. Int. J. Food Sci. Technol. 2015, 50, 1988–1995. [Google Scholar] [CrossRef]
- Kim, J.-S.; Wang, S.-B.; Kang, S.-K.; Cho, Y.-S.; Park, S.-K. Quality properties of white lotus leaf fermented by mycelial Paecilomyces japonica. J. Korean Soc. Food Sci. Nutr. 2009, 38, 594–600. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Galvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, E.; Yermolenko, E.; Donets, V.; Sundukova, Z.; Bochkareva, A.; Borschev, I.; Suvorova, M.; Ilyasov, I.; Simanenkov, V.; Suvorov, A. The influence of probiotic Enterococcus faecium strain L5 on the microbiota and cytokines expression in rats with dysbiosis induced by antibiotics. Benef. Microbes 2010, 1, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, J.; Jiang, Y. Effects of Enterococcus faecium (SF68) on immune function in mice. Food Chem. 2010, 123, 63–68. [Google Scholar] [CrossRef]
- Sarantinopoulos, P.; Kalantzopoulos, G.; Tsakalidou, E. Effect of Enterococcus faecium on microbiological, physicochemical and sensory characteristics of Greek Feta cheese. Int. J. Food Microbiol. 2002, 76, 93–105. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Janchot, K.; Kanacharoen, S.; Lertmongkolaksorn, T.; Wongsaroj, L.; Somboonna, N.; Ngamwongsatit, N.; Leelahavanichkul, A. Lactiplantibacillus plantarum dfa1 outperforms Enterococcus faecium dfa1 on anti-obesity in high fat-induced obesity mice possibly through the differences in gut dysbiosis attenuation, despite the similar anti-Inflammatory properties. Nutrients 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Shimada, T.; Fukada, K.; Morita, M.; Katada, K.; Higashimura, Y.; Mizushima, K.; Okamori, M.; Naito, Y.; Yoshikawa, T. Beneficial effects of heat-treated Enterococcus faecalis FK-23 on high-fat diet-induced hepatic steatosis in mice. Br. J. Nutr. 2014, 112, 868–875. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, M.; Wan, C.; Chen, X.; Chen, X.; Tao, X.; Shah, N.P.; Hua, W. Screening probiotic strains for safety: Evaluation of virulence and antimicrobial susceptibility of enterococci from healthy Chinese infants. J. Dairy Sci. 2016, 99, 4282–4290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiu, L.; Xu, X.; Liu, Z.; Zhan, H.; Tao, X.; Shah, N.P.; Wei, H. Beneficial effects of probiotic cholesterol-lowering strain of Enterococcus faecium WEFA23 from infants on diet-induced metabolic syndrome in rats. J. Dairy Sci. 2017, 100, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, Z.; Qiu, L.; Tao, X.; Lihua, H.E.; Wei, H. Antibacterial and antioxidant evaluation of supernatant of the fermented lotus leaf by Enterococcus faecium WEFA23 and Enterococci hirae WHEHI01. J. Nanchang Univ. 2018, 101, 7757–7767. [Google Scholar]
- Liu, H.; Liu, M.; Jin, Z.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J.; Guo, S. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019, 10, 3603–3614. [Google Scholar] [CrossRef] [PubMed]
- Lumbera, W.; Cruz, J.D.; Yang, S.H.; Hwang, S.G. Heat shock protein augmentation of angelica gigas nakai root hot water extract on adipogenic differentiation in murine 3T3-L1 preadipocytes. Asian-Australas J. Anim. Sci. 2016, 29, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, H.; Xie, J.; Xu, Q.; Liu, J. Antiobesity effects of zeaxanthin on 3T3-L1 preadipocyte and high fat induced obese mice. Food Funct. 2017, 8, 3327. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Tian, L.; Zhan, H.; He, Y.; Zhong, C.; Wei, H. In vitro and in vivo assessments of Artemisia argyi fermented with Lactobacillus plantarum WLPL01 as an alternative anti-Salmonella agent. Food Control 2021, 126, 108079. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, D.-S.; Lee, M.-C.; Park, S.; Lee, J.-W.; Om, A.-S. Effects of Bacillus Subtilis-fermented white sword bean extract on adipogenesis and lipolysis of 3T3-L1 adipocytes. Foods 2021, 10, 1423. [Google Scholar] [CrossRef]
- So, K.-H.; Suzuki, Y.; Yonekura, S.; Suzuki, Y.; Lee, C.H.; Kim, S.W.; Katoh, K.; Roh, S.-G. Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes. Nutr. Res. Pract. 2015, 9, 439–444. [Google Scholar] [CrossRef]
- Shao, H.-Y.; Hsu, H.-Y.; Wu, K.-S.; Hee, S.-W.; Chuang, L.-M.; Yeh, J.-I. Prolonged induction activates Cebpα independent adipogenesis in NIH/3T3 cells. PLoS ONE 2013, 8, e51459. [Google Scholar] [CrossRef]
- Kotzka, J.; Müller-Wieland, D. Sterol regulatory element-binding protein (SREBP)-1: Gene regulatory target for insulin resistance? Expert Opin. Ther. Targets 2004, 8, 141–149. [Google Scholar] [CrossRef]
- Hwang, J.W.; Do, H.J.; Kim, O.Y.; Chung, J.H.; Lee, J.-Y.; Park, Y.S.; Hwang, K.Y.; Seong, S.-I.; Shin, M.-J. Fermented soy bean extract suppresses differentiation of 3T3-L1 preadipocytes and facilitates its glucose utilization. J. Funct. Foods 2015, 15, 516–524. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jang, M.-S. Anti-obesity effects of Laminaria japonica fermentation on 3T3-L1 adipocytes are mediated by the inhibition of C/EBP-alpha/beta and PPAR-gamma. Cell. Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Jung, D.-W.; Lee, O.-H.; Kang, I.-J. Fermented platycodon grandiflorum extract inhibits lipid accumulation in 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Med. Food 2016, 19, 1004–1014. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-X.; Shen, W.; Sun, H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol. Int. 2010, 4, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Shimomura, I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, N.; Wang, S.-Q.; Wang, Y.; Zhao, Y.-K.; Zhu, X.-D. Effect of Gegen Qinlian decoction on hepatic gluconeogenesis in ZDF Rats with type 2 diabetes mellitus based on the farnesol X receptor/ceramide signaling pathway regulating mitochondrial metabolism and endoplasmic reticulum stress. Evid.-Based Complementary Altern. Med. 2021, 2021, 9922292. [Google Scholar] [CrossRef]
- Joung, H.; Kim, B.; Park, H.; Lee, K.; Kim, H.-H.; Sim, H.-C.; Do, H.-J.; Hyun, C.-K.; Do, M.-S. Fermented Moringa oleifera decreases hepatic adiposity and ameliorates glucose intolerance in high-fat diet-induced obese mice. J. Med. Food 2017, 20, 439–447. [Google Scholar] [CrossRef]


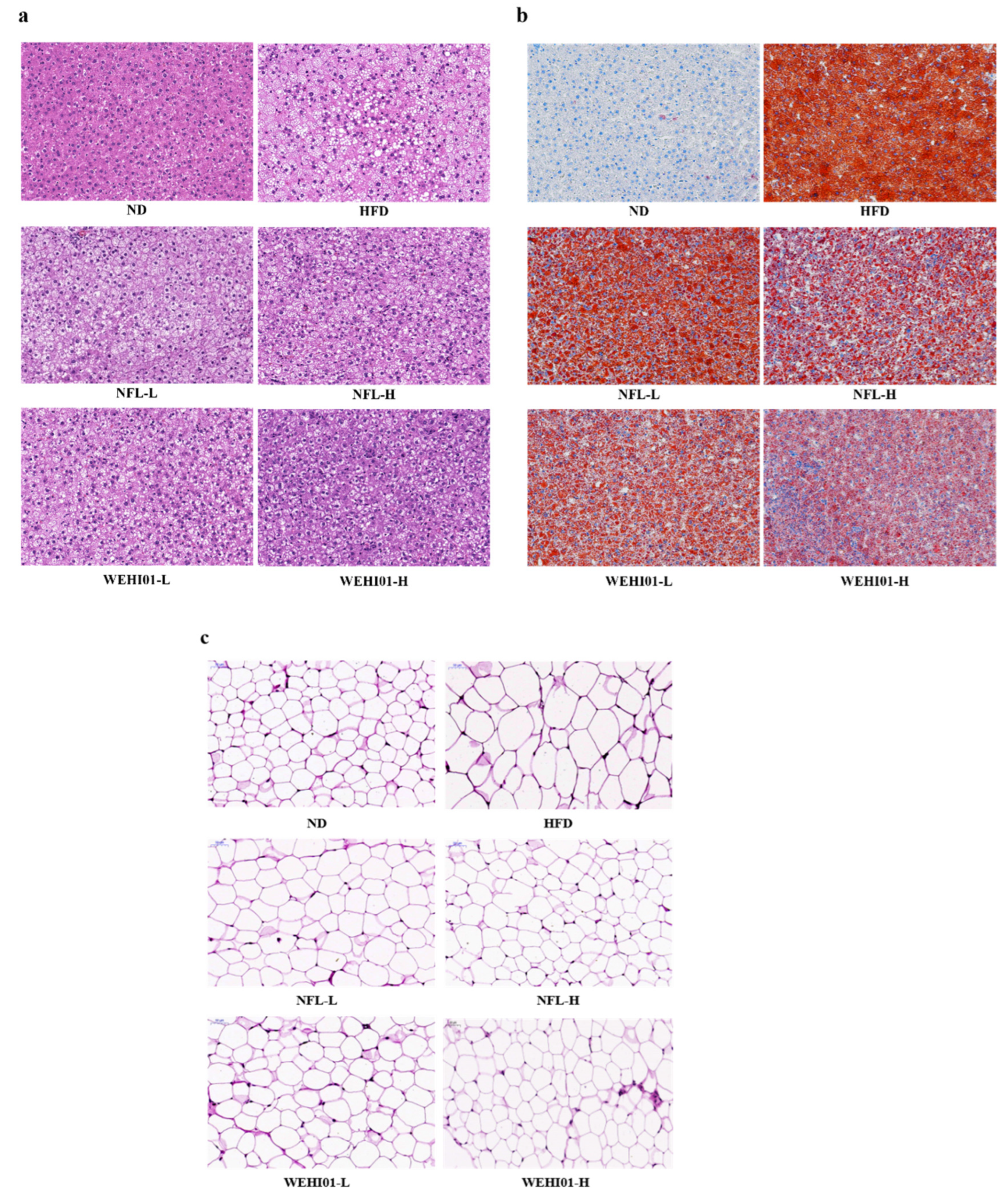
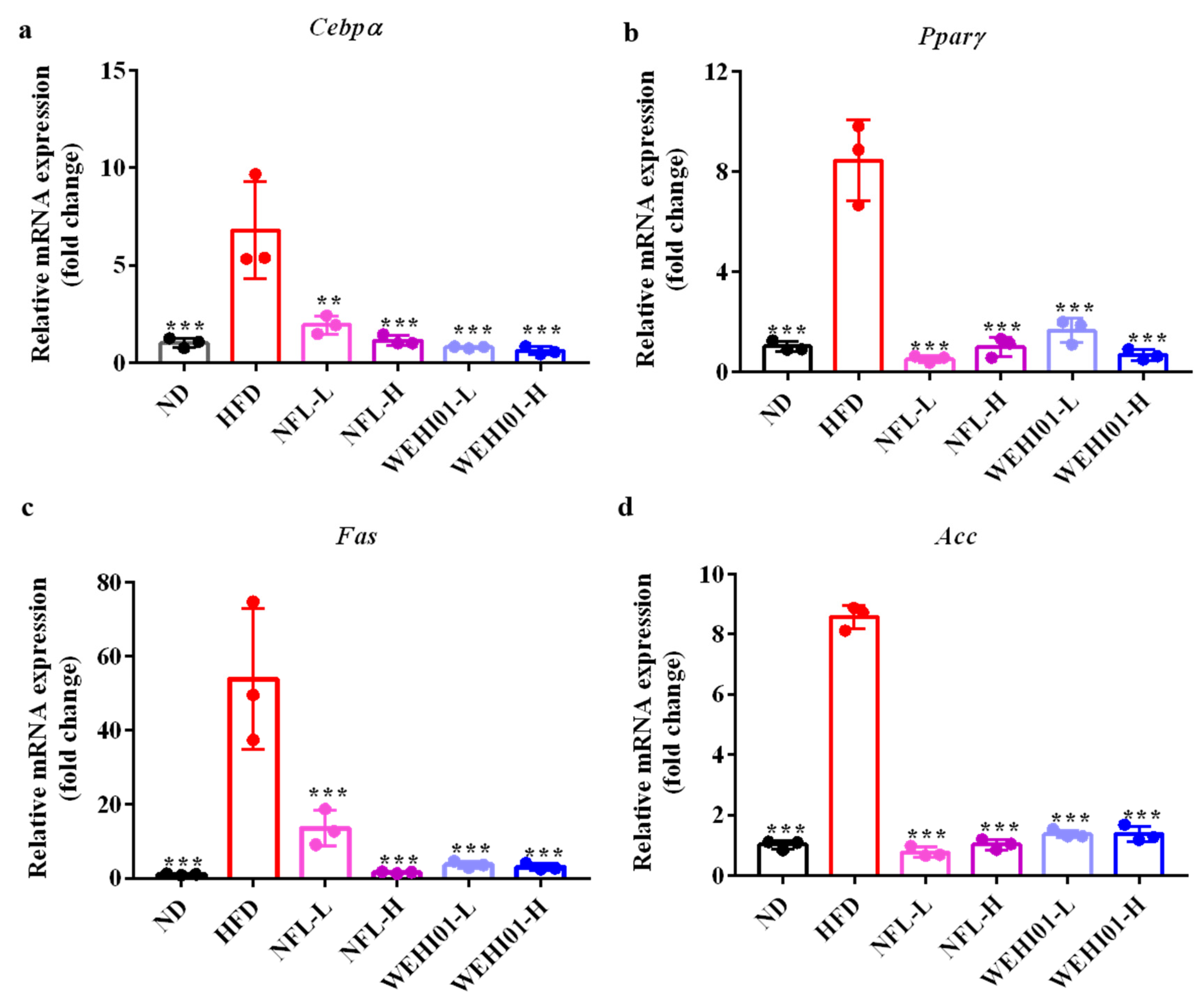
| Weight (g) | ND | HFD | NFL-L | NFL-H | WEHI01-L | WEHI01-H |
|---|---|---|---|---|---|---|
| Liver | 11.13 ± 0.93 *** | 20.37 ± 3.10 | 20.31 ± 1.63 | 20.42 ± 2.50 | 18.08 ± 1.26 * | 16.78 ± 0.77 * |
| Epididymis fat | 2.15 ± 0.68 * | 4.69 ± 1.15 | 3.83 ± 1.45 | 3.72 ± 1.22 | 3.30 ± 0.96 | 2.84 ± 0.64 * |
| Kidney | 2.11 ± 0.19 | 2.31 ± 0.22 | 2.31 ± 0.11 | 2.24 ± 0.16 | 2.09 ± 0.18 | 2.10 ± 0.21 |
| Spleen | 0.59 ± 0.09 | 0.89 ± 0.20 | 1.00 ± 0.31 | 1.01 ± 0.19 | 0.87 ± 0.11 | 0.95 ± 0.22 |
| Food intake | 20.99 ± 1.48 | 19.90 ± 2.37 | 20.06 ± 2.76 | 19.87 ± 3.22 | 19.06 ± 3.42 | 19.80 ± 2.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Tao, Y.; Qiu, L.; Xu, W.; Huang, X.; Wei, H.; Tao, X. Lotus (Nelumbo nucifera Gaertn.) Leaf-Fermentation Supernatant Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Induced Obese Rats. Nutrients 2022, 14, 4348. https://doi.org/10.3390/nu14204348
He Y, Tao Y, Qiu L, Xu W, Huang X, Wei H, Tao X. Lotus (Nelumbo nucifera Gaertn.) Leaf-Fermentation Supernatant Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Induced Obese Rats. Nutrients. 2022; 14(20):4348. https://doi.org/10.3390/nu14204348
Chicago/Turabian StyleHe, Yao, Yue Tao, Liang Qiu, Wenfeng Xu, Xiaoli Huang, Hua Wei, and Xueying Tao. 2022. "Lotus (Nelumbo nucifera Gaertn.) Leaf-Fermentation Supernatant Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Induced Obese Rats" Nutrients 14, no. 20: 4348. https://doi.org/10.3390/nu14204348
APA StyleHe, Y., Tao, Y., Qiu, L., Xu, W., Huang, X., Wei, H., & Tao, X. (2022). Lotus (Nelumbo nucifera Gaertn.) Leaf-Fermentation Supernatant Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Induced Obese Rats. Nutrients, 14(20), 4348. https://doi.org/10.3390/nu14204348





