CoQ10 and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dysfunctions
Abstract
1. Introduction
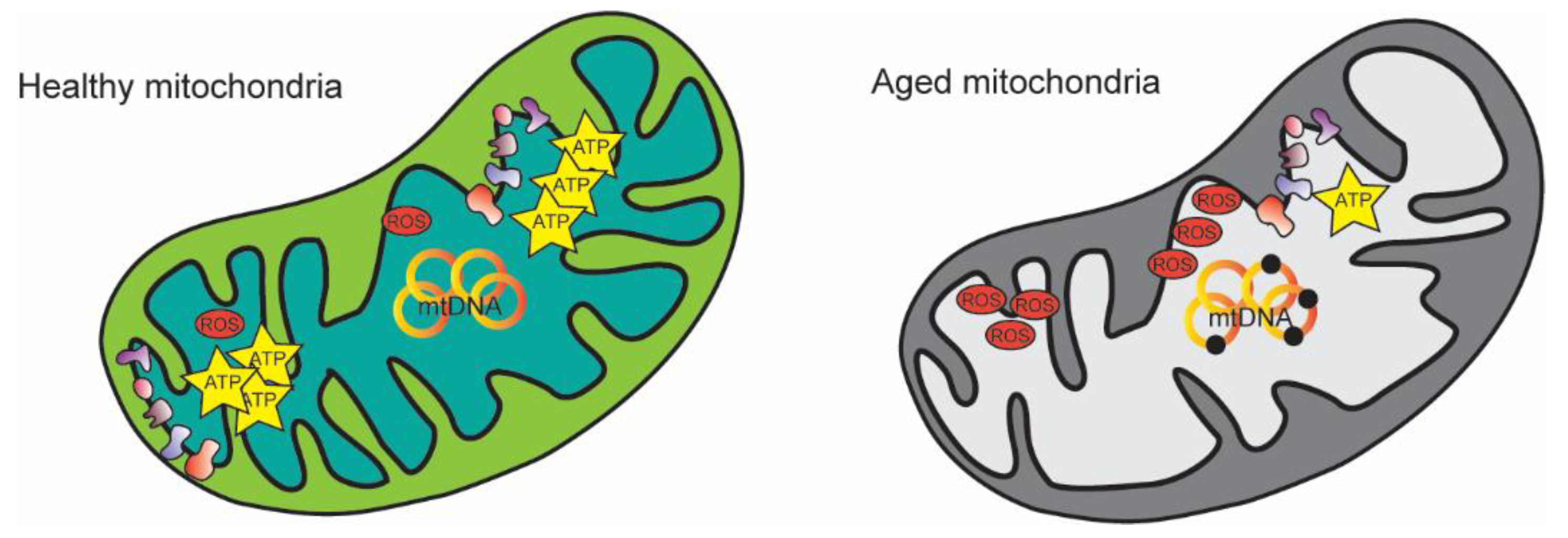
2. Mitochondrial Alterations during Aging
2.1. Re-Evaluation of the “Mitochondrial Free Radical Theory of Aging”
2.2. mtDNA Mutations during Aging
2.3. PGC1-α Function Is Critical during Aging, Especially in Skeletal Muscle
3. Resveratrol
3.1. Chemistry, Safety, and Bioavailability of Resveratrol
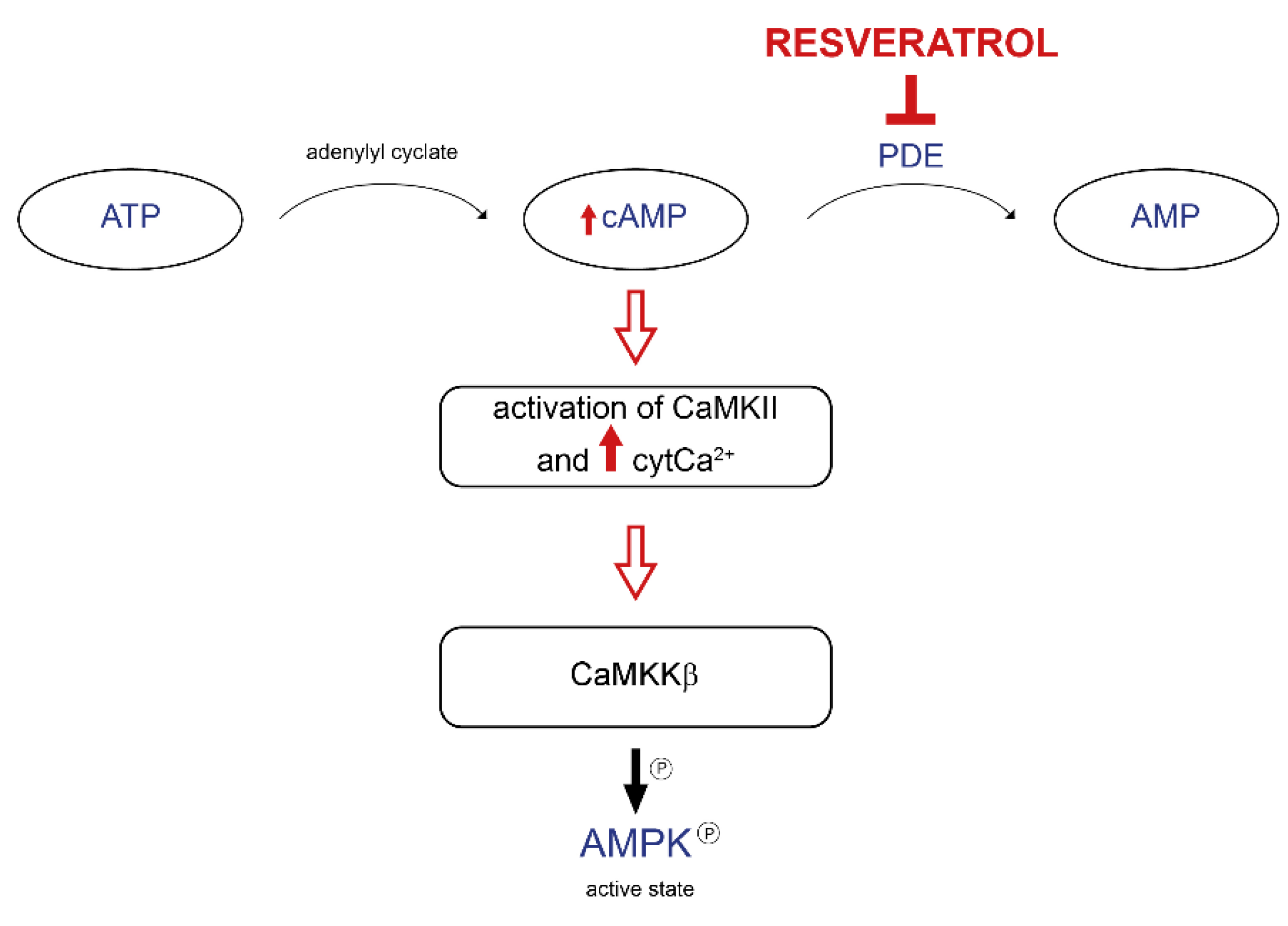
3.2. Mitochondrial Mechanisms of Action of Resveratrol
3.3. Beneficial Effects of Resveratrol during Aging and Age-Associated Diseases
| Effects of Resveratrol On | Beneficial Effect On | Studies On | Ref. |
|---|---|---|---|
| skeletal muscle | muscle mass | rodents | [52] |
| muscle performance | rodents | [53] | |
| sarcomere structure | rodents | [54] | |
| activation of AMPK-SIRT1 pathway | humans | [55] | |
| no difference in the inflammation state | humans | [56] | |
| cardiovascular impairment | systolic function | rodents | [61] |
| renin-angiotensin axis | rodents | [62] | |
| aged-related mitochondrial dysfunctions | rodents | [63] | |
| glycemic control | T2DM patients | [65] | |
| decreased cholesterol levels | patients with angina | [66] | |
| neurodegenerative diseases | increased long-term memory formation | rodents | [69] |
| increased neurogenesis and vascularization | rodents | [70] | |
| increased cerebellar blood flow | humans | [71] | |
| increased memory performance | humans | [72] | |
| diabetes | enhanced cardiometabolic markers without affecting glycemia | humans | [76] |
| increased glucose control | humans | [77] | |
| normal glycemia restoration | gestational diabetes | [78,79] |
4. CoenzymeQ10
4.1. Chemistry of CoQ10
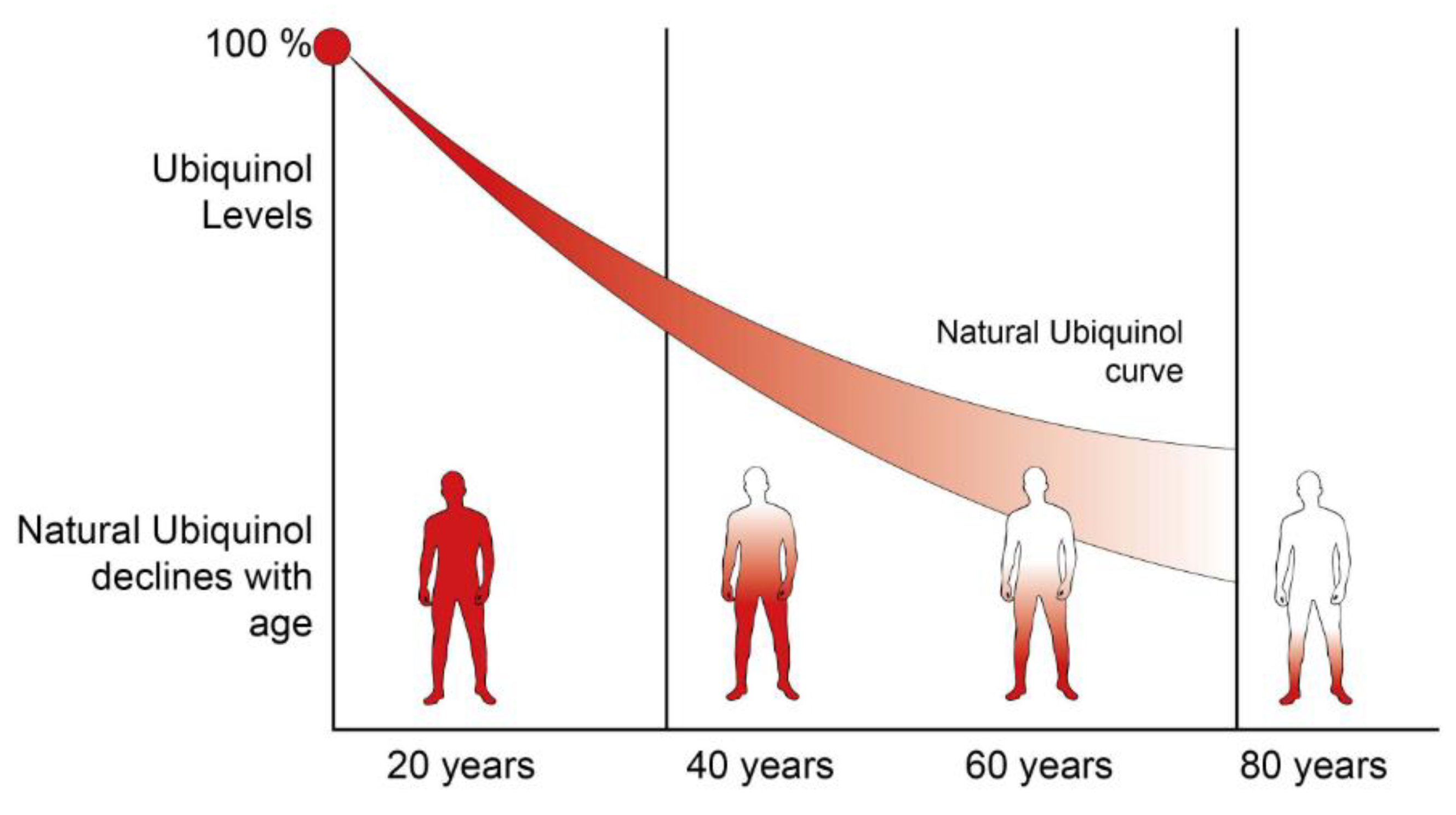
4.2. CoQ10 as a Key Factor in Controlling Cellular Homeostasis
4.3. Beneficial Effects of CoQ10 during Aging and Age-Associated Diseases
| Effect of Coenzyme Q10 On | Beneficial Effect On | Ref. |
|---|---|---|
| elderly subjects | exercise performance | [109] |
| Parkinson’s disease patients | decreased development of disability | [116] |
| no effect on motor symptoms | [105,113] | |
| cardiovascular impairment | decreased systemic blood pressure | [107] |
| preventing arrhythmias in cardiac surgery-subjected patients | [114] | |
| increased left ventricular ejection fraction | [115] | |
| decreased cardiovascular events in patients with chronic heart failure | [116] | |
| diabetes | decreased blood glucose | [117] |
| no differences in glycemic control | [118] | |
| counteracting statins side-effects | decreased muscle impairment | [122] |
4.4. CoQ Deficiency Syndrome
5. Challenges and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F.; Engstrom, G.W. Calcium Uptake by Rat Kidney Mitochondria. Proc. Natl. Acad. Sci. USA 1961, 47, 1744–1750. Available online: http://www.ncbi.nlm.nih.gov/pubmed/13885269 (accessed on 14 October 2016). [CrossRef] [PubMed]
- Vasington, F.D.; Murphy, J.V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J. Biol. Chem. 1962, 237, 2670–2677. Available online: http://www.ncbi.nlm.nih.gov/pubmed/13925019 (accessed on 14 October 2016). [CrossRef]
- Bernardi, P.; Di Lisa, F.; Fogolari, F.; Lippe, G. From ATP to PTP and Back: A Dual Function for the Mitochondrial ATP Synthase. Circ. Res. 2015, 116, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002, 80, 780–787. [Google Scholar] [CrossRef]
- Wickens, A.P. Ageing and the free radical theory. Respir. Physiol. 2001, 128, 379–391. [Google Scholar] [CrossRef]
- Aguilar, T.A.F.; Hernández Navarro, B.C.; Pérez, J.A.M. Endogenous Antioxidants: A Review of their Role in Oxidative Stres. In A Master Regulator of Oxidative Stress—The Transcription Factor Nrf2; IntechOpen Limited: London, UK, 2016. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Li, T.Y.; Mottis, A.; Auwerx, J. Pleiotropic effects of mitochondria in aging. Nat. Aging 2022, 2, 199–213. [Google Scholar] [CrossRef]
- Miyazawa, T.; Abe, C.; Burdeos, G.C.; Matsumoto, A.; Toda, M. Food Antioxidants and Aging: Theory, Current Evidence and Perspectives. Nutraceuticals 2022, 2, 181–204. [Google Scholar] [CrossRef]
- Rockstein, M.; Brandt, K.F. Enzyme Changes in Flight Muscle Correlated with Aging and Flight Ability in the Male Housefly. Science 1963, 139, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.D.; Franks, L. The Effect of Age on Mitochondrial Ultrastructure. Gerontology 1975, 21, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Bratic, I.; Trifunovic, A. Mitochondrial energy metabolism and ageing. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M.; Hekimi, S. Deletion of the Mitochondrial Superoxide Dismutase sod-2 Extends Lifespan in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000361. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.; Weinberger, M.; Silva, A.; Sampaio-Marques, B.; Almeida, B.; Leão, C.; Costa, V.; Rodrigues, F.; Burhans, W.C.; Ludovico, P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc. Natl. Acad. Sci. USA 2010, 107, 15123–15128. [Google Scholar] [CrossRef]
- Doonan, R.; McElwee, J.J.; Matthijssens, F.; Walker, G.A.; Houthoofd, K.; Back, P.; Matscheski, A.; Vanfleteren, J.R.; Gems, D. Against the oxidative damage theory of aging: Superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008, 22, 3236–3241. [Google Scholar] [CrossRef]
- Zhang, Y.; Ikeno, Y.; Qi, W.; Chaudhuri, A.; Li, Y.; Bokov, A.; Thorpe, S.R.; Baynes, J.W.; Epstein, C.; Richardson, A.; et al. Mice Deficient in Both Mn Superoxide Dismutase and Glutathione Peroxidase-1 Have Increased Oxidative Damage and a Greater Incidence of Pathology but No Reduction in Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1212–1220. [Google Scholar] [CrossRef]
- Van Remmen, H.; Ikeno, Y.; Hamilton, M.; Pahlavani, M.; Wolf, N.; Thorpe, S.R.; Alderson, N.L.; Baynes, J.W.; Epstein, C.J.; Huang, T.-T.; et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genom. 2003, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.I.; Van Remmen, H.; Bokov, A.; Epstein, C.J.; Vijg, J.; Richardson, A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 2009, 8, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Edgar, D.; Shabalina, I.; Camara, Y.; Wredenberg, A.; Calvaruso, M.A.; Nijtmans, L.; Nedergaard, J.; Cannon, B.; Larsson, N.-G.; Trifunovic, A. Random Point Mutations with Major Effects on Protein-Coding Genes Are the Driving Force behind Premature Aging in mtDNA Mutator Mice. Cell Metab. 2009, 10, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The Aging Stress Response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Hardie, D.G. Use of Cells Expressing γ Subunit Variants to Identify Diverse Mechanisms of AMPK Activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Feige, J.N.; Auwerx, J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007, 17, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Anderson, R.; Prolla, T. PGC-1α in aging and anti-aging interventions. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1059–1066. [Google Scholar] [CrossRef]
- Wenz, T.; Rossi, S.G.; Rotundo, R.L.; Spiegelman, B.M.; Moraes, C.T. Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. USA 2009, 106, 20405–20410. [Google Scholar] [CrossRef]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T.; Jones, D.L.; Walker, D.W. Modulation of Longevity and Tissue Homeostasis by the Drosophila PGC-1 Homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Timperio, A.M.; D’Alessandro, A.; Fagioni, M.; Magro, P.; Zolla, L. Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiol. Biochem. 2012, 50, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Andreazza, A.A.C.; Salvador, M. Sequestering Ability of Butylated Hydroxytoluene, Propyl Gallate, Resveratrol, and Vitamins C and E against ABTS, DPPH, and Hydroxyl Free Radicals in Chemical and Biological Systems. J. Agric. Food Chem. 2003, 51, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2019, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okuda, H. Resveratrol Isolated from Polygonum cuspidatum Root Prevents Tumor Growth and Metastasis to Lung and Tumor-Induced Neovascularization in Lewis Lung Carcinoma-Bearing Mice. J. Nutr. 2001, 131, 1844–1849. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107–111. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of synthetic trans-resveratrol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4368. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Hart, N.; Sarga, L.; Csende, Z.; Koltai, E.; Koch, L.G.; Britton, S.L.; Davies, K.J.; Kouretas, D.; Wessner, B.; Radak, Z. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem. Toxicol. 2013, 61, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Haramizu, S.; Ota, N.; Hase, T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology 2009, 10, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. J. Cereb. Blood Flow Metab. 2000, 130, 1115–1123. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated Protein Kinase Signaling Activation by Resveratrol Modulates Amyloid-β Peptide Metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Soeur, J.; Eilstein, J.; Léreaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic. Biol. Med. 2014, 78, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef]
- Bloom, D.E.; Chatterji, S.; Kowal, P.; Lloyd-Sherlock, P.; McKee, M.; Rechel, B.; Rosenberg, L.; Smith, J.P. Macroeconomic implications of population ageing and selected policy responses. Lancet 2014, 385, 649–657. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.-M.; Malamo, A.G.; Silvestre, J.; Wawrzyniak, N.; Carey-Love, S.; Nguyen, L.M.-D.; Dutta, D.; Xu, J.; Leeuwenburgh, C.; Adhihetty, P.J. Short-term caloric restriction, resveratrol, or combined treatment regimens initiated in late-life alter mitochondrial protein expression profiles in a fiber-type specific manner in aged animals. Exp. Gerontol. 2013, 48, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Allam, M.M. Resveratrol and/or exercise training counteract aging-associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function. J. Physiol. Sci. 2017, 68, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-Y.; Chen, J.-L.; Xiao, M.-H.; Sun, Y.; Zhao, Y.-X.; Pu, D.; Lv, A.-K.; Wang, M.-L.; Zhou, J.; Zhu, S.-Y.; et al. The effect of exercise, resveratrol or their combination on Sarcopenia in aged rats via regulation of AMPK/Sirt1 pathway. Exp. Gerontol. 2017, 98, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Gliemann, L.; Biensø, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Zoratti, M. Resveratrol and Health: The Starting Point. ChemBioChem 2012, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Tasatargil, A.; Tanriover, G.; Barutcigil, A.; Turkmen, E. Protective effect of resveratrol on methylglyoxal-induced endothelial dysfunction in aged rats. Aging 2018, 31, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, E.; Wu, T.; Ruan, Y.; Wu, S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Des. Dev. Ther. 2019, 13, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Sin, T.K.; Tam, B.T.; Yung, B.Y.; Yip, S.P.; Chan, L.W.; Wong, S.C.C.; Ying, M.; Rudd, J.A.; Siu, P.M. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J. Physiol. 2015, 593, 1887–1899. [Google Scholar] [CrossRef]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.-S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mi, S.-L.; Hu, N.; Doser, T.A.; Sun, A.; Ge, J.; Ren, J. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: Role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med. 2014, 71, 208–220. [Google Scholar] [CrossRef]
- Novelle, M.G.; Wahl, D.; Diéguez, C.; Bernier, M.; De Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Cuzzola, V.F.; Ciurleo, R.; Giacoppo, S.; Marino, S.; Bramanti, P. Role of resveratrol and its analogues in the treatment of neurodegenerative diseases: Focus on recent discoveries. CNS Neurol. Disord. Drug Targets 2011, 10, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pérez, M.; Tellez-Ballesteros, R.I.; Ortiz-López, L.; Ichwan, M.; Vega-Rivera, N.M.; Castro-García, M.; Gómez-Sánchez, A.; Kempermann, G.; Ramirez-Rodriguez, G.B. Resveratrol Enhances Neuroplastic Changes, Including Hippocampal Neurogenesis, and Memory in Balb/C Mice at Six Months of Age. PLoS ONE 2015, 10, e0145687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-N.; Li, W.-F.; Li, F.; Zhang, Z.; Dai, Y.-D.; Xu, A.-L.; Qi, C.; Gao, J.-M.; Gao, J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem. Biophys. Res. Commun. 2013, 435, 597–602. [Google Scholar] [CrossRef]
- Gocmez, S.S.; Gacar, N.; Utkan, T.; Gacar, G.; Scarpace, P.J.; Tumer, N. Protective effects of resveratrol on aging-induced cognitive impairment in rats. Neurobiol. Learn. Mem. 2016, 131, 131–136. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Fiori, J.L.; Shin, Y.-K.; Kim, W.; Krzysik-Walker, S.M.; González-Mariscal, I.; Carlson, O.D.; Sanghvi, M.; Moaddel, R.; Farhang, K.; Gadkaree, S.K.; et al. Resveratrol Prevents β-Cell Dedifferentiation in Nonhuman Primates Given a High-Fat/High-Sugar Diet. Diabetes 2013, 62, 3500–3513. [Google Scholar] [CrossRef]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol Improves Adipose Insulin Signaling and Reduces the Inflammatory Response in Adipose Tissue of Rhesus Monkeys on High-Fat, High-Sugar Diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol. Nutr. Food Res. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Msc, G.M.B.; Bsc, S.M.K.; Brar, N.; Cole, L.K.; Seshadri, N.; Pereira, T.J.; Xiang, B.; Hunt, K.L.; Fonseca, M.A.; Hatch, G.M.; et al. Maternal resveratrol administration protects against gestational diabetes-induced glucose intolerance and islet dysfunction in the rat offspring. J. Physiol. 2019, 597, 4175–4192. [Google Scholar] [CrossRef]
- Zheng, T.; Chen, H. Resveratrol ameliorates the glucose uptake and lipid metabolism in gestational diabetes mellitus mice and insulin-resistant adipocytes via miR-23a-3p/NOV axis. Mol. Immunol. 2021, 137, 163–173. [Google Scholar] [CrossRef]
- Crane, F.L.; Navas, P. The diversity of coenzyme Q function. Mol. Asp. Med. 1997, 18, 1–6. [Google Scholar] [CrossRef]
- Crane, F.; Hatefi, Y.; Lester, R.; Widmer, C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 1957, 25, 220–221. [Google Scholar] [CrossRef]
- Crane, F.L. Biochemical Functions of Coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, R.; Takeshige, K.; Minakami, S. NADH- and NADPH-dependent lipid peroxidation in bovine heart submitochondrial particles. Dependence on the rate of electron flow in the respiratory chain and an antioxidant role of ubiquinol. Biochem. J. 1980, 192, 853–860. [Google Scholar] [CrossRef]
- Mellors, A.; Tappel, A.L. Quinones and quinols as inhibitors of lipid peroxidation. Lipids 1966, 1, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.M.; Xia, L.; Eriksson, L.C.; Björnstedt, M. Ubiquinone is reduced by lipoamide dehydrogenase and this reaction is potently stimulated by zinc. FEBS Lett. 1999, 448, 190–192. [Google Scholar] [CrossRef]
- Landi, L.; Fiorentini, D.; Galli, M.; Segura-Aguilar, J.; Beyer, R.E. DT-Diaphorase Maintains the Reduced State of Ubiquinones in Lipid Vesicles thereby Promoting their Antioxidant Function. Free Radic. Biol. Med. 1997, 22, 329–335. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamaguchi, T.; Shitashige, M.; Okamoto, T.; Kishi, T. Reduction of ubiquinone in membrane lipids by rat liver cytosol and its involvement in the cellular defence system against lipid peroxidation. Biochem. J. 1995, 309, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Tran, U.C.; Clarke, C.F. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 2007, 7, S62–S71. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.; López, L. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants 2021, 10, 520. [Google Scholar] [CrossRef]
- Rauchová, H.; Battino, M.; Fato, R.; Lenaz, G.; Drahota, Z. Coenzyme Q-pool function in glycerol-3-phosphate oxidation in hamster brown adipose tissue mitochondria. J. Bioenerg. Biomembr. 1992, 24, 235–241. [Google Scholar] [CrossRef]
- Missaglia, S.; Tavian, D.; Angelini, C. ETF dehydrogenase advances in molecular genetics and impact on treatment. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 360–372. [Google Scholar] [CrossRef]
- González-García, P.; Hidalgo-Gutiérrez, A.; Mascaraque, C.; Barriocanal-Casado, E.; Bakkali, M.; Ziosi, M.; Abdihankyzy, U.B.; Sánchez-Hernández, S.; Escames, G.; Prokisch, H.; et al. Coenzyme Q10 modulates sulfide metabolism and links the mitochondrial respiratory chain to pathways associated to one carbon metabolism. Hum. Mol. Genet. 2020, 29, 3296–3311. [Google Scholar] [CrossRef] [PubMed]
- Summitt, C.B.; Johnson, L.C.; Jönsson, T.J.; Parsonage, D.; Holmes, R.P.; Lowther, W.T. Proline dehydrogenase 2 (PRODH2) is a hydroxyproline dehydrogenase (HYPDH) and molecular target for treating primary hyperoxaluria. Biochem. J. 2015, 466, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Bartoschek, S.; Johansson, M.; Geierstanger, B.H.; Okun, J.G.; Lancaster, C.R.D.; Humpfer, E.; Yu, L.; Yu, C.-A.; Griesinger, C.; Brandt, U. Three Molecules of Ubiquinone Bind Specifically to Mitochondrial Cytochrome bc1 Complex. J. Biol. Chem. 2001, 276, 35231–35234. [Google Scholar] [CrossRef]
- Tocilescu, M.A.; Zickermann, V.; Zwicker, K.; Brandt, U. Quinone binding and reduction by respiratory complex I. Biochim. Biophys. Acta 2010, 1797, 1883–1890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos-Ocaña, C.; Do, T.Q.; Clarke, C.F.; Padilla, S.; Navas, P. Uptake of Exogenous Coenzyme Q and Transport to Mitochondria Is Required for bc1 Complex Stability in Yeast coq Mutants. J. Biol. Chem. 2002, 277, 10973–10981. [Google Scholar] [CrossRef] [PubMed]
- Guarás, A.; Perales-Clemente, E.; Calvo, E.; Acin-Perez, R.; Loureiro, M.; Pujol, C.; Martínez-Carrascoso, I.; Nuñez, E.; Garcia-Marques, F.; Rodríguez-Hernández, Á.; et al. The CoQH2/CoQ Ratio Serves as a Sensor of Respiratory Chain Efficiency. Cell Rep. 2016, 15, 197–209. [Google Scholar] [CrossRef]
- Acín-Pérez, R.; Fernández-Silva, P.; Peleato, M.L.; Pérez-Martos, A.; Enriquez, J.A. Respiratory Active Mitochondrial Supercomplexes. Mol. Cell 2008, 32, 529–539. [Google Scholar] [CrossRef]
- Takahashi, K.; Ohsawa, I.; Shirasawa, T.; Takahashi, M. Optic atrophy 1 mediates coenzyme Q-responsive regulation of respiratory complex IV activity in brain mitochondria. Exp. Gerontol. 2017, 98, 217–223. [Google Scholar] [CrossRef]
- Björnstedt, M.; Nordman, T.; Olsson, J.M. Extramitochondrial Reduction of Ubiquinone by Flavoenzymes. Methods Enzymol. 2004, 378, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Noguchi, N.; Gotoh, N. Inhibition of Oxidative Modification of Low Density Lipoprotein by Antioxidants. J. Nutr. Sci. Vitaminol. 1993, 39, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; Neuzil, J.; Stocker, R. Inhibition of LDL oxidation by ubiquinol-10. A protective mechanism for coenzyme Q in atherogenesis? Mol. Asp. Med. 1997, 18, 85–103. [Google Scholar] [CrossRef]
- Takahashi, T.; Mine, Y.; Okamoto, T. Extracellular coenzyme Q10 (CoQ10) is reduced to ubiquinol-10 by intact Hep G2 cells independent of intracellular CoQ10 reduction. Arch. Biochem. Biophys. 2019, 672, 108067. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.E.; Burnett, B.-A.; Cartwright, K.J.; Edington, D.W.; Falzon, M.J.; Kreitman, K.R.; Kuhn, T.W.; Ramp, B.J.; Rhee, S.Y.S.; Rosenwasser, M.J.; et al. Tissue coenzyme Q (ubiquinone) and protein concentrations over the life span of the laboratory rat. Mech. Ageing Dev. 1985, 32, 267–281. [Google Scholar] [CrossRef]
- Lapointe, J.; Hekimi, S. Early Mitochondrial Dysfunction in Long-lived Mclk1+/− Mice. J. Biol. Chem. 2008, 283, 26217–26227. [Google Scholar] [CrossRef]
- Tian, G.; Sawashita, J.; Kubo, H.; Nishio, S.-Y.; Hashimoto, S.; Suzuki, N.; Yoshimura, H.; Tsuruoka, M.; Wang, Y.; Liu, Y.; et al. Ubiquinol-10 Supplementation Activates Mitochondria Functions to Decelerate Senescence in Senescence-Accelerated Mice. Antioxid. Redox Signal. 2014, 20, 2606–2620. [Google Scholar] [CrossRef]
- del Pozo-Cruz, J.; Rodríguez-Bies, E.; Navas-Enamorado, I.; Cruz, B.D.P.; Navas, P.; López-Lluch, G. Relationship between functional capacity and body mass index with plasma coenzyme Q10 and oxidative damage in community-dwelling elderly-people. Exp. Gerontol. 2014, 52, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Onur, S.; Niklowitz, P.; Menke, T.; Laudes, M.; Rimbach, G.; Döring, F. Coenzyme Q10 Status as a Determinant of Muscular Strength in Two Independent Cohorts. PLoS ONE 2016, 11, e0167124. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Dahlström, Ö.; Dahlström, U.; Alehagen, U. Improved health-related quality of life, and more days out of hospital with supplementation with selenium and coenzyme Q10 combined. Results from a double blind, placebo-controlled prospective study. J. Nutr. Health Aging 2015, 19, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of Coenzyme Q10 in Early Parkinson Disease: Evidence of Slowing of the Functional Decline. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.-N. Mitochondrial Enhancement for Neurodegenerative Movement Disorders: A Systematic Review of Trials Involving Creatine, Coenzyme Q10, Idebenone and Mitoquinone. CNS Drugs 2013, 28, 63–68. [Google Scholar] [CrossRef]
- Zhu, Z.-G.; Sun, M.-X.; Zhang, W.-L.; Wang, W.-W.; Jin, Y.-M.; Xie, C.-L. The efficacy and safety of coenzyme Q10 in Parkinson’s disease: A meta-analysis of randomized controlled trials. Neurol. Sci. 2016, 38, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Flowers, N.; Hartley, L.; Todkill, D.; Stranges, S.; Rees, K. Co-enzyme Q10 supplementation for primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- De Frutos, F.; Gea, A.; Hernandez-Estefania, R.; Rabago, G. Prophylactic treatment with coenzyme Q10 in patients undergoing cardiac surgery: Could an antioxidant reduce complications? A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2014, 20, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Fotino, A.D.; Thompson-Paul, A.M.; Bazzano, L.A. Effect of coenzyme Q10 supplementation on heart failure: A meta-analysis. Am. J. Clin. Nutr. 2012, 97, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The Effect of Coenzyme Q10 on Morbidity and Mortality in Chronic Heart Failure: Results from Q-SYMBIO: A Randomized Double-Blind Trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Haghighatdoost, F.; Feizi, A.; Larijani, B.; Azadbakht, L. Effect of Coenzyme Q10 Supplementation on Diabetes Biomarkers: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Arch. Iran. Med. 2016, 19, 588–596. [Google Scholar] [PubMed]
- Suksomboon, N.; Poolsup, N.; Juanak, N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2015, 40, 413–418. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta -analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Aaseth, J.; Alexander, J.; Brismar, K. Increase in insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 1 after supplementation with selenium and coenzyme Q10. A prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. PLoS ONE 2017, 12, e0178614. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Clarkson, P.; Karas, R.H. Statin-Associated Myopathy. JAMA 2003, 289, 1681–1690. [Google Scholar] [CrossRef]
- Fedacko, J.; Pella, D.; Fedackova, P.; Hänninen, O.; Tuomainen, P.; Jarcuska, P.; Lopuchovsky, T.; Jedlickova, L.; Merkovska, L.; Littarru, G.P. Coenzyme Q10 and selenium in statin-associated myopathy treatment. Can. J. Physiol. Pharmacol. 2013, 91, 165–170. [Google Scholar] [CrossRef]
- Baschiera, E.; Sorrentino, U.; Calderan, C.; Desbats, M.A.; Salviati, L. The multiple roles of coenzyme Q in cellular homeostasis and their relevance for the pathogenesis of coenzyme Q deficiency. Free Radic. Biol. Med. 2021, 166, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ogasahara, S.; Engel, A.G.; Frens, D.; Mack, D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc. Natl. Acad. Sci. USA 1989, 86, 2379–2382. [Google Scholar] [CrossRef]
- Desbats, M.A.; Lunardi, G.; Doimo, M.; Trevisson, E.; Salviati, L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ10) deficiency. J. Inherit. Metab. Dis. 2014, 38, 145–156. [Google Scholar] [CrossRef]
- Navas, P.; Cascajo, M.V.; Alcázar-Fabra, M.; Hernández-Camacho, J.D.; Sánchez-Cuesta, A.; Rodríguez, A.B.C.; Ballesteros-Simarro, M.; Arroyo-Luque, A.; Rodríguez-Aguilera, J.C.; Fernández-Ayala, D.J.M.; et al. Secondary CoQ10 deficiency, bioenergetics unbalance in disease and aging. BioFactors 2021, 47, 551–569. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L. Prodrugs of Quercetin and Resveratrol: A Strategy under Development. Curr. Drug Metab. 2014, 15, 77–95. [Google Scholar] [CrossRef] [PubMed]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000 mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G. The Important Role of CoQ10 in Aging. Antioxidants 2019, 8, 570. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherardi, G.; Corbioli, G.; Ruzza, F.; Rizzuto, R. CoQ10 and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dysfunctions. Nutrients 2022, 14, 4326. https://doi.org/10.3390/nu14204326
Gherardi G, Corbioli G, Ruzza F, Rizzuto R. CoQ10 and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dysfunctions. Nutrients. 2022; 14(20):4326. https://doi.org/10.3390/nu14204326
Chicago/Turabian StyleGherardi, Gaia, Giovanni Corbioli, Filippo Ruzza, and Rosario Rizzuto. 2022. "CoQ10 and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dysfunctions" Nutrients 14, no. 20: 4326. https://doi.org/10.3390/nu14204326
APA StyleGherardi, G., Corbioli, G., Ruzza, F., & Rizzuto, R. (2022). CoQ10 and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dysfunctions. Nutrients, 14(20), 4326. https://doi.org/10.3390/nu14204326





