Systems Medicine Approach for Tinnitus with Comorbid Disorders
Abstract
1. Introduction
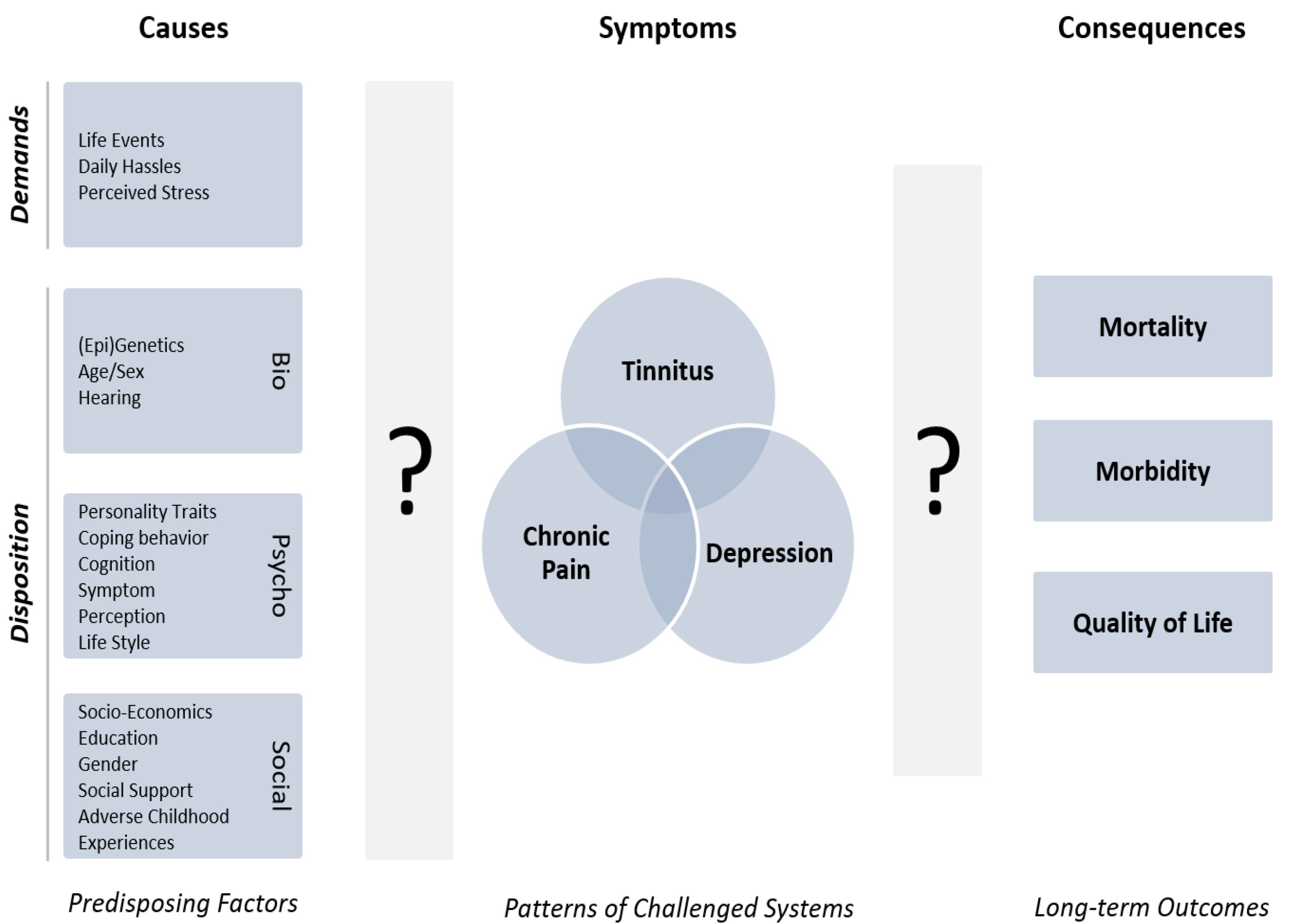
2. Comorbidity in Chronic Tinnitus and Its Challenge to the Health Care Systems
2.1. A Common Basis of Tinnitus and Depression
2.2. A Common Basis of Tinnitus and Chronic Pain
2.3. A Common Basis of Chronic Pain and Depression
3. Tinnitus and Comorbid Conditions as Neural Network Disorders
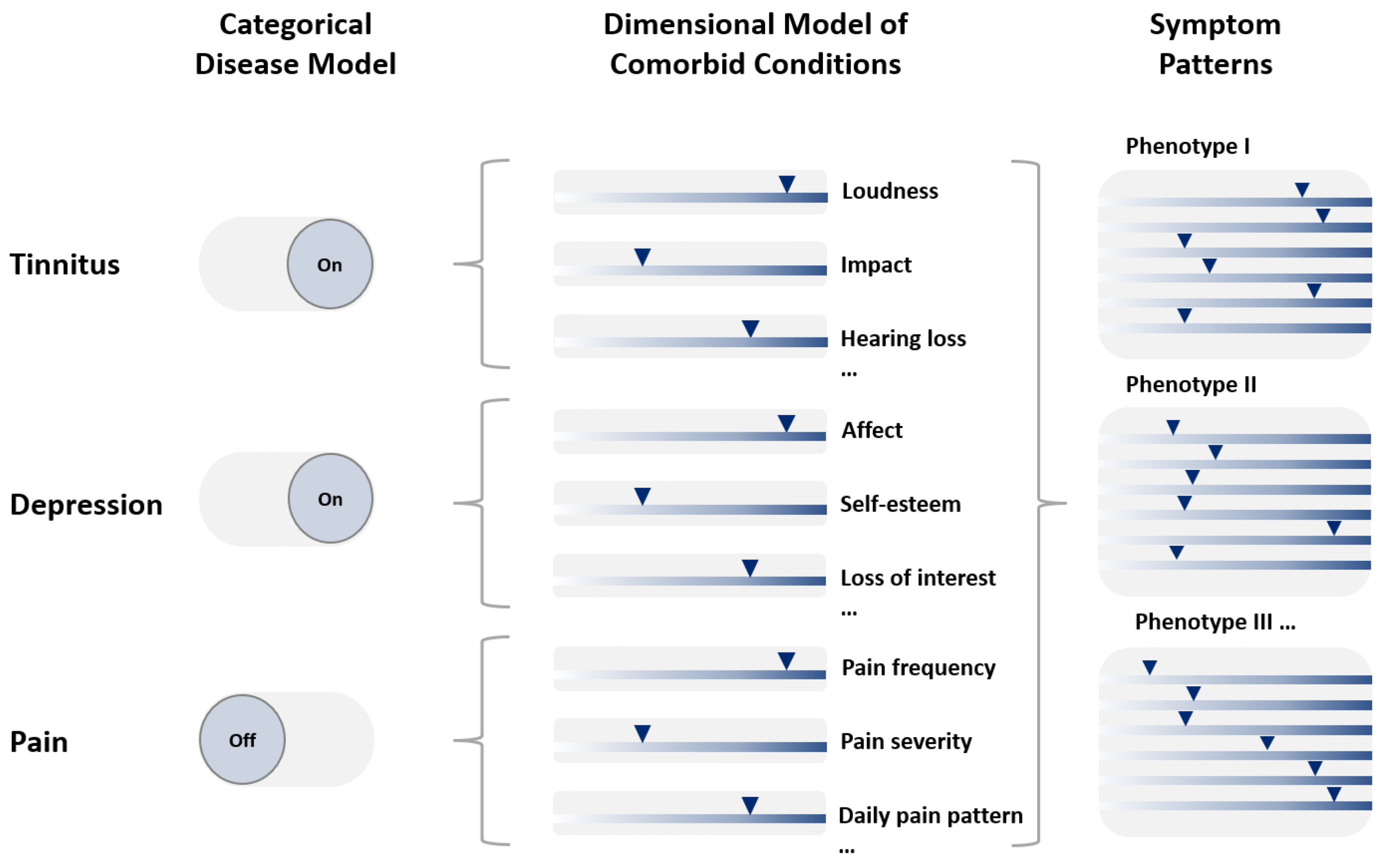
4. Comorbidity as a Collection of Symptoms
5. Identification of Phenotypes
6. Conclusions: System Medicine Approaches—A Change from Classical Phenotypes to New Phenotypes Based on All Complex Components of Comorbid Disorders
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.J.; Andersson, G.; et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 2021, 260, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Sterk, P.J.; Adcock, I.M.; Chung, K.F.; Roca, J.; Agusti, A.; Brightling, C.; Cambon-Thomsen, A.; Cesario, A.; et al. Systems medicine and integrated care to combat chronic noncommunicable diseases. Genome Med. 2011, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Charron, D.; Hood, L. Predictive, preventive, personalized and participatory medicine: Back to the future. Genome Med. 2010, 2, 57. [Google Scholar] [CrossRef]
- Bousquet, J.; Jorgensen, C.; Dauzat, M.; Cesario, A.; Camuzat, T.; Bourret, R.; Best, N.; Anto, J.M.; Abecassis, F.; Aubas, P.; et al. Systems medicine approaches for the definition of complex phenotypes in chronic diseases and ageing. From concept to implementation and policies. Curr. Pharm. Des. 2014, 20, 5928–5944. [Google Scholar] [CrossRef]
- Zeman, F.; Koller, M.; Langguth, B.; Landgrebe, M. Which tinnitus-related aspects are relevant for quality of life and depression: Results from a large international multicentre sample. Health Qual. Life Outcomes 2014, 12, 7. [Google Scholar] [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Bauer, C.A.; Berry, J.; Brozoski, T.J. Clinical trials supported by the Tinnitus Research Consortium: Lessons learned, the Southern Illinois University experience. Hear. Res. 2016, 334, 65–71. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Breivik, H.; Eisenberg, E.; O’Brien, T. The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef]
- Maes, I.H.; Cima, R.F.; Vlaeyen, J.W.; Anteunis, L.J.; Joore, M.A. Tinnitus: A cost study. Ear Hear. 2013, 34, 508–514. [Google Scholar] [CrossRef]
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016, 337, 70–79. [Google Scholar] [CrossRef]
- Olesen, J.; Gustavsson, A.; Svensson, M.; Wittchen, H.-U.; Jönsson, B. The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012, 19, 155–162. [Google Scholar] [CrossRef]
- Mansell, W.; McEvoy, P.M. A test of the core process account of psychopathology in a heterogenous clinical sample of anxiety and depression: A case of the blind men and the elephant? J. Anxiety Disord. 2017, 46, 4–10. [Google Scholar] [CrossRef]
- Sharp, P.B.; Miller, G.A.; Heller, W. Transdiagnostic dimensions of anxiety: Neural mechanisms, executive functions, and new directions. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2015, 98, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Geocze, L.; Mucci, S.; Abranches, D.C.; Marco, M.A.d.; Penido, N.d.O. Systematic review on the evidences of an association between tinnitus and depression. Braz. J. Otorhinolaryngol. 2013, 79, 106–111. [Google Scholar] [CrossRef]
- Hasson, D.; Theorell, T.; Bergquist, J.; Canlon, B. Acute stress induces hyperacusis in women with high levels of emotional exhaustion. PLoS ONE 2013, 8, e52945. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Effect of acute stress on auditory processing: A systematic review of human studies. Rev. Neurosci. 2017, 28, 1–13. [Google Scholar] [CrossRef]
- Ivansic, D.; Guntinas-Lichius, O.; Muller, B.; Volk, G.F.; Schneider, G.; Dobel, C. Impairments of Speech Comprehension in Patients with Tinnitus-A Review. Front. Aging Neurosci 2017, 9, 224. [Google Scholar] [CrossRef]
- Pergamin-Hight, L.; Naim, R.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H.; Bar-Haim, Y. Content specificity of attention bias to threat in anxiety disorders: A meta-analysis. Clin. Psychol. Rev. 2015, 35, 10–18. [Google Scholar] [CrossRef]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 2015, 20, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-M.; Ko, Y.-H.; Shin, C.; Lee, J.-H.; Choi, J.; Kwon, D.-Y.; Yoon, H.-K.; Han, C.; Kim, Y.-K. Tinnitus, depression, and suicidal ideation in adults: A nationally representative general population sample. J. Psychiatr. Res. 2018, 98, 124–132. [Google Scholar] [CrossRef]
- Trevis, K.J.; McLachlan, N.M.; Wilson, S.J. Psychological mediators of chronic tinnitus: The critical role of depression. J. Affect. Disord. 2016, 204, 234–240. [Google Scholar] [CrossRef]
- Wallhäusser-Franke, E.; Repik, I.; Delb, W.; Glauner, A.; Hörmann, K. Long-term Development of Acute Tinnitus. Laryngo- Rhino- Otologie 2015, 94, 759–769. [Google Scholar] [CrossRef]
- Hébert, S.; Canlon, B.; Hasson, D.; Magnusson Hanson, L.L.; Westerlund, H.; Theorell, T. Tinnitus severity is reduced with reduction of depressive mood--a prospective population study in Sweden. PLoS ONE 2012, 7, e37733. [Google Scholar] [CrossRef]
- Chen, Y.-C.T.; Tsai, S.-J.; Chen, J.-C.; Hwang, J.-H. Risks of tinnitus, sensorineural hearing impairment, and sudden deafness in patients with non-migraine headache. PLoS ONE 2019, 9, e0222041. [Google Scholar] [CrossRef]
- Erlandsson, S.I.; Hallberg, L.R.; Axelsson, A. Psychological and audiological correlates of perceived tinnitus severity. Audiol. Off. Organ. Int. Soc. Audiol. 1992, 31, 168–179. [Google Scholar] [CrossRef]
- Fernandes, G.; Siqueira, J.T.T.d.; Godoi Gonçalves, D.A.d.; Camparis, C.M. Association between painful temporomandibular disorders, sleep bruxism and tinnitus. Braz. Oral Res. 2014, 28. [Google Scholar] [CrossRef]
- Guichard, E.; Montagni, I.; Tzourio, C.; Kurth, T. Association Between Headaches and Tinnitus in Young Adults: Cross-Sectional Study. Headache 2016, 56, 987–994. [Google Scholar] [CrossRef]
- Isaacson, J.E.M.; Matthew, T.; Schuler, H. Gregg; Blackall, George F. Clinical associations between tinnitus and chronic pain. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2003, 5, 706–710. [Google Scholar] [CrossRef]
- Khedr, E.M.; Ahmed, M.A.; Shawky, O.A.; Mohamed, E.S.; El Attar, G.S.; Mohammad, K.A. Epidemiological study of chronic tinnitus in Assiut, Egypt. Neuroepidemiology 2010, 35, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Hund, V.; Landgrebe, M.; Schecklmann, M. Tinnitus Patients with Comorbid Headaches: The Influence of Headache Type and Laterality on Tinnitus Characteristics. Front. Neurol. 2017, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R. Similarities between chronic pain and tinnitus. Am. J. Otol. 1997, 18, 577–585. [Google Scholar] [CrossRef]
- Tonndorf, J. The analogy between tinnitus and pain: A suggestion for a physiological basis of chronic tinnitus. Hear. Res. 1987, 28, 271–275. [Google Scholar] [CrossRef]
- Flores, E.N.; Duggan, A.; Madathany, T.; Hogan, A.K.; Márquez, F.G.; Kumar, G.; Seal, R.P.; Edwards, R.H.; Liberman, M.C.; García-Añoveros, J. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr. Biol. CB 2015, 25, 606–612. [Google Scholar] [CrossRef]
- Eggermont, J.J. Tinnitus: Neurobiological substrates. Drug Discov. Today 2005, 10, 1283–1290. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; May, E.S.; Maudoux, A.; Ploner, M. Frontostriatal Gating of Tinnitus and Chronic Pain. Trends Cogn. Sci. 2015, 19, 567–578. [Google Scholar] [CrossRef]
- Jeanmonod, D.; Magnin, M.; Morel, A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain J. Neurol. 1996, 119 Pt 2, 363–375. [Google Scholar] [CrossRef]
- Linton, S.J. A review of psychological risk factors in back and neck pain. Spine 2000, 25, 1148–1156. [Google Scholar] [CrossRef]
- Trevis, K.J.; McLachlan, N.M.; Wilson, S.J. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin. Psychol. Rev. 2018, 60, 62–86. [Google Scholar] [CrossRef]
- Pilgramm, M.; Lenders, H.; Schumann, K. [A method for relieving tinnitus complaints in long-enlisted soldiers with multiple acoustic traumas--external electrostimulation]. Hno 1986, 34, 280–284. [Google Scholar]
- Tyler, R.S. Tinnitus Handbook; Singular: San Diego, CA, USA, 2000. [Google Scholar]
- Ivansic, D.; Besteher, B.; Gantner, J.; Guntinas-Lichius, O.; Pantev, C.; Nenadic, I.; Dobel, C. Psychometric assessment of mental health in tinnitus patients, depressive and healthy controls. Psychiatry Res. 2019, 281, 112582. [Google Scholar] [CrossRef]
- Zöger, S.; Svedlund, J.; Holgers, K.-M. Relationship between tinnitus severity and psychiatric disorders. Psychosomatics 2006, 47, 282–288. [Google Scholar] [CrossRef]
- McCormack, A.; Edmondson-Jones, M.; Fortnum, H.; Dawes, P.D.; Middleton, H.; Munro, K.J.; Moore, D.R. Investigating the association between tinnitus severity and symptoms of depression and anxiety, while controlling for neuroticism, in a large middle-aged UK population. Int. J. Audiol. 2015, 54, 599–604. [Google Scholar] [CrossRef]
- Andersson, G. Psychological aspects of tinnitus and the application of cognitive–behavioral therapy. Clin. Psychol. Rev. 2002, 22, 977–990. [Google Scholar] [CrossRef]
- Hiller, W.; Janca, A.; Burke, K.C. Association between tinnitus and somatoform disorders. J. Psychosom. Res. 1997, 43, 613–624. [Google Scholar] [CrossRef]
- Banks, S.M.; Kerns, R.D. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychol. Bull. 1996, 119, 95–110. [Google Scholar] [CrossRef]
- Fishbain, D.A.; Lewis, J.E.; Gao, J.; Cole, B.; Steele Rosomoff, R. Is chronic pain associated with somatization/hypochondriasis? An evidence-based structured review. Pain Pract. Off. J. World Inst. Pain 2009, 9, 449–467. [Google Scholar] [CrossRef]
- Pincus, T.; Morley, S. Cognitive-processing bias in chronic pain: A review and integration. Psychol. Bull. 2001, 127, 599–617. [Google Scholar] [CrossRef]
- Fernandez, E.; Milburn, T.W. Sensory and affective predictors of overall pain and emotions associated with affective pain. Clin. J. Pain 1994, 10, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.F.; Rose, M. Scoring Depression on a Common Metric: A Comparison of EAP Estimation, Plausible Value Imputation, and Full Bayesian IRT Modeling. Multivar. Behav. Res. 2019, 54, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef]
- Gellatly, R.; Beck, A.T. Catastrophic thinking: A transdiagnostic process across psychiatric disorders. Cogn. Ther. Res. 2016, 40, 441–452. [Google Scholar] [CrossRef]
- Keogh, E.; Cochrane, M. Anxiety sensitivity, cognitive biases, and the experience of pain. J. Pain 2002, 3, 320–329. [Google Scholar] [CrossRef]
- Pincus, T.; Burton, A.K.; Vogel, S.; Field, A.P. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine 2002, 27, E109–E120. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Lynch, M.E.; Clark, A.J. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain 2005, 113, 310–315. [Google Scholar] [CrossRef]
- Vowles, K.E.; McCracken, L.M.; Christopher Eccleston. Patient functioning and catastrophizing in chronic pain: The mediating effects of acceptance. Health Psychol. 2008, 25, 136–143. [Google Scholar] [CrossRef]
- Bujak, B.K.; Regan, E.; Beattie, P.F.; Harrington, S. The effectiveness of interdisciplinary intensive outpatient programs in a population with diverse chronic pain conditions: A systematic review and meta-analysis. Pain Manag. 2019, 9, 417–429. [Google Scholar] [CrossRef]
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Noreña, A.; Hoare, D.J. A multidisciplinary European guideline for tinnitus: Diagnostics, assessment, and treatment. Hno 2019, 67, 10–42. [Google Scholar] [CrossRef]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Baliki, M.N.; Geha, P.Y. Towards a theory of chronic pain. Prog. Neurobiol. 2009, 87, 81–97. [Google Scholar] [CrossRef]
- Radat, F.; Margot-Duclot, A.; Attal, N. Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: A multicentre cohort study. Eur. J. Pain 2013, 17, 1547–1557. [Google Scholar] [CrossRef]
- Orenius, T.I.; Raij, T.T.; Nuortimo, A.; Näätänen, P.; Lipsanen, J.; Karlsson, H. The interaction of emotion and pain in the insula and secondary somatosensory cortex. Neuroscience 2017, 349, 185–194. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Centeno, M.V.; Ren, W.; Berger, S.E.; Tétreault, P.; Ghantous, M.; Baria, A.; Farmer, M.; Baliki, M.N.; Schnitzer, T.J.; et al. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J. Dent. Res. 2016, 95, 605–612. [Google Scholar] [CrossRef]
- Yalcin, I.; Barthas, F.; Barrot, M. Emotional consequences of neuropathic pain: Insight from preclinical studies. Neurosci. Biobehav. Rev. 2014, 47, 154–164. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Calderon, P.d.S.; Hilgenberg, P.B.; Rossetti, L.M.N.; Laurenti, J.V.E.H.; Conti, P.C.R. Influence of tinnitus on pain severity and quality of life in patients with temporomandibular disorders. J. Appl. Oral Sci. Rev. FOB 2012, 20, 170–173. [Google Scholar] [CrossRef][Green Version]
- Leaver, A.M.; Seydell-Greenwald, A.; Rauschecker, J.P. Auditory-limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear. Res. 2016, 334, 49–57. [Google Scholar] [CrossRef]
- Husain, F.T.; Schmidt, S.A. Using resting state functional connectivity to unravel networks of tinnitus. Hear. Res. 2014, 307, 153–162. [Google Scholar] [CrossRef]
- Paraskevopoulos, E.; Dobel, C.; Wollbrink, A.; Salvari, V.; Bamidis, P.D.; Pantev, C. Maladaptive alterations of resting state cortical network in Tinnitus: A directed functional connectivity analysis of a larger MEG data set. Sci. Rep. 2019, 9, 15452. [Google Scholar] [CrossRef]
- Besteher, B.; Gaser, C.; Ivansic, D.; Guntinas-Lichius, O.; Dobel, C.; Nenadic, I. Chronic tinnitus and the limbic system: Reappraising brain structural effects of distress and affective symptoms. NeuroImage. Clin. 2019, 24, 101976. [Google Scholar] [CrossRef]
- Eden, A.S.; Schreiber, J.; Anwander, A.; Keuper, K.; Laeger, I.; Zwanzger, P.; Zwitserlood, P.; Kugel, H.; Dobel, C. Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Roesmann, K.; Dellert, T.; Junghoefer, M.; Kissler, J.; Zwitserlood, P.; Zwanzger, P.; Dobel, C. The causal role of prefrontal hemispheric asymmetry in valence processing of words - Insights from a combined cTBS-MEG study. Neuroimage 2019, 191, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Penalba, V.; Deshields, T.L.; Klinkenberg, D. Gaps in communication between cancer patients and healthcare providers: Symptom distress and patients’ intentions to disclose. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2019, 27, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.F.; Rose, M. www.common-metrics.org: A web application to estimate scores from different patient-reported outcome measures on a common scale. BMC Med. Res. Methodol. 2016, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.M.; Vach, W.; Kent, P.; Hestbaek, L.; Kongsted, A. Using existing questionnaires in latent class analysis: Should we use summary scores or single items as input? A methodological study using a cohort of patients with low back pain. Clin. Epidemiol. 2016, 8, 73–89. [Google Scholar] [CrossRef]
- Collins, L.M.; Graham, J.W.; Rousculp, S.S.; Fidler, P.L.; Pan, J.; Hansen, W.B. Latent transition analysis and how it can address prevention research questions. NIDA Res. Monogr. 1994, 142, 81–111. [Google Scholar]
- Arik, S.O.; Pfister, T. Protoattend: Attention-based prototypical learning. arXiv 2019, arXiv:1902.06292. [Google Scholar] [CrossRef]
- Qureshi, S.A.; Saha, S.; Hasanuzzaman, M.; Dias, G. Multitask Representation Learning for Multimodal Estimation of Depression Level. IEEE Intell. Syst. (IEEE Intell. Syst.) 2019, 34, 45–52. [Google Scholar] [CrossRef]
- Krauss, P.; Metzner, C.; Schilling, A.; Tziridis, K.; Traxdorf, M.; Wollbrink, A.; Rampp, S.; Pantev, C.; Schulze, H. A statistical method for analyzing and comparing spatiotemporal cortical activation patterns. Sci. Rep. 2018, 8, 5433. [Google Scholar] [CrossRef]
- Langguth, B.; Landgrebe, M.; Schlee, W.; Schecklmann, M.; Vielsmeier, V.; Steffens, T.; Staudinger, S.; Frick, H.; Frick, U. Different Patterns of Hearing Loss among Tinnitus Patients: A Latent Class Analysis of a Large Sample. Front. Neurol. 2017, 8, 46. [Google Scholar] [CrossRef]
- Niemann, U.; Brueggemann, P.; Boecking, B.; Mebus, W.; Rose, M.; Spiliopoulou, M.; Mazurek, B. Phenotyping chronic tinnitus patients using self-report questionnaire data: Cluster analysis and visual comparison. Sci. Rep. 2020, 10, 16411. [Google Scholar] [CrossRef]
- Obbarius, A.; Fischer, F.; Liegl, G.; Obbarius, N.; van Bebber, J.; Hofmann, T.; Rose, M. A Step Towards a Better Understanding of Pain Phenotypes: Latent Class Analysis in Chronic Pain Patients Receiving Multimodal Inpatient Treatment. J. Pain Res. 2020, 13, 1023–1038. [Google Scholar] [CrossRef]
- Exley, D.; Norman, A.; Hyland, M. Adverse childhood experience and asthma onset: A systematic review. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2015, 24, 299–305. [Google Scholar] [CrossRef]
- Herzog, J.I.; Schmahl, C. Adverse Childhood Experiences and the Consequences on Neurobiological, Psychosocial, and Somatic Conditions Across the Lifespan. Front. Psychiatry 2018, 9, 420. [Google Scholar] [CrossRef]
- Jaworska-Andryszewska, P.; Rybakowski, J.K. Childhood trauma in mood disorders: Neurobiological mechanisms and implications for treatment. Pharmacol. Rep. PR 2019, 71, 112–120. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Maes, M.; Carvalho, A.F.; Puri, B.K. Socioeconomic Deprivation, Adverse Childhood Experiences and Medical Disorders in Adulthood: Mechanisms and Associations. Mol. Neurobiol. 2019, 56, 5866–5890. [Google Scholar] [CrossRef]
- Ports, K.A.; Holman, D.M.; Guinn, A.S.; Pampati, S.; Dyer, K.E.; Merrick, M.T.; Lunsford, N.B.; Metzler, M. Adverse Childhood Experiences and the Presence of Cancer Risk Factors in Adulthood: A Scoping Review of the Literature From 2005 to 2015. J. Pediatric. Nurs. 2019, 44, 81–96. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, Y.H. Relationship Between Diet and Tinnitus: Korea National Health and Nutrition Examination Survey. Clin. Exp. Otorhinolaryngol. 2018, 11, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Dawes, P.; Cruickshanks, K.J.; Marsden, A.; Moore, D.R.; Munro, K.J. Relationship Between Diet, Tinnitus, and Hearing Difficulties. Ear Hear. 2020, 41, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Medina, M. Systems biology for molecular life sciences and its impact in biomedicine. Cell Mol. Life Sci 2013, 70, 1035–1053. [Google Scholar] [CrossRef] [PubMed]
- Cesario, A.; Auffray, C.; Agusti, A.; Apolone, G.; Balling, R.; Barbanti, P.; Bellia, A.; Boccia, S.; Bousquet, J.; Cardaci, V.; et al. A systems medicine clinical platform for understanding and managing non- communicable diseases. Curr. Pharm. Des. 2014, 20, 5945–5956. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Vogelzangs, N.; De Jonge, P.; Smit, J.; Bahn, S.; Penninx, B. Cytokine production capacity in depression and anxiety. Transl. Psychiatry 2016, 6, e825. [Google Scholar] [CrossRef]

| Disorder | Prevalence | Preventable Burden (B EUR/y) |
|---|---|---|
| Tinnitus | 11−30% | 117–325 |
| Depression | 25% | 170 |
| Chronic Pain | 19−30% | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, B.; Rose, M.; Schulze, H.; Dobel, C. Systems Medicine Approach for Tinnitus with Comorbid Disorders. Nutrients 2022, 14, 4320. https://doi.org/10.3390/nu14204320
Mazurek B, Rose M, Schulze H, Dobel C. Systems Medicine Approach for Tinnitus with Comorbid Disorders. Nutrients. 2022; 14(20):4320. https://doi.org/10.3390/nu14204320
Chicago/Turabian StyleMazurek, Birgit, Matthias Rose, Holger Schulze, and Christian Dobel. 2022. "Systems Medicine Approach for Tinnitus with Comorbid Disorders" Nutrients 14, no. 20: 4320. https://doi.org/10.3390/nu14204320
APA StyleMazurek, B., Rose, M., Schulze, H., & Dobel, C. (2022). Systems Medicine Approach for Tinnitus with Comorbid Disorders. Nutrients, 14(20), 4320. https://doi.org/10.3390/nu14204320










