Preventive Effects of Ginkgo-Extract EGb 761® on Noise Trauma-Induced Cochlear Synaptopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
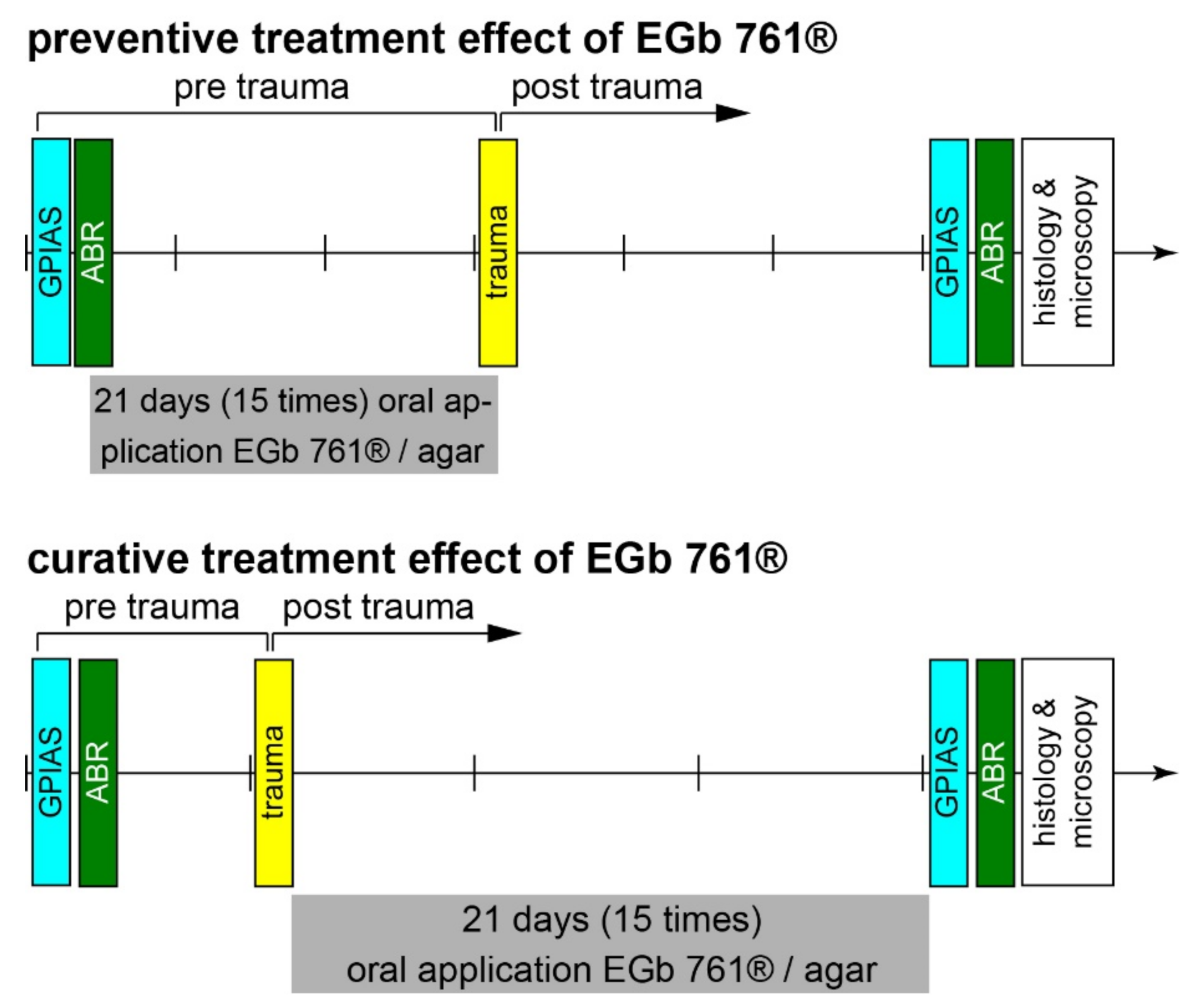
2.2. Time Regime of Experiments
2.3. Agar Medium and Oral Application
2.4. Behavioral Tinnitus Assessment (by GPIAS)
2.5. Assessment of Hearing Thresholds (by ABR)
2.6. Monaural Acoustic Trauma
2.7. Immunohistology
2.8. Data Evaluation and Statistical Analysis
3. Results
3.1. Hearing and Hearing Loss
3.2. Behavioral Evidences of Tinnitus Percepts
3.3. Changes in Ribbon Synapse Numbers at the IHC of the Cochlea
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, A.; Refaie, A.; Tyler, R. Tinnitus Handbook; Thompson Learning: Novato, CA, USA, 2000; Volume 1. [Google Scholar]
- Andersson, G. Psychological aspects of tinnitus and the application of cognitive-behavioral therapy. Clin. Psychol. Rev. 2002, 22, 977–990. [Google Scholar] [CrossRef]
- Heller, A.J. Classification and epidemiology of tinnitus. Otolaryngol. Clin. N. Am. 2003, 36, 239–248. [Google Scholar] [CrossRef]
- Nondahl, D.M.; Cruickshanks, K.J.; Huang, G.H.; Klein, B.E.; Klein, R.; Tweed, T.S.; Zhan, W. Generational differences in the reporting of tinnitus. Ear Hear. 2012, 33, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus. Trends Neurosci. 2004, 27, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Gerken, G.M. Central tinnitus and lateral inhibition: An auditory brainstem model. Hear. Res. 1996, 97, 75–83. [Google Scholar] [CrossRef]
- Schaette, R.; McAlpine, D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J. Neurosci. 2011, 31, 13452–13457. [Google Scholar] [CrossRef] [PubMed]
- Krauss, P.; Schilling, A.; Tziridis, K.; Schulze, H. Models of tinnitus development: From cochlea to cortex. HNO 2019, 69, 172–177. [Google Scholar] [CrossRef]
- Krauss, P.; Tziridis, K.; Metzner, C.; Schilling, A.; Hoppe, U.; Schulze, H. Stochastic resonance controlled upregulation of internal noise after hearing loss as a putative cause of tinnitus-related neuronal hyperactivity. Front. Neurosci. 2016, 10, 597. [Google Scholar] [CrossRef]
- Sunwoo, W.; Jeon, Y.J.; Bae, Y.J.; Jang, J.H.; Koo, J.-W.; Song, J.-J. Typewriter tinnitus revisited: The typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci. Rep. 2017, 7, 10615. [Google Scholar] [CrossRef]
- Barth, S.W.; Lehner, M.D.; Dietz, G.P.H.; Schulze, H. Pharmacologic treatments in preclinical tinnitus models with special focus on Ginkgo biloba leaf extract EGb 761(R). Mol. Cell. Neurosci. 2021, 116, 103669. [Google Scholar] [CrossRef]
- Okamoto, H.; Stracke, H.; Stoll, W.; Pantev, C. Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc. Natl. Acad. Sci. USA 2010, 107, 1207–1210. [Google Scholar] [CrossRef]
- Stein, A.; Wunderlich, R.; Lau, P.; Engell, A.; Wollbrink, A.; Shaykevich, A.; Kuhn, J.-T.; Holling, H.; Rudack, C.; Pantev, C. Clinical trial on tonal tinnitus with tailor-made notched music training. BMC Neurol. 2016, 16, 38. [Google Scholar] [CrossRef]
- Haab, L.; Lehser, C.; Corona-Strauss, F.I.; Bernarding, C.; Seidler, H.; Hannemann, R.; Strauss, D.J. Implementation and long-term evaluation of a hearing aid supported tinnitus treatment using notched environmental sounds. IEEE J. Trans. Eng. Health Med. 2019, 7, 1600109. [Google Scholar] [CrossRef]
- Engineer, N.D.; Riley, J.R.; Seale, J.D.; Vrana, W.A.; Shetake, J.A.; Sudanagunta, S.P.; Borland, M.S.; Kilgard, M.P. Reversing pathological neural activity using targeted plasticity. Nature 2011, 470, 101–104. [Google Scholar] [CrossRef]
- Konstantina, G.; Fildissis, G.; Zyga, S.; Baltopoulos, G. The Clinical Efficacy of Hyperbaric Oxygen Therapy in Idiopathic Sudden Sensorineural Hearing Loss and Tinnitus. Health Sci. J. 2016, 10, 1. [Google Scholar]
- Távora-Vieira, D.; Marino, R.; Krishnaswamy, J.; Kuthbutheen, J.; Rajan, G.P. Cochlear implantation for unilateral deafness with and without tinnitus: A case series. Laryngoscope 2013, 123, 1251–1255. [Google Scholar] [CrossRef]
- Tziridis, K.; Brunner, S.; Schilling, A.; Krauss, P.; Schulze, H. Spectrally matched near-threshold noise for subjective tinnitus loudness attenuation based on stochastic resonance. Front. Neurosci. 2022, 16, 831581. [Google Scholar] [CrossRef]
- Schilling, A.; Krauss, P.; Hannemann, R.; Schulze, H.; Tziridis, K. Reduktion der Tinnituslautstärke. HNO 2020, 69, 891–898. [Google Scholar] [CrossRef]
- Tziridis, K.; Forster, J.; Buchheidt-Dorfler, I.; Krauss, P.; Schilling, A.; Wendler, O.; Sterna, E.; Schulze, H. Tinnitus development is associated with synaptopathy of inner hair cells in Mongolian gerbils. Eur. J. Neurosci. 2021, 54, 4768–4780. [Google Scholar] [CrossRef]
- Schilling, A.; Tziridis, K.; Schulze, H.; Krauss, P. The Stochastic Resonance model of auditory perception: A unified explanation of tinnitus development, Zwicker tone illusion, and residual inhibition. bioRxiv 2020. [Google Scholar] [CrossRef]
- Krauss, P.; Tziridis, K. Simulated transient hearing loss improves auditory sensitivity. Sci. Rep. 2021, 11, 14791. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Liberman, L.D.; Suzuki, J.; Liberman, M.C. Dynamics of cochlear synaptopathy after acoustic overexposure. J. Assoc. Res. Otolaryngol. 2015, 16, 205–219. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Tziridis, K.; Korn, S.; Ahlf, S.; Schulze, H. Protective effects of Ginkgo biloba extract EGb 761 against noise trauma-induced hearing loss and tinnitus development. Neural Plast. 2014, 2014, 427298. [Google Scholar] [CrossRef]
- Krauss, P.; Tziridis, K.; Buerbank, S.; Schilling, A.; Schulze, H. Therapeutic Value of Ginkgo biloba Extract EGb 761(R) in an Animal Model (Meriones unguiculatus) for Noise Trauma Induced Hearing Loss and Tinnitus. PLoS ONE 2016, 11, e0157574. [Google Scholar] [CrossRef]
- Ahlf, S.; Tziridis, K.; Korn, S.; Strohmeyer, I.; Schulze, H. Predisposition for and prevention of subjective tinnitus development. PLoS ONE 2012, 7, e44519. [Google Scholar] [CrossRef]
- Tziridis, K.; Ahlf, S.; Jeschke, M.; Happel, M.F.; Ohl, F.W.; Schulze, H. Noise Trauma Induced Neural Plasticity throughout the Auditory System of Mongolian Gerbils: Differences between Tinnitus Developing and Non-Developing Animals. Front. Neurol. 2015, 6, 22. [Google Scholar] [CrossRef]
- Schilling, A.; Krauss, P.; Gerum, R.; Metzner, C.; Tziridis, K.; Schulze, H. A New Statistical Approach for the Evaluation of Gap-prepulse Inhibition of the Acoustic Startle Reflex (GPIAS) for Tinnitus Assessment. Front. Behav. Neurosci. 2017, 11, 198. [Google Scholar] [CrossRef]
- Lanaia, V.; Tziridis, K.; Schulze, H. Salicylate-Induced Changes in Hearing Thresholds in Mongolian Gerbils Are Correlated with Tinnitus Frequency but Not with Tinnitus Strength. Front. Behav. Neurosci. 2021, 15, 698516. [Google Scholar] [CrossRef]
- Schilling, A.; Gerum, R.; Krauss, P.; Metzner, C.; Tziridis, K.; Schulze, H. Objective estimation of sensory thresholds based on neurophysiological parameters. arXiv 2018, arXiv:1811.02335. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Brozoski, T.J.; Bauer, C.A.; Parrish, J.L.; Myers, K.; Hughes, L.F.; Caspary, D.M. Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behav. Neurosci. 2006, 120, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Gerum, R.; Rahlfs, H.; Streb, M.; Krauss, P.; Grimm, J.; Metzner, C.; Tziridis, K.; Günther, M.; Schulze, H.; Kellermann, W. Open (G) PIAS: An open-source solution for the construction of a high-precision acoustic startle response setup for tinnitus screening and threshold estimation in rodents. Front. Behav. Neurosci. 2019, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear. Res. 1996, 94, 148–156. [Google Scholar] [CrossRef]
- Sjostrand, A.P.; Dogan, R.; Kocyigit, A.; Karatas, E.; Budak, B.B.; Ozturan, O. Therapeutic efficacy of Ginkgo biloba for early-period noise-induced hearing loss: An experimental animal study. Am. J. Otolaryngol. 2016, 37, 416–424. [Google Scholar] [CrossRef]
- Dogan, R.; Sjostrand, A.P.; Yenıgun, A.; Karatas, E.; Kocyigit, A.; Ozturan, O. Influence of Ginkgo Biloba extract (EGb 761) on expression of IL-1 Beta, IL-6, TNF-alfa, HSP-70, HSF-1 and COX-2 after noise exposure in the rat cochlea. Auris Nasus Larynx 2018, 45, 680–685. [Google Scholar] [CrossRef]
- Gargouri, B.; Carstensen, J.; Bhatia, H.S.; Huell, M.; Dietz, G.P.; Fiebich, B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 2018, 44, 45–55. [Google Scholar] [CrossRef]
- Nevado, J.; Sanz, R.; Sánchez-Rodríguez, C.; García-Berrocal, J.R.; Martín-Sanz, E.; González-García, J.Á.; Esteban-Sánchez, J.; Ramírez-Camacho, R. Ginkgo biloba extract (EGb761) protects against aging-related caspase-mediated apoptosis in rat cochlea. Acta Otolaryngol. 2010, 130, 1101–1112. [Google Scholar] [CrossRef]
- Finkler, A.D.; da Silveira, A.F.; Munaro, G.; Zanrosso, C.D. Otoprotection in guinea pigs exposed to pesticides and ginkgo biloba. Braz. J. Otorhinolaryngol. 2012, 78, 122–128. [Google Scholar] [CrossRef]
- Szasz, B.K.; Lenkey, N.; Barth, A.M.; Mike, A.; Somogyvari, Z.; Farkas, O.; Lendvai, B. Converging effects of Ginkgo biloba extract at the level of transmitter release, NMDA and sodium currents and dendritic spikes. Planta Med. 2008, 74, 1235–1239. [Google Scholar] [CrossRef]
- Oestreicher, E.; Arnold, W.; Ehrenberger, K.; Felix, D. Dopamine regulates the glutamatergic inner hair cell activity in guinea pigs. Hear. Res. 1997, 107, 46–52. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z. Ginkgo biloba extract enhances differentiation and performance of neural stem cells in mouse cochlea. Cell. Mol. Neurobiol. 2015, 35, 861–869. [Google Scholar] [CrossRef]
- Szczepek, A.J.; Dietz, G.P.; Reich, U.; Hegend, O.; Olze, H.; Mazurek, B. Differences in stress-induced modulation of the auditory system between Wistar and Lewis rats. Front. Neurosci. 2018, 12, 828. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Kim, J.S.; Cho, S.W.; Yang, H.C.; Jung, K.H.; Kim, J.Y.; Choi, C.H.; Lim, Y.; Park, H. Effect of Ginkgo biloba extract on endotoxin-induced labyrinthitis. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 905–909. [Google Scholar] [CrossRef]
- Eggermont, J.J. Hearing loss, hyperacusis, or tinnitus: What is modeled in animal research? Hear. Res. 2013, 295, 140–149. [Google Scholar] [CrossRef]
- Eggermont, J.J. Animal Models of Stress and Tinnitus, Tinnitus and Stress; Springer: Cham, Switzerland, 2017; pp. 77–94. [Google Scholar]
- Lobarinas, E.; Hayes, S.H.; Allman, B.L. The gap-startle paradigm for tinnitus screening in animal models: Limitations and optimization. Hear. Res. 2013, 295, 150–160. [Google Scholar] [CrossRef]
- Krauss, P.; Metzner, C.; Schilling, A.; Schutz, C.; Tziridis, K.; Fabry, B.; Schulze, H. Adaptive stochastic resonance for unknown and variable input signals. Sci. Rep. 2017, 7, 2450. [Google Scholar] [CrossRef]
- Krauss, P.; Tziridis, K.; Schilling, A.; Schulze, H. Cross-modal stochastic resonance as a universal principle to enhance sensory processing. Front. Neurosci. 2018, 12, 578. [Google Scholar] [CrossRef]
- Gollnast, D.; Tziridis, K.; Krauss, P.; Schilling, A.; Hoppe, U.; Schulze, H. Analysis of Audiometric Differences of Patients with and without Tinnitus in a Large Clinical Database. Front. Neurol. 2017, 8, 31. [Google Scholar] [CrossRef]
- Woelk, H.; Arnoldt, K.; Kieser, M.; Hoerr, R. Ginkgo biloba special extract EGb 761® in generalized anxiety disorder and adjustment disorder with anxious mood: A randomized, double-blind, placebo-controlled trial. J. Psychiatr. Res. 2007, 41, 472–480. [Google Scholar] [CrossRef]





| Treatment | Factor | F-Statistics | p-Value |
|---|---|---|---|
| Preventive | Ear | F(1, 50) = 0.91 | 0.34 |
| Frequency | F(3, 50) = 8.45 | <0.001 | |
| Substance | F(1, 50) = 2.19 | 0.15 | |
| Ear × frequency | F(3, 50) = 0.64 | 0.59 | |
| Ear × substance | F(1, 50) = 0.79 | 0.38 | |
| Frequency × substance | F(3, 50) = 1.30 | 0.29 | |
| Ear × frequency × substance | F(3, 50) = 0.58 | 0.63 | |
| Curative | Ear | F(1, 64) = 1.41 | 0.24 |
| Frequency | F(3, 64) = 3.85 | 0.02 | |
| Substance | F(1, 64) = 1.11 | 0.30 | |
| Ear × frequency | F(3, 64) = 1.30 | 0.28 | |
| Ear × substance | F(1, 64) = 0.72 | 0.40 | |
| Frequency × substance | F(3, 64) = 0.49 | 0.69 | |
| Ear × frequency × substance | F(3, 64) = 0.43 | 0.84 |
| Treatment | Factor | F-Statistics | p-Value |
|---|---|---|---|
| Preventive | Ear | F(1, 39) = 0.46 | 0.50 |
| Frequency | F(3, 39) = 1.39 | 0.21 | |
| Substance | F(1, 39) = 0.03 | 0.87 | |
| Ear × frequency | F(3, 39) = 0.16 | 0.92 | |
| Ear × substance | F(1, 39) = 0.48 | 0.48 | |
| Frequency × substance | F(3, 39) = 0.92 | 0.44 | |
| Ear × frequency × substance | F(3, 39) = 1.77 | 0.12 | |
| Curative | Ear | F(1, 50) = 4.17 | 0.03 |
| Frequency | F(3, 50) = 0.67 | 0.57 | |
| Substance | F(1, 50) = 0.01 | 0.93 | |
| Ear × frequency | F(3, 50) = 0.31 | 0.82 | |
| Ear × substance | F(1, 50) = 0.82 | 0.37 | |
| Frequency × substance | F(3, 50) = 0.65 | 0.59 | |
| Ear × frequency × substance | F(3, 50) = 0.44 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tziridis, K.; Schulze, H. Preventive Effects of Ginkgo-Extract EGb 761® on Noise Trauma-Induced Cochlear Synaptopathy. Nutrients 2022, 14, 3015. https://doi.org/10.3390/nu14153015
Tziridis K, Schulze H. Preventive Effects of Ginkgo-Extract EGb 761® on Noise Trauma-Induced Cochlear Synaptopathy. Nutrients. 2022; 14(15):3015. https://doi.org/10.3390/nu14153015
Chicago/Turabian StyleTziridis, Konstantin, and Holger Schulze. 2022. "Preventive Effects of Ginkgo-Extract EGb 761® on Noise Trauma-Induced Cochlear Synaptopathy" Nutrients 14, no. 15: 3015. https://doi.org/10.3390/nu14153015
APA StyleTziridis, K., & Schulze, H. (2022). Preventive Effects of Ginkgo-Extract EGb 761® on Noise Trauma-Induced Cochlear Synaptopathy. Nutrients, 14(15), 3015. https://doi.org/10.3390/nu14153015







