Improving Food-Related Quality of Life in Inflammatory Bowel Disease through a Novel Web Resource: A Feasibility Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Web Resource (Intervention)
2.2. Study Design and Participants
2.3. Randomisation
2.4. Trial Protocol and Procedures
2.5. FR-QoL, HR-QoL and Psychological Outcomes
2.6. Disease Activity and Disease Control
2.7. Acceptability and Usage of the Web Resource
2.8. Patient Identification, Screening, Randomisation and Completion
2.9. Statistical Analysis
3. Results
3.1. Food-Related Quality of Life (FR-QoL)
3.2. Quality of Life and Psychological Outcomes
3.3. Disease Activity and Disease Control
3.4. Acceptability and Usage of the Web Resource
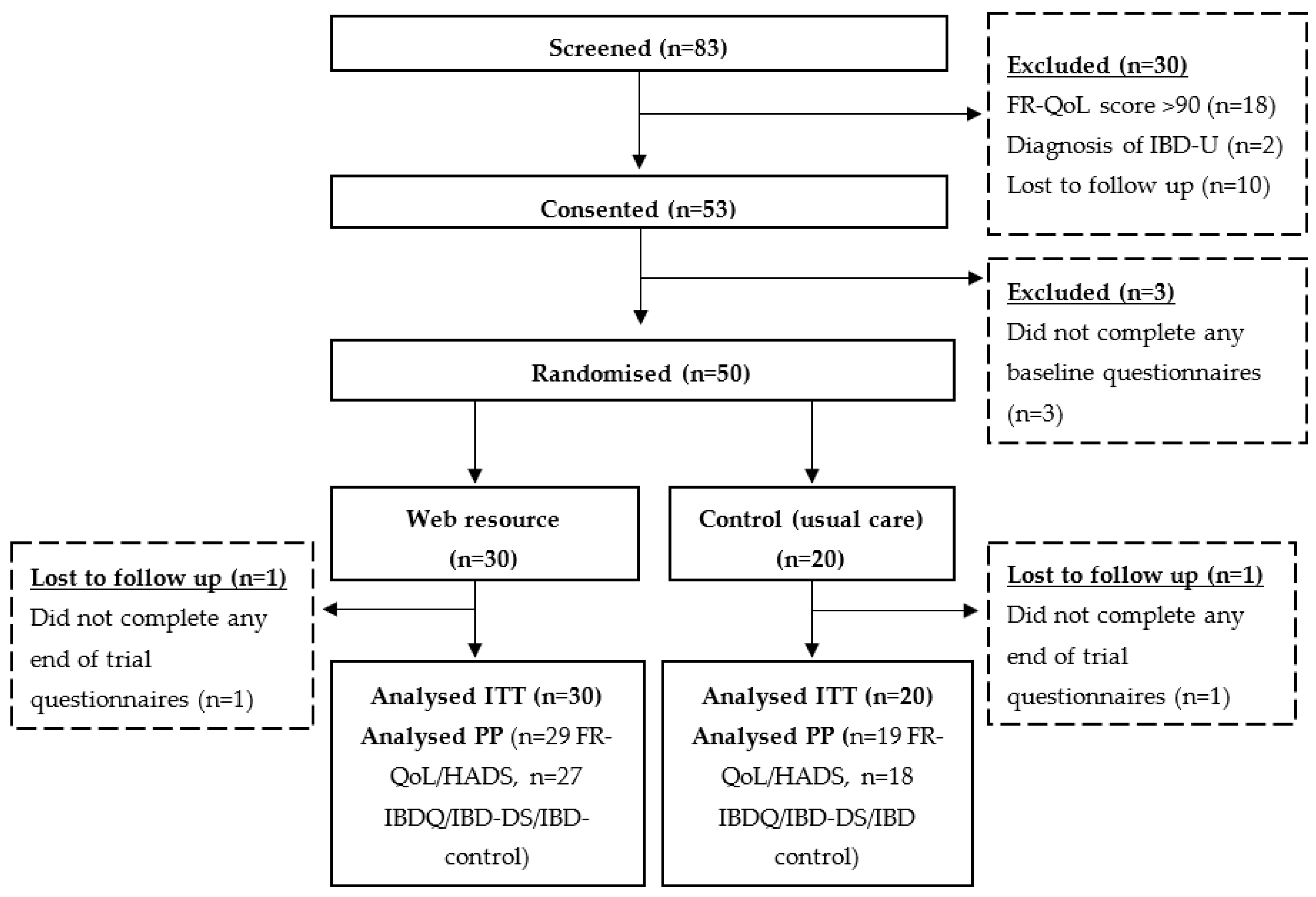
3.5. Patient Identification, Screening, Randomisation and Completion
4. Discussion
4.1. Future Research
4.2. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Vries, J.H.; Dijkhuizen, M.; Tap, P.; Witteman, B.J. Patient’s Dietary Beliefs and Behaviour in Inflammatory Bowel Disease. Dig. Dis. 2019, 37, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Yao, C.K.; Costello, S.P.; Andrews, J.M.; Bryant, R.V. Food avoidance, restrictive eating behaviour and association with quality of life in adults with inflammatory bowel disease: A systematic scoping review. Appetite 2021, 167, 105650. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Clarke, H.; O’Keeffe, M.; Dubois, P.; Irving, P.M.; Lindsay, J.O.; Whelan, K. Nutrient, fibre and FODMAP intakes and food-related quality of life in patients with inflammatory bowel disease and their relationship with gastrointestinal symptoms of differing aetiologies: A case-control study. J. Crohn’s Colitis 2021, 15, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Vidarsdottir, J.B.; Johannsdottir, S.E.; Thorsdottir, I.; Bjornsson, E.; Ramel, A. A cross-sectional study on nutrient intake and -status in inflammatory bowel disease patients. Nutr. J. 2016, 15, 61. [Google Scholar] [CrossRef]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef]
- Gustafsson, U.; Draper, A. The social aspects of food and nutrition. J. Hum. Nutr. Diet. 2009, 22, 87–88. [Google Scholar] [CrossRef]
- Czuber-Dochan, W.; Morgan, M.; Hughes, L.D.; Lomer, M.C.; Lindsay, J.O.; Whelan, K. Perceptions and psychosocial impact of food, nutrition, eating and drinking in people with inflammatory bowel disease: A qualitative investigation of food-related quality of life. J. Hum. Nutr. Diet. 2019, 33, 115–127. [Google Scholar] [CrossRef]
- Nowlin, S.; Manning, L.; Keefer, L.; Gorbenko, K. Perceptive eating as part of the journey in inflammatory bowel disease: Lessons learned from lived experience. Clin. Nutr. 2021, 41, 299–304. [Google Scholar] [CrossRef]
- Jamieson, A.E.; Fletcher, P.C.; Schneider, M.A. Seeking control through the determination of diet: A qualitative investigation of women with irritable bowel syndrome and inflammatory bowel disease. Clin. Nurse Spec. 2007, 21, 152–160. [Google Scholar] [CrossRef]
- Daniel, J.M. Young adults’ perceptions of living with chronic inflammatory bowel disease. Gastroenterol. Nurs. 2002, 25, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Hart, A.L.; Verjee, A.; Daly, A.; Solomon, J.; Mclaughlin, J. What are the dietary treatment research priorities for inflammatory bowel disease? A short report based on a priority setting partnership with the James Lind Alliance. J. Hum. Nutr. Diet. 2017, 30, 709–713. [Google Scholar] [CrossRef]
- Hughes, L.D.; King, L.; Morgan, M.; Ayis, S.; Direkze, N.; Lomer, M.C.; Lindsay, J.O.; Whelan, K. Food-related Quality of Life in Inflammatory Bowel Disease: Development and Validation of a Questionnaire. J. Crohn’s Colitis 2015, 10, 194–201. [Google Scholar] [CrossRef]
- Whelan, K.; Murrells, T.; Morgan, M.; Cummings, F.; Stansfield, C.; Todd, A.; Sebastian, S.; Lobo, A.; Lomer, M.C.E.; Lindsay, J.O.; et al. Food-related quality of life is impaired in inflammatory bowel disease and associated with reduced intake of key nutrients. Am. J. Clin. Nutr. 2021, 113, 832–844. [Google Scholar] [CrossRef]
- Guadagnoli, L.; Mutlu, E.A.; Doerfler, B.; Ibrahim, A.; Brenner, D.; Taft, T.H. Food-related quality of life in patients with inflammatory bowel disease and irritable bowel syndrome. Qual. Life Res. 2019, 28, 2195–2205. [Google Scholar] [CrossRef]
- Day, A.S.; Yao, C.K.; Costello, S.P.; Andrews, J.M.; Bryant, R.V. Food-related quality of life in adults with inflammatory bowel disease is associated with restrictive eating behaviour, disease activity and surgery: A prospective multicentre observational study. J. Hum. Nutr. Diet. 2022, 35, 234–244. [Google Scholar] [CrossRef]
- Brown, S.C.; Whelan, K.; Frampton, C.; Wall, C.L.; Gearry, R.B.; Day, A.S. Food-Related Quality of Life in Children and Adolescents with Crohn’s Disease. Inflamm. Bowel Dis. 2022, 15, izac010. [Google Scholar] [CrossRef]
- Aslan Çin, N.N.; Whelan, K.; Özçelik, A.Ö. Food-related quality of life in inflammatory bowel disease: Measuring the validity and reliability of the Turkish version of FR-QOL-29. Health Qual. Life Outcomes 2022, 20, 103. [Google Scholar] [CrossRef]
- Robert, G.; Cornwell, J.; Locock, L.; Purushotham, A.; Sturmey, G.; Gager, M. Patients and staff as codesigners of healthcare services. BMJ 2015, 350, g7714. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.D.; Gray, K.; Knowles, S.R.; de Cruz, P. EHealth Technologies in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2016, 10, 1103–1121. [Google Scholar] [CrossRef]
- Barlow, C.; Cooke, D.; Mulligan, K.; Beck, E.; Newman, S. A Critical Review of Self-Management and Educational Interventions in Inflammatory Bowel Disease. Gastroenterol. Nurs. 2010, 33, 11–18. [Google Scholar] [CrossRef]
- Kim, A.H.; Girgis, A.; de Cruz, P.; Siegel, C.A.; Karimi, N.; Ruban, S.O.; Sechi, A.J.; Ng, W.S.W.; Andrews, J.M.; Connor, S.J. Development and Feasibility of a Web-Based Decision Aid for Patients with Ulcerative Colitis: Qualitative Pilot Study. J. Med. Internet Res. 2021, 23, e15946. [Google Scholar] [CrossRef]
- Lancaster, G.A. Pilot and feasibility studies come of age! Pilot Feasibility Stud. 2015, 1, 1. [Google Scholar] [CrossRef]
- Blatch-Jones, A.J.; Pek, W.; Kirkpatrick, E.; Ashton-Key, M. Role of feasibility and pilot studies in randomised controlled trials: A cross-sectional study. BMJ Open 2018, 8, e022233. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Garratt, A.M.; Russell, I.T.; Williams, J.G. The UK IBDQ—A British version of the inflammatory bowel disease questionnaire: Development and validation. J. Clin. Epidemiol. 2000, 53, 297–306. [Google Scholar] [CrossRef]
- Dibley, L.; Czuber-Dochan, W.; Woodward, S.; Wade, T.; Bassett, P.; Sturt, J.; Norton, C. Development and Psychometric Properties of the Inflammatory Bowel Disease Distress Scale (IBD-DS): A New Tool to Measure Disease-Specific Distress. Inflamm. Bowel Dis. 2018, 24, 2068–2077. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Zou, G.; D’Haens, G.; Feagan, B.G.; Sandborn, W.J.; Vandervoort, M.K.; Rolleri, R.L.; Bortey, E.; Paterson, C.; Forbes, W.P.; et al. A retrospective analysis: The development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment. Pharmacol. Ther. 2015, 41, 77–86. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the Non-invasive components of the Mayo Score to Assess Clinical Response in Ulcerative Colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef]
- Bodger, K.; Ormerod, C.; Shackcloth, D.; Harrison, M. Development and validation of a rapid, generic measure of disease control from the patient’s perspective: The IBD-Control questionnaire. Gut 2014, 63, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.A. “Challenging“ Research Practices: Turning a Critical Lens on the Work of Transcription. Qual. Inquiry 2003, 9, 750. [Google Scholar] [CrossRef]
- Fade, S. Using interpretive phenomenological analysis for public health nutrition and dietetic research: A practical guide. Proc. Nutr. Soc. 2004, 63, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.; Campbell, M.J.; Cooper, C.L.; Lancaster, G.A. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med. Res. Methodol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Julious, S. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Sim, J.; Lewis, M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wardle, R.A.; Thapaliya, G.; Nowak, A.; Radford, S.; Dalton, M.; Finlayson, G.; Moran, G.W. An Examination of Appetite and Disordered Eating in Active Crohn’s Disease. J. Crohn’s Colitis 2018, 12, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Robelin, K.; Senada, P.; Ghoz, H.; Sim, L.; Lebow, J.; Picco, M.; Cangemi, J.; Farraye, F.A.; Werlang, M. Prevalence and Clinician Recognition of Avoidant/Restrictive Food Intake Disorder in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2021, 17, 510–514. [Google Scholar]
- Yamada, J.; Kouri, A.; Simard, S.N.; Segovia, S.A.; Gupta, S. Barriers and Enablers to Using a Patient-Facing Electronic Questionnaire: A Qualitative Theoretical Domains Framework Analysis. J. Med. Internet Res. 2020, 22, e19474. [Google Scholar] [CrossRef]
- Prince, A.C.; Moosa, A.; Lomer, M.C.; Reidlinger, D.P.; Whelan, K. Variable access to quality nutrition information regarding inflammatory bowel disease: A survey of patients and health professionals and objective examination of written information. Health Expect. 2015, 18, 2501–2512. [Google Scholar] [CrossRef]
- Khalil, C.; van Deen, W.; Dupuy, T.; Bonthala, N.; Almario, C.; Spiegel, B. Developing Patient-Centered Inflammatory Bowel Disease–Related Educational Videos Optimized for Social Media: Qualitative Research Study. JMIR Med. Educ. 2020, 6, e21639. [Google Scholar] [CrossRef]
- Selinger, C.P.; Carbery, I.; Warren, V.; Rehman, A.F.; Williams, C.J.; Mumtaz, S.; Bholah, H.; Sood, R.; Gracie, D.J.; Hamlin, P.J.; et al. The relationship between different information sources and disease-related patient knowledge and anxiety in patients with inflammatory bowel disease. Aliment. Pharmacol. Therap. 2017, 45, 63–74. [Google Scholar] [CrossRef]
- Elliott, D.; Husbands, S.; Hamdy, F.C.; Holmberg, L.; Donovan, J.L. Understanding and Improving Recruitment to Randomised Controlled Trials: Qualitative Research Approaches. Eur. Urol. 2017, 72, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Brøgger-Mikkelsen, M.; Ali, Z.; Zibert, J.R.; Andersen, A.D.; Thomsen, S.F. Online Patient Recruitment in Clinical Trials: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e22179. [Google Scholar] [CrossRef] [PubMed]

| Web Resource (n = 30) | Control (n = 20) | p-Value | |
|---|---|---|---|
| Age (years), mean (SD) | 34 (11) | 30 (6) | 0.141 |
| Male | 14 (47) | 12 (60) | 0.355 |
| Ethnicity | 0.705 | ||
| Asian or Asian British | 6 (20) | 4 (20) | |
| Black, Black British, Caribbean or African | 1 (3) | 0 (0) | |
| Mixed or multiple ethnic groups | 0 (0) | 0 (0) | |
| White | 22 (73) | 16 (80) | |
| Other ethnic group | 1 (3) | 0 (0) | |
| Education level | 0.159 | ||
| No formal qualifications | 1 (3) | 4 (20) | |
| School level qualifications | 3 (10) | 1 (5) | |
| Advanced school level qualifications | 3 (10) | 0 (0) | |
| University Degree | 17 (57) | 13 (65) | |
| Postgraduate Degree | 6 (20) | 2 (10) | |
| Relationship status | 0.856 | ||
| Married | 12 (40) | 8 (40) | |
| Living with partner | 8 (27) | 5 (25) | |
| Divorced | 1 (3) | 0 (0) | |
| Single | 9 (30) | 7 (35) | |
| Accommodation status | 0.029 | ||
| Homeowner | 12 (40) a | 3 (15) a | |
| Renting | 11 (37) a | 15 (75) b | |
| Living with family | 7 (23) a | 2 (10) a | |
| Employment status | 0.850 | ||
| Full-time employed | 20 (67) | 16 (80) | |
| Part-time employed | 1 (3) | 1 (5) | |
| Full-time education | 2 (7) | 1 (5) | |
| Home duties | 2 (7) | 1 (5) | |
| Retired | 1 (3) | 0 (0) | |
| Unemployed | 4 (13) | 1 (5) | |
| Smoking status | 0.008 | ||
| Current smoker | 1 (3) a | 7 (35) b | |
| Previous smoker | 11 (37) a | 3 (15) a | |
| Non-smoker | 18 (60) a | 10 (50) a |
| Web Resource (n = 30) | Control (n = 20) | p-Value | |
|---|---|---|---|
| Diagnosis | 0.186 | ||
| Crohn’s disease | 13 (43) | 5 (25) | |
| Ulcerative colitis | 17 (57) | 15 (75) | |
| Crohn’s disease location | 0.990 | ||
| Ileal (L1) | 5 (17) | 2 (10) | |
| Colonic (L2) | 3 (10) | 1 (5) | |
| Ileocolonic (L3) | 5 (17) | 2 (10) | |
| Crohn’s disease behaviour | 0.758 | ||
| Non-stricturing, non-penetrating (B1) | 9 (69) | 3 (60) | |
| Stricturing (B2) | 3 (23) | 1 (20) | |
| Penetrating (B3) | 1 (8) | 1 (20) | |
| Baseline PRO-2 score, mean (SD) | 2.9 (2.8) | 4.0 (3.3) | 0.469 |
| Ulcerative colitis extent | 0.328 | ||
| Proctitis (E1) | 7 (41) | 3 (20) | |
| Left-sided (E2) | 4 (24) | 3 (20) | |
| Extensive (E3) | 6 (35) | 9 (60) | |
| Ulcerative colitis severity | 0.279 | ||
| Clinical remission (S0) | 9 (53) | 4 (27) | |
| Mild (S1) | 6 (35) | 7 (47) | |
| Moderate (S2) | 2 (12) | 4 (27) | |
| Baseline Partial Mayo Score, mean (SD) | 1.7 (1.6) | 2.2 (1.9) | 0.405 |
| Age of IBD onset | 0.214 | ||
| 17–40 years | 25 (83) | 19 (95) | |
| >40 years | 5 (17) | 1 (5) | |
| IBD diagnosis (years), mean (SD) | 0.4 (0.2) | 0.5 (0.3) | 0.548 |
| Previous surgery | 2 (7) | 0 (0) | 0.510 |
| Current medications at randomisation | |||
| 5-aminosalicylates | 12 (40) | 5 (25) | 0.365 |
| Thiopurines | 7 (23) | 3 (15) | 0.720 |
| Methotrexate | 1 (3) | 0 (0) | 1.000 |
| Biologics | 8 (27) | 3 (15) | 0.489 |
| Corticosteroids | 4 (13) | 3 (15) | 1.000 |
| Allopurinol | 1 (3) | 1 (5) | 1.000 |
| Baseline FR-QoL-29 score, mean (SD) | 63.2 (12.3) | 69.7 (10.5) | 0.060 |
| Questionnaire | Score at End of Trial | Change in Score from Baseline to End | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | p-Value | Intervention | Control | p-Value | |
| Intention to Treat | (n = 30) | (n = 20) | (n = 30) | (n = 20) | ||
| FR-QoL (FR-QoL-29) | 75.0 (24.3) | 71.1 (19.0) | 0.552 | +11.7 (18.2) | +1.4 (20.4) | 0.067 |
| IBD HRQoL (IBD-Q) | 77.5 (13.1) | 78.4 (11.3) | 0.821 | +7.6 (10.0) | +9.8 (19.3) | 0.607 |
| IBD Distress (IBD-DS) | 91.1 (35.4) | 94.5 (29.9) | 0.727 | −6.8 (26.6) | +8.3 (25.5) | 0.052 |
| HADS | 13.4 (7.9) | 12.9 (4.7) | 0.779 | −3.8 (5.7) | −3.8 (7.9) | 0.986 |
| IBD-Control | 73.2 (33.2) | 69.7 (24.5) | 0.559 | +8.2 (26.6) | +5.6 (34.4) | 0.761 |
| CD activity (PRO-2) | 3.1 (2.8) | 5.2 (1.3) | 0.046 | +0.2 (3.6) | +1.2 (3.1) | 0.607 |
| UC activity (Partial Mayo) | 1.3 (1.3) | 1.0 (1.0) | 0.475 | −0.4 (1.3) | −0.9 (1.8) | 0.301 |
| Per protocol | ||||||
| FR-QoL (FR-QoL-29) | 75.2 (24.7) | 71.6 (19.4) | 0.589 | +12.1 (18.4) | +1.5 (21.0) | 0.069 |
| IBD HRQoL (IBD-Q) | 77.0 (13.2), [n = 27] | 76.9 (10.7) [n = 18] | 0.969 | +8.4 (10.2) [n = 27] | +10.8 (20.0) [n = 18] | 0.601 |
| IBD Distress (IBD-DS) | 91.9 (36.8), [n = 27] | 99.8 (26.3) [n = 18] | 0.435 | −7.5 (28.0) [n = 27] | +9.2 (26.7) [n = 18] | 0.053 |
| HADS | 13.1 (7.8), [n = 29] | 12.8 (4.8) [n = 19] | 0.864 | −4.0 (5.8) [n = 29] | −4.0 (8.1) [n = 19] | 0.986 |
| IBD-Control | 71.6 (33.2) | 69.5 (25.2) | 0.819 | +9.1 (27.9) | +6.2 (36.4) | 0.760 |
| CD activity (PRO-2) | 3.1 (2.8) | 5.5 (1.3) | 0.034 | +0.1 (2.5) | +0.3 (1.6) | 0.738 |
| UC activity (Partial Mayo) | 1.4 (1.3) | 1.1 (1.0) | 0.431 | −0.2 (1.0) | −0.8 (1.7) | 0.224 |
| Topics | Number of Patients Accessing (% of Total) (n = 25) |
|---|---|
| 1.1 What is IBD? | 7 (28) |
| 2.1 An introduction to diet and IBD | 3 (12) |
| 2.2 What is a healthy diet in IBD? | 5 (20) |
| 2.3 Can altering my diet help reduce IBD activity? | 6 (24) |
| 2.4 Eating during an IBD flare | 5 (20) |
| 2.5 Should I exclude foods from my diet? | 8 (32) |
| 2.6 Can I eat fruits and vegetables? | 5 (20) |
| 2.7 What types of fibre should I eat? | 5 (20) |
| 3.1 How can a liquid diet help with Crohn’s disease? | 4 (16) |
| 3.2 Practical advice for following a liquid diet | 0 (0) |
| 3.3 Should I take probiotics? | 3 (12) |
| 3.4 Should I follow a specific diet? | 2 (8) |
| 3.5 Why are iron, calcium and minerals important in IBD? | 2 (8) |
| 3.6 Which foods contain the vitamins and minerals I need? | 3 (12) |
| 4.1 Can changing my diet help manage symptoms? (overview) | 6 (24) |
| 4.2 Dietary management of gut symptoms | 11 (44) |
| 4.3 Identifying foods that trigger symptoms | 6 (24) |
| 5.1 Cooking for your family | 5 (20) |
| 5.2 Meal planning | 4 (16) |
| 5.3 Eating out and practical advice | 5 (20) |
| 5.4 Can I drink alcohol? | 7 (28) |
| 5.5 Guide for family and friends cooking for patients | 4 (16) |
| All Participants | Guy’s and St Thomas’ NHS Foundation Trust | Barts Health NHS Trust | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recruitment Method | Screened | Randomised (% Screened) | All End of Trial Data Available * | Screened | Randomised (% Screened) | All End of Trial Data Available * | Screened | Randomised (% Screened) | All End of Trial Data Available * |
| Untargeted clinic recruitment | 40 | 26 (65%) | 24/26 (92%) | 12 | 6 (50%) | 6/6 (100%) | 28 | 20 (71%) | 18/20 (90%) |
| Targeted clinic recruitment | 33 | 21 (64%) | 18/21 (86%) | 24 | 16 (67%) | 13/16 (81%) | 9 | 5 (56%) | 5/5 (100%) |
| Clinician referral | 1 | 0 (0%) | - | 1 | 0 (0%) | - | 0 | - | - |
| Letter and self-referral | 9 | 3 (33%) | 3/3 (100%) | 9 | 3 (33%) | 3/3 (100%) | 0 | - | - |
| Advertising and self-referral | 0 | 0 (0) | 0 (0) | 0 | 0 (0) | 0 (0) | 0 | 0 (0) | 0 (0) |
| TOTAL | 83 | 50 (60%) 3.3 per month | 45 (90%) | 46 | 25 (54%) 1.7 per month | 22/25 (88%) | 37 | 25 (68%) 2.1 per month | 23/25 (92%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, S.R.; Czuber-Dochan, W.; Wall, C.L.; Clarke, H.; Drysdale, C.; Lomer, M.C.; Lindsay, J.O.; Whelan, K. Improving Food-Related Quality of Life in Inflammatory Bowel Disease through a Novel Web Resource: A Feasibility Randomised Controlled Trial. Nutrients 2022, 14, 4292. https://doi.org/10.3390/nu14204292
Cox SR, Czuber-Dochan W, Wall CL, Clarke H, Drysdale C, Lomer MC, Lindsay JO, Whelan K. Improving Food-Related Quality of Life in Inflammatory Bowel Disease through a Novel Web Resource: A Feasibility Randomised Controlled Trial. Nutrients. 2022; 14(20):4292. https://doi.org/10.3390/nu14204292
Chicago/Turabian StyleCox, Selina R., Wladyslawa Czuber-Dochan, Catherine L. Wall, Hazel Clarke, Candice Drysdale, Miranda C. Lomer, James O. Lindsay, and Kevin Whelan. 2022. "Improving Food-Related Quality of Life in Inflammatory Bowel Disease through a Novel Web Resource: A Feasibility Randomised Controlled Trial" Nutrients 14, no. 20: 4292. https://doi.org/10.3390/nu14204292
APA StyleCox, S. R., Czuber-Dochan, W., Wall, C. L., Clarke, H., Drysdale, C., Lomer, M. C., Lindsay, J. O., & Whelan, K. (2022). Improving Food-Related Quality of Life in Inflammatory Bowel Disease through a Novel Web Resource: A Feasibility Randomised Controlled Trial. Nutrients, 14(20), 4292. https://doi.org/10.3390/nu14204292





