Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Energy Consumption, Weight, Fasting Glucose, Insulin Sensitivity and Insulin Resistance
2.3. Biomarkers in Serum
2.4. Lipid Peroxidation Assessment
2.5. Histological Analysis of Liver
2.6. Immunohistochemistry
2.7. microRNAs Expression
2.8. Analysis of Gene Expression by Real-Time PCR
2.9. Western Blot Analysis
2.10. Transcriptome Analysis
2.11. Statistical Analysis
3. Results
3.1. Moringa Extract Showed Antioxidant Capacity
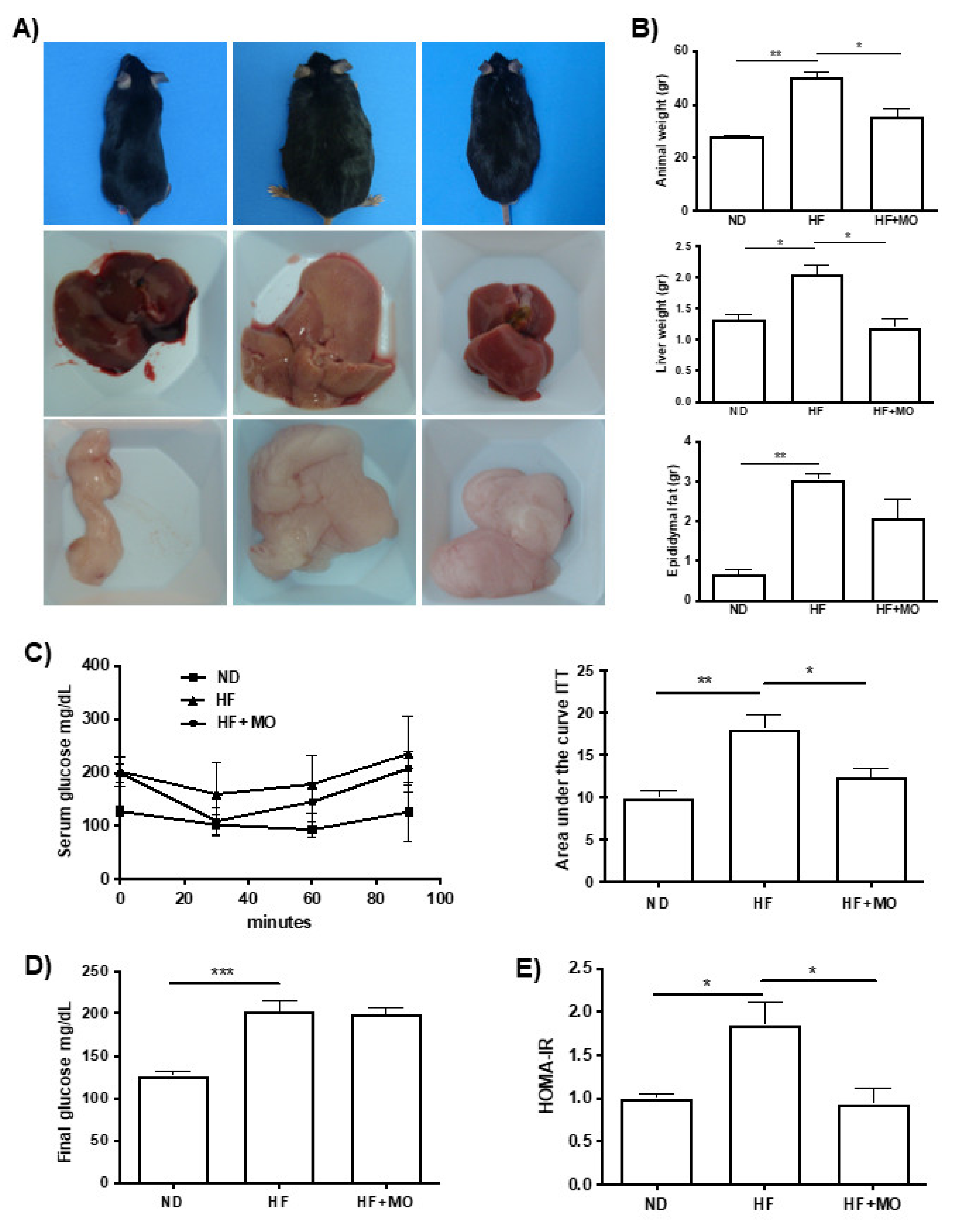
3.2. Moringa Diminished Animal, Liver and Epididymal Fat Weight
3.3. Moringa Reduced Daily Food Intake
3.4. Moringa Preserves Insulin Sensitivity in MAFLD/NASH Animals
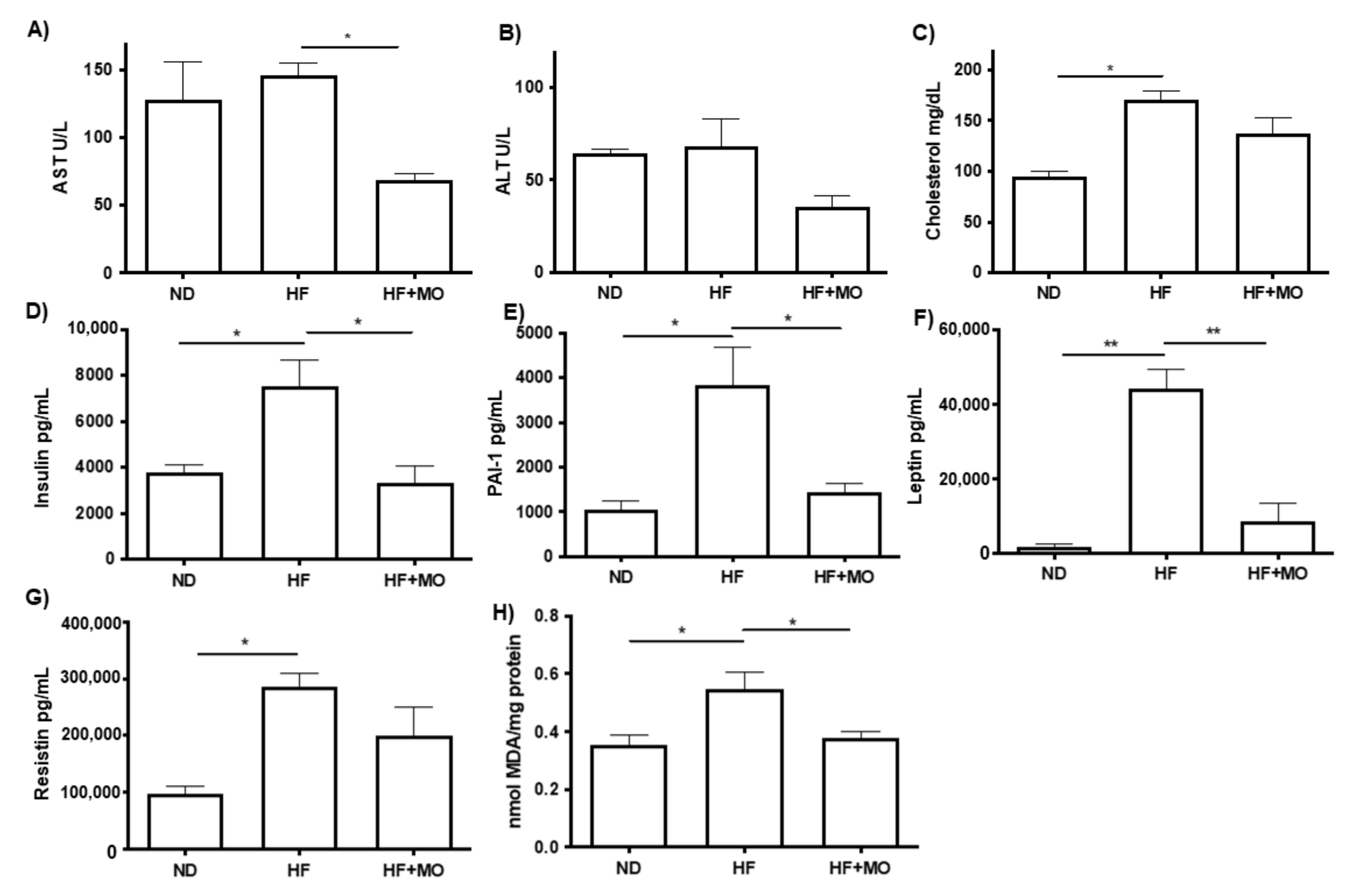
3.5. Moringa Improves Biochemical Hepatic Test and Adipokines Serum Levels
3.6. Moringa Reduced Lipid Peroxidation
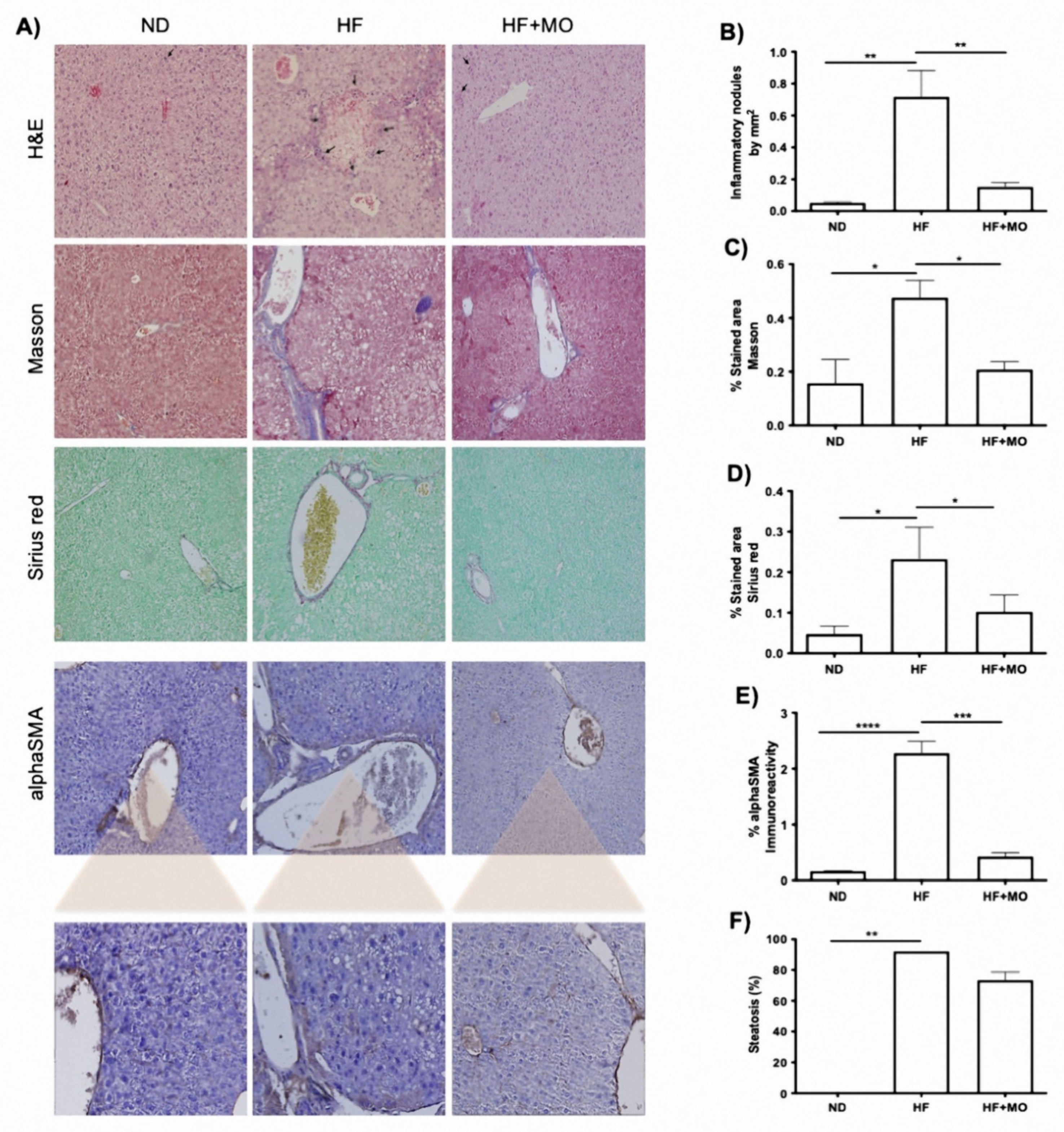
3.7. Liver Histology Improved after Moringa Administration
3.8. Moringa Modifies Hepatic miRNAs Expression
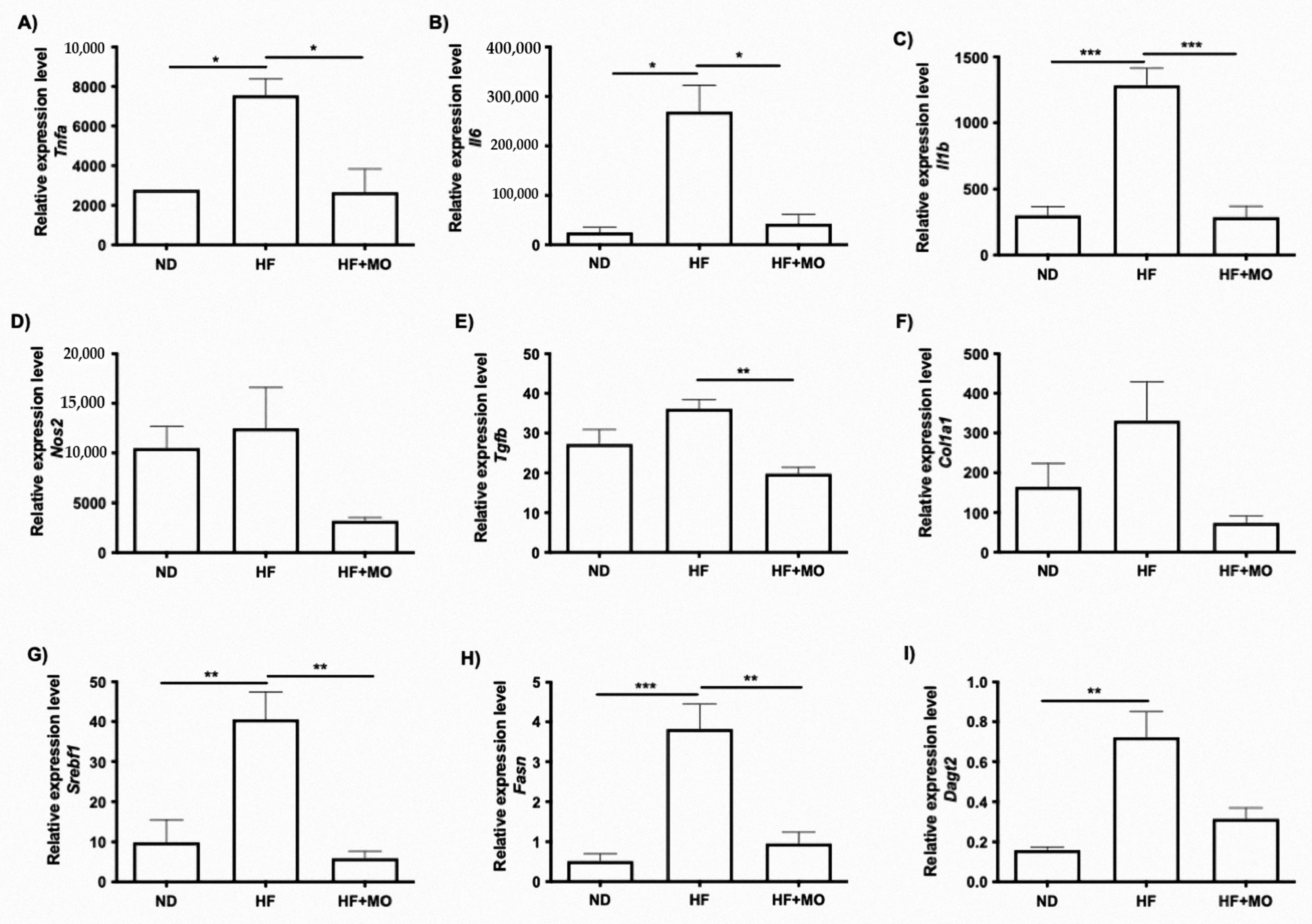
3.9. Moringa Extract Reduces Expression of Inflammation-Related Genes
3.10. Moringa Extract Reduces Fibrosis-Related Genes Expression
3.11. Moringa Extract Reduces Expression of Lipogenic-Related Genes
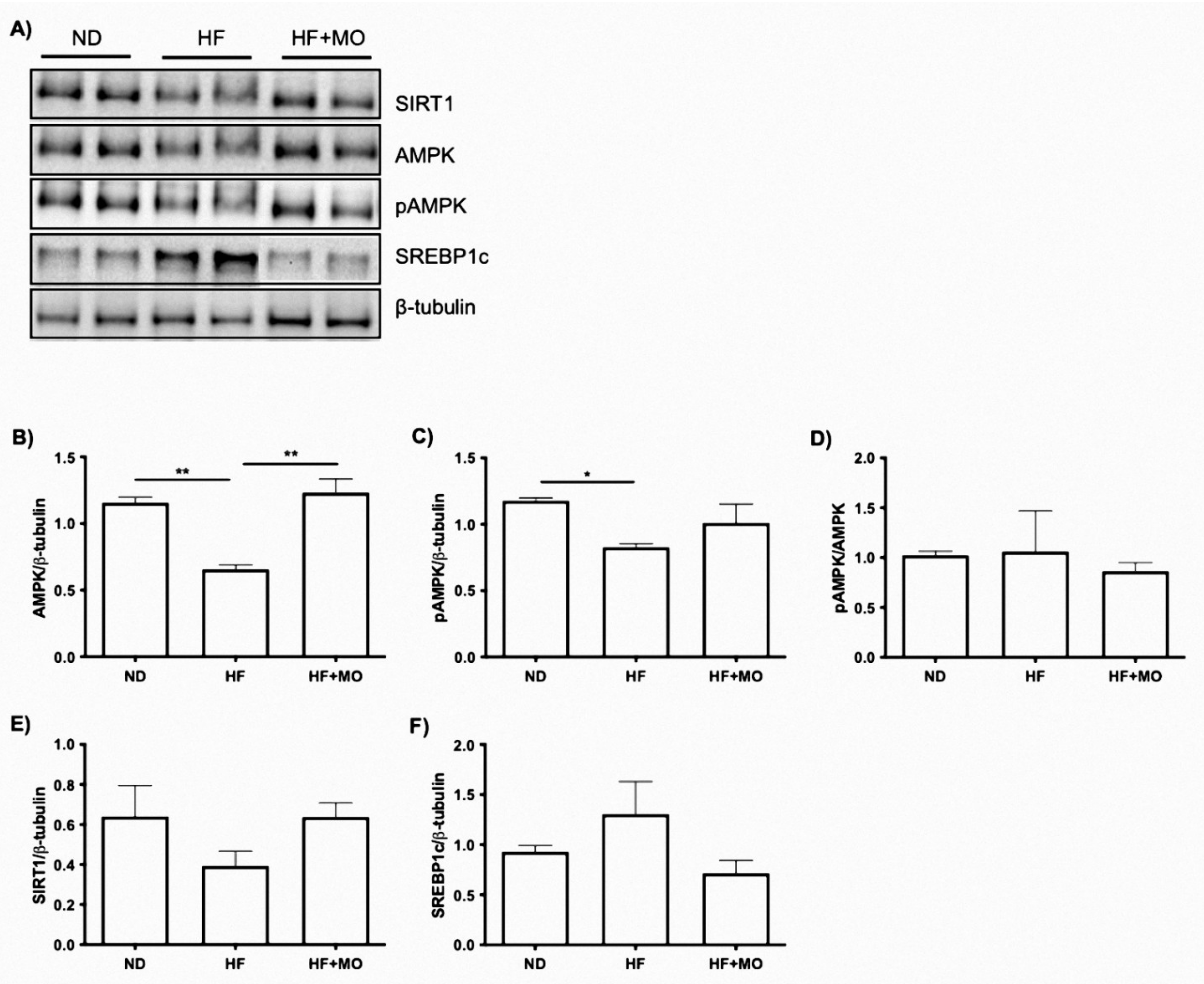
3.12. Effect of Moringa Extract on The SIRT1/AMPKα/SREBP1C/FAS Signaling Axis and Protein
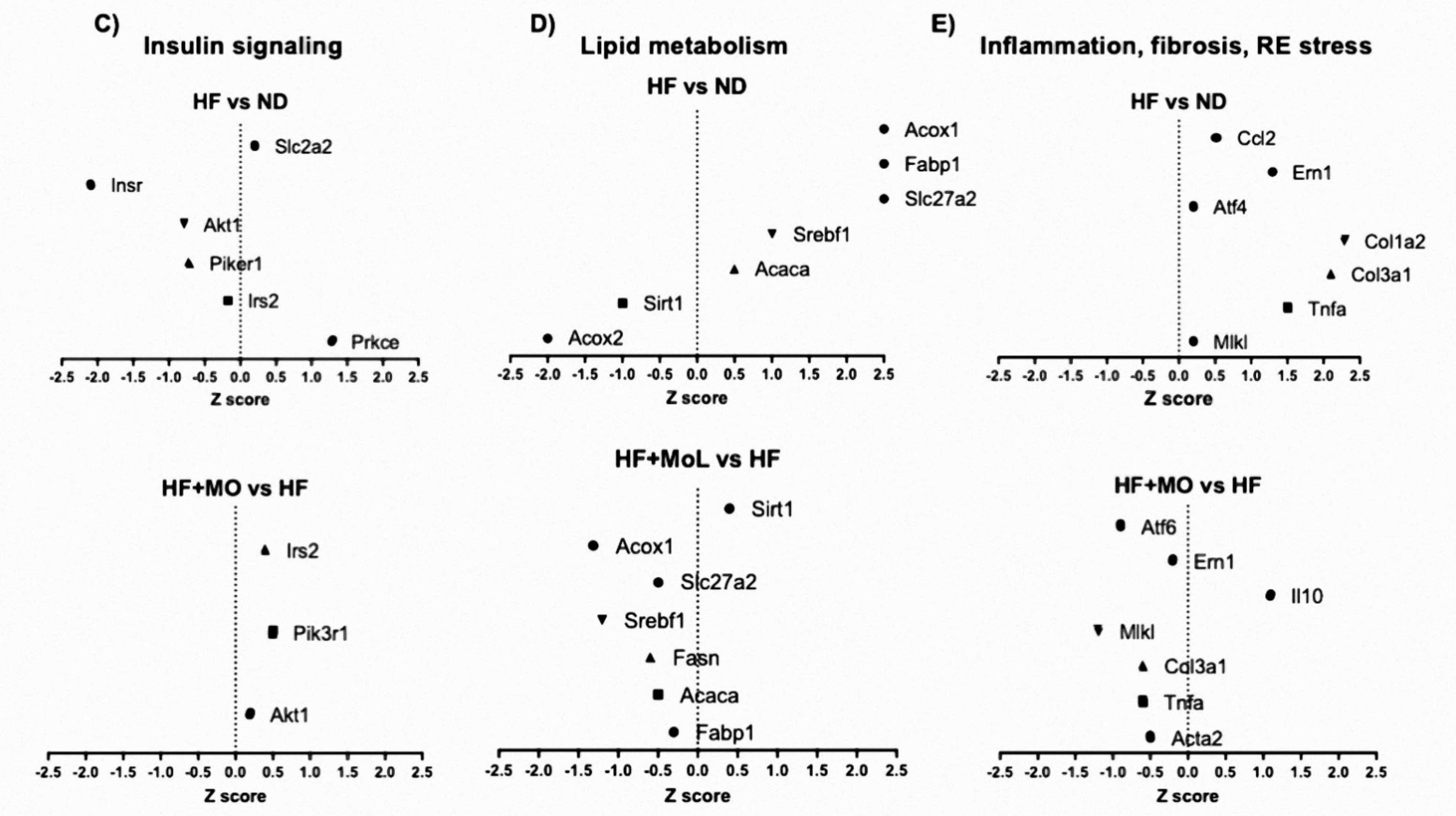
3.13. Effect of Moringa Extract on Liver Transcriptome in A MAFLD Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tilg, H.; Effenberger, M. From NAFLD to MAFLD: When pathophysiology succeeds. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2015, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.F.; Cogliati, B.; Otton, R. Green Tea Prevents NAFLD by Modulation of miR-34a and miR-194 Expression in a High-Fat Diet Mouse Model. Oxid. Med. Cell. Longev. 2019, 2019, 4168380. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.A.; Beasley, J.M.; Aebersold, K.; Jhangiani, S.S.; Wylie-Rosett, J. Nutritional Management of Insulin Resistance in Nonalcoholic Fatty Liver Disease (NAFLD). Nutrients 2013, 5, 4093–4114. [Google Scholar] [CrossRef]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothi-ocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef]
- Feustel, S.; Ayón-Pérez, F.; Sandoval-Rodriguez, A.; Rodríguez-Echevarría, R.; Contreras-Salinas, H.; Armendáriz-Borunda, J.; Sánchez-Orozco, L.V. Protective Effects of Moringa oleifera on HBV Genotypes C and H Transiently Transfected Huh7 Cells. J. Immunol. Res. 2017, 2017, 6063850. [Google Scholar] [CrossRef]
- Khan, W.; Parveen, R.; Chester, K.; Parveen, S.; Ahmad, S. Hypoglycemic Potential of Aqueous Extract of Moringa oleifera Leaf and In Vivo GC-MS Metabolomics. Front. Pharmacol. 2017, 8, 577. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Ahmed, M.A.; El Sayed, R.A. Molecular effects of Moringa leaf extract on insulin resistance and reproductive function in hyperinsulinemic male rats. J. Diabetes Metab. Disord. 2019, 18, 487–494. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Jiang, W.-W.; Luo, X.-F.; Dai, T.-Y.; Peng, L.; Song, S.; Li, L.-F.; Tao, L.; Shi, C.-Y.; et al. Moringa oleifera Leaf Petroleum Ether Extract Inhibits Lipogenesis by Activating the AMPK Signaling Pathway. Front. Pharmacol. 2018, 9, 1447. [Google Scholar] [CrossRef]
- Bao, Y.; Xiao, J.; Weng, Z.; Lu, X.; Shen, X.; Wang, F. A phenolic glycoside from Moringa oleifera Lam. improves the carbohydrate and lipid metabolisms through AMPK in db/db mice. Food Chem. 2019, 311, 125948. [Google Scholar] [CrossRef]
- Schueller, F.; Roy, S.; Vucur, M.; Trautwein, C.; Luedde, T.; Roderburg, C. The Role of miRNAs in the Pathophysiology of Liver Diseases and Toxicity. Int. J. Mol. Sci. 2018, 19, 261. [Google Scholar] [CrossRef]
- Szabo, G.; Bala, S. MicroRNAs in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 542–552. [Google Scholar] [CrossRef]
- Pirola, C.J.; Gianotti, T.F.; Castano, G.O.; Mallardi, P.; San Martino, J.; Ledesma, M.M.G.L.; Flichman, D.; Mirshahi, F.; Sanyal, A.J.; Sookoian, S. Circulating microRNA signature in non-alcoholic fatty liver disease: From serum non-coding RNAs to liver histology and disease pathogenesis. Gut 2015, 64, 800–812. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Jung, C.H.; Ha, T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol. Nutr. Food Res. 2012, 56, 1665–1674. [Google Scholar] [CrossRef]
- Sun, C.; Huang, F.; Liu, X.; Xiao, X.; Yang, M.; Hu, G.; Liu, H.; Liao, L. miR-21 regulates triglyceride and cholesterol metabolism in non-alcoholic fatty liver disease by targeting HMGCR. Int. J. Mol. Med. 2015, 35, 847–853. [Google Scholar] [CrossRef]
- Xu, Y.; Zalzala, M.; Xu, J.; Li, Y.; Yin, L.; Zhang, Y. A metabolic stress-inducible miR-34a-HNF4α pathway regulates lipid and lipoprotein metabolism. Nat. Commun. 2015, 6, 7466. [Google Scholar] [CrossRef]
- Joven, J.; Espinel, E.; Rull, A.; Aragonès, G.; Rodríguez-Gallego, E.; Camps, J.; Micol, V.; Herranz-López, M.; Menéndez, J.A.; Borrás, I.; et al. Plant-derived polyphenols regulate expression of miRNA paralogs miR-103/107 and miR-122 and prevent diet-induced fatty liver disease in hyperlipidemic mice. Biochim. Biophys. Acta 2012, 1820, 894–899. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Arola-Arnal, A.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvadó, M.J.; Arola, L.; Blade, C. Chronic admin-istration of proanthocyanidins or docosahexaenoic acid reverses the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS ONE 2013, 8, e69817. [Google Scholar] [CrossRef]
- Gracia, A.; Fernández-Quintela, A.; Miranda, J.; Eseberri, I.; González, M.; Portillo, M.P. Are miRNA-103, miRNA-107 and miRNA-122 Involved in the Prevention of Liver Steatosis Induced by Resveratrol? Nutrients 2017, 9, 360. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanarin. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.I.L.; Moorillón, G.V.N.; Aguilar, C.N. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac. J. Trop. Med. 2015, 8, 104–111. [Google Scholar] [CrossRef]
- Sandoval-Rodriguez, A.; Monroy-Ramirez, H.C.; Meza-Rios, A.; Garcia-Bañuelos, J.; Vera-Cruz, J.; Gutiérrez-Cuevas, J.; Silva-Gomez, J.; Staels, B.; Dominguez-Rosales, J.; Galicia-Moreno, M.; et al. Pirfenidone is an Agonistic Ligand for PPARα and Improves NASH by Activation of SIRT1/LKB1/pAMPK. Hepatol. Commun. 2020, 4, 434–449. [Google Scholar] [CrossRef]
- Kohli, R.; Kirby, M.; Xanthakos, S.A.; Softic, S.; Feldstein, A.E.; Saxena, V.; Tang, P.H.; Miles, L.; Miles, M.V.; Balistreri, W.F.; et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010, 52, 934–944. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J. Lipid Res. 1978, 19, 1053–1057. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Rosique-Oramas, D.; Medina-Avila, Z.; Álvarez-Torres, T.; Falcón, D.; La Tijera, F.H.-D.; Béjar, Y.L.; Cordero-Pérez, P.; Muñoz-Espinosa, L.; Pérez-Hernández, J.L.; et al. Behavior of Oxidative Stress Markers in Alcoholic Liver Cirrhosis Patients. Oxid. Med. Cell. Longev. 2016, 2016, 9370565. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Sadek, K.M.; Abouzed, T.K.; Abouelkhair, R.; Nasr, S. The chemo-prophylactic efficacy of an ethanol Moringa oleifera leaf extract against hepatocellular carcinoma in rats. Pharm. Biol. 2017, 55, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Almatrafi, M.M.; Vergara-Jimenez, M.; Murillo, A.G.; Norris, G.H.; Blesso, C.N.; Fernandez, M.L. Moringa Leaves Prevent Hepatic Lipid Accumulation and Inflammation in Guinea Pigs by Reducing the Expression of Genes Involved in Lipid Metabolism. Int. J. Mol. Sci. 2017, 18, 1330. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Ajmera, V.; Perito, E.R.; Bass, N.M.; Terrault, N.A.; Yates, K.P.; Gill, R.; Loomba, R.; Diehl, A.M.; Aouizerat, B.E.; for the NASH Clinical Research Network. Novel plasma biomarkers associated with liver disease severity in adults with nonalcoholic fatty liver disease. Hepatology 2017, 65, 65–77. [Google Scholar] [CrossRef]
- Hossain, I.A.; Akter, S.; Rahman, M.K.; Ali, L. Gender Specific Association of Serum Leptin and Insulinemic Indices with Nonalcoholic Fatty Liver Disease in Prediabetic Subjects. PLoS ONE 2015, 10, e0142165. [Google Scholar] [CrossRef]
- Jamali, R.; Razavizade, M.; Arj, A.; Aarabi, M.H. Serum adipokines might predict liver histology findings in non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 5096–5103. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Eccleston, H.B.; Bailey, S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008, 44, 1259–1272. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.-J. Preventive effects of dietary walnuts on high-fat-induced hepatic fat accumulation, oxidative stress and apoptosis in mice. J. Nutr. Biochem. 2016, 38, 70–80. [Google Scholar] [CrossRef]
- Das, N.; Sikder, K.; Ghosh, S.; Fromenty, B.; Dey, S. Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian J. Exp. Biol. 2012, 50. [Google Scholar]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proin-flammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef]
- Hamza, A.A. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010, 48, 345–355. [Google Scholar] [CrossRef]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta 2007, 381, 107–113. [Google Scholar] [CrossRef]
- Li, X.; Jin, Q.; Yao, Q.; Xu, B.; Li, L.; Zhang, S.; Tu, C. The Flavonoid Quercetin Ameliorates Liver Inflammation and Fibrosis by Regulating Hepatic Macrophages Activation and Polarization in Mice. Front. Pharmacol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2013, 25, 138–145. [Google Scholar] [CrossRef]
- Ponugoti, B.; Kim, D.-H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.-Y.; Chiang, C.-M.; Veenstra, T.D.; Kemper, J.K. SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism. J. Biol. Chem. 2010, 285, 33959–33970. [Google Scholar] [CrossRef]
- Ding, R.-B.; Bao, J.; Deng, C.-X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- He, Z.; Hu, C.; Jia, W. miRNAs in non-alcoholic fatty liver disease. Front. Med. 2016, 10, 389–396. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Drosatos, K.; Hiyama, Y.; Goldberg, I.J.; Zannis, V.I. MicroRNA-370 controls the expression of MicroRNA-122 and Cpt1α and affects lipid metabolism. J. Lipid Res. 2010, 51, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, G.M.; Al-Qahtani, W.H.; AlFaris, N.A.; Alzahrani, N.S.; Alkhateeb, M.A.; Yahya, M.A. Quercetin prevents cadmium chlo-ride-induced hepatic steatosis and fibrosis by downregulating the transcription of miR-21. Biofactors 2021, 47, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Dattaroy, D.; Pourhoseini, S.; Das, S.; Alhasson, F.; Seth, R.K.; Nagarkatti, M.; Michelotti, G.A.; Diehl, A.M.; Chatterjee, S. Micro-RNA 21 in-hibition of SMAD7 enhances fibrogenesis via leptin-mediated NADPH oxidase in experimental and human nonalcoholic steato-hepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G298–G312. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ng, R.; Chen, X.; Steer, C.J.; Song, G. MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut 2015, 65, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yan, Y.; Xv, W.; Qian, G.; Li, C.; Zou, H.; Li, Y. A New Insight into the Roles of MiRNAs in Metabolic Syndrome. BioMed Res. Int. 2018, 2018, 7372636. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Escutia-Gutiérrez, R.; Rodríguez-Sanabria, J.S.; Monraz-Méndez, C.A.; García-Bañuelos, J.; Santos-García, A.; Sandoval-Rodríguez, A.; Armendáriz-Borunda, J. Pirfenidone modifies hepatic miRNAs expression in a model of MAFLD/NASH. Sci. Rep. 2021, 11, 11709. [Google Scholar] [CrossRef]


| ND | HF | HF + MO | ||
|---|---|---|---|---|
| Prior treatment | ||||
| Daily energy intake | Kcal | 10.4 ± 1.42 | 13.5 ± 1.26 +++ | 14.2 ± 1.26 ++++ |
| Daily food intake | g | 3.35 ± 0.47 | 2.49 ± 0.26 ++++ | 2.6 ± 0.25 +++ |
| Diet fat percentage | % | 18 | 60 | 60 |
| Daily fat intake | Kcal | 1.80 ± 0.26 | 7.7 ± 0.8 ++++ | 8.03 ± 0.79 ++++ |
| During treatment | ||||
| Daily energy intake | Kcal | 10.2 ± 1.11 | 13.7 ± 1.18 | 11.5 ± 1.52 ** |
| Daily food intake | g | 3.28 ± 0.35 | 2.54 ± 0.21 | 2.1 ± 0.26 ** |
| Diet fat percentage | % | 18 | 60 | 60 |
| Daily fat intake | Kcal | 1.84 ± 0.20 | 7.7 ± 0.7 | 6.47 ± 0.79 * |
| Moringa Dose | mg/kg | - | - | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monraz-Méndez, C.A.; Escutia-Gutiérrez, R.; Rodriguez-Sanabria, J.S.; Galicia-Moreno, M.; Monroy-Ramírez, H.C.; Sánchez-Orozco, L.; García-Bañuelos, J.; De la Rosa-Bibiano, R.; Santos, A.; Armendáriz-Borunda, J.; et al. Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications. Nutrients 2022, 14, 4225. https://doi.org/10.3390/nu14204225
Monraz-Méndez CA, Escutia-Gutiérrez R, Rodriguez-Sanabria JS, Galicia-Moreno M, Monroy-Ramírez HC, Sánchez-Orozco L, García-Bañuelos J, De la Rosa-Bibiano R, Santos A, Armendáriz-Borunda J, et al. Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications. Nutrients. 2022; 14(20):4225. https://doi.org/10.3390/nu14204225
Chicago/Turabian StyleMonraz-Méndez, C. Alejandra, Rebeca Escutia-Gutiérrez, Jonathan Samael Rodriguez-Sanabria, Marina Galicia-Moreno, Hugo Christian Monroy-Ramírez, Laura Sánchez-Orozco, Jesus García-Bañuelos, Ricardo De la Rosa-Bibiano, Arturo Santos, Juan Armendáriz-Borunda, and et al. 2022. "Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications" Nutrients 14, no. 20: 4225. https://doi.org/10.3390/nu14204225
APA StyleMonraz-Méndez, C. A., Escutia-Gutiérrez, R., Rodriguez-Sanabria, J. S., Galicia-Moreno, M., Monroy-Ramírez, H. C., Sánchez-Orozco, L., García-Bañuelos, J., De la Rosa-Bibiano, R., Santos, A., Armendáriz-Borunda, J., & Sandoval-Rodríguez, A. (2022). Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications. Nutrients, 14(20), 4225. https://doi.org/10.3390/nu14204225







