Dietary Hempseed Decreases Femur Maximum Load in a Young Female C57BL/6 Mouse Model but Does Not Influence Bone Mineral Density or Micro-Architecture
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Diet, and Animals
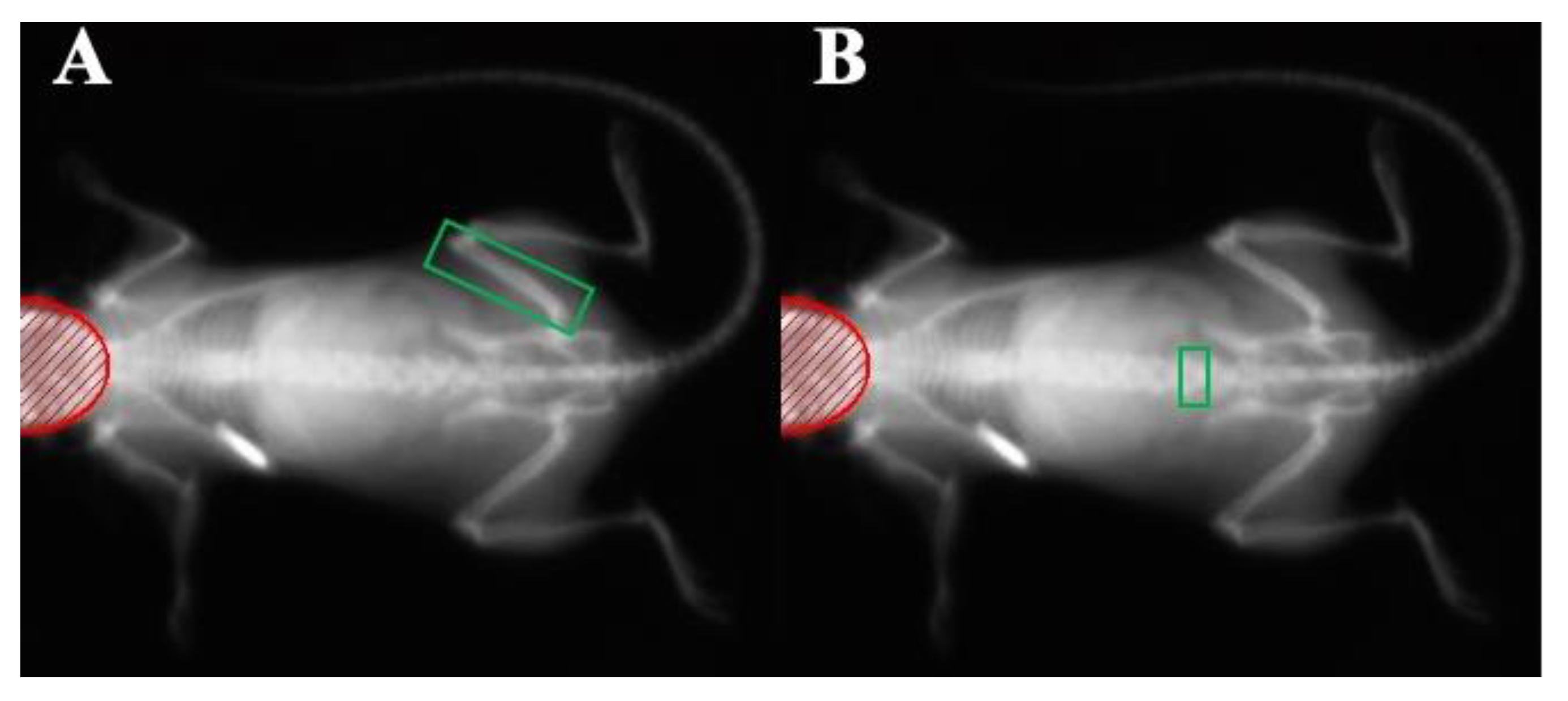
2.2. In Vivo DXA Scanning of the Femur and L5 Bone Mineralization
2.3. Ex Vivo Micro-Computed Tomography Evaluation of the Femur and L5 Micro-Architecture
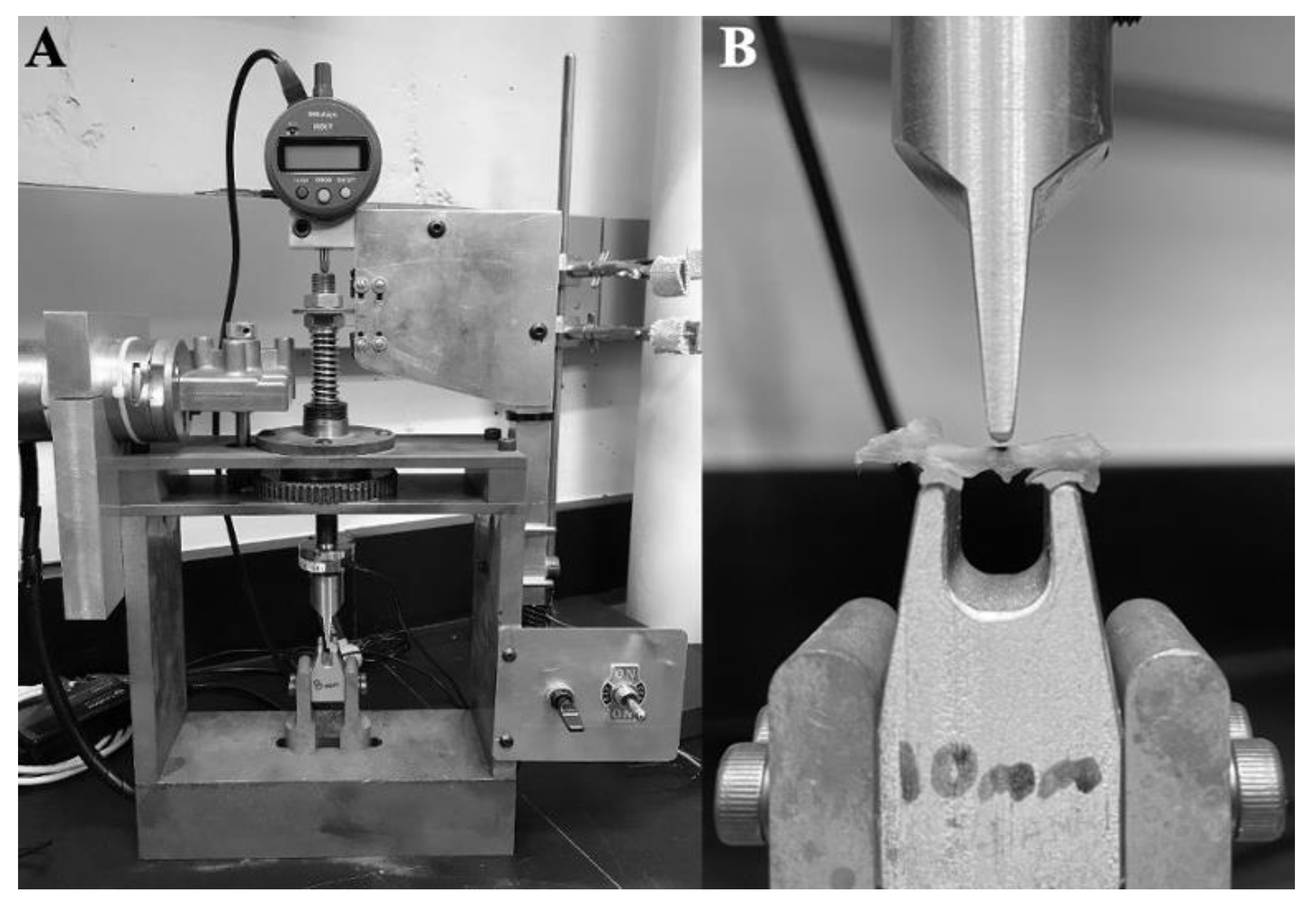
2.4. Three-Point Bending of the Femur
2.5. Analysis of Load-Displacement Curves
2.6. Statistical Analysis
3. Results
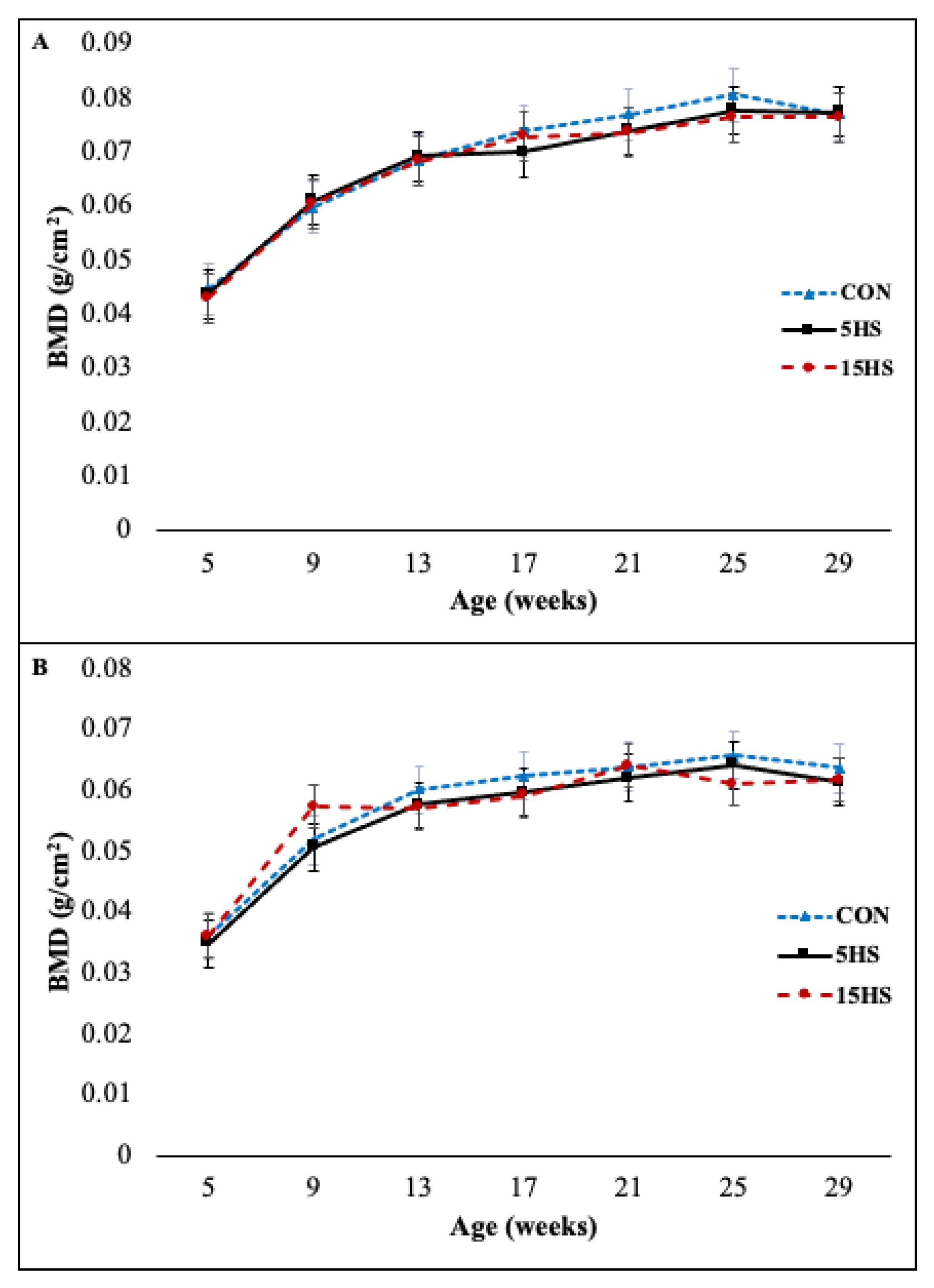
3.1. Right Femur and L5 BMD
3.2. Cortical and Trabecular Micro-Architecture
3.3. Femur Biomechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilezikian, J.P.; Raisz, L.G.; Rodan, G.A. Principles of Bone Biology; Academic Press: San Diego, CA, USA, 2002; Volume 2. [Google Scholar]
- Emami, A.J.; Toupadakis, C.A.; Telek, S.M.; Fyhrie, D.P.; Yellowley, C.E.; Christiansen, B.A. Age Dependence of Systemic Bone Loss and Recovery Following Femur Fracture in Mice. J. Bone Miner. Res. 2019, 34, 157–170. [Google Scholar] [CrossRef]
- Kemmak, A.R.; Reazpour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med J. Islam. Repub. Iran 2020, 34, 1054–1061. [Google Scholar] [CrossRef]
- Tucker, K.L.; Chen, H.; Hannan, M.T.; Cupples, L.A.; Wilson, P.W.; Felson, D.; Kiel, D.P. Bone mineral density and dietary patterns in older adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2002, 76, 245–252. [Google Scholar] [CrossRef]
- Tenkumo, T.; Aobulikasimu, A.; Asou, Y.; Shirato, M.; Shishido, S.; Kanno, T.; Niwano, Y.; Sasaki, K.; Nakamura, K. Proanthocyanidin-rich grape seed extract improves bone loss, bone healing, and implant osseointegration in ovariectomized animals. Sci. Rep. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Spilmont, M.; Léotoing, L.; Davicco, M.-J.; Lebecque, P.; Mercier, S.; Miot-Noirault, E.; Pilet, P.; Rios, L.; Wittrant, Y.; Coxam, V. Pomegranate seed oil prevents bone loss in a mice model of osteoporosis, through osteoblastic stimulation, osteoclastic inhibition and decreased inflammatory status. J. Nutr. Biochem. 2013, 24, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Ren, Y.; Qiao, Z.; Stevenson, D.; Bartlett, M. Protective Effects of Curcumin and Broccoli Seed Extract Against Age-Related Bone Loss in Mice. Curr. Dev. Nutr. 2020, 4, 46. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Wang, H.; Dong, Z.; Zhang, M.; Li, J.; Shao, S.; Yu, C.; Huan, Z.; Xu, J. Flaxseed oil ameliorated high-fat-diet-induced bone loss in rats by promoting osteoblastic function in rat primary osteoblasts. Nutr. Metab. 2019, 16, 71. [Google Scholar] [CrossRef]
- Chañi, E.M.M.; Pacheco, S.O.S.; Martínez, G.A.; Freitas, M.R.; Ivona, J.G.; Ivona, J.A.; Craig, W.J.; Pacheco, F.J. Long-Term Dietary Intake of Chia Seed Is Associated with Increased Bone Mineral Content and Improved Hepatic and Intestinal Morphology in Sprague-Dawley Rats. Nutrients 2018, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, K.; Bhat, K.M.; Srinivasa, S.R.; Devkar, R.A.; Henry, T. Beneficial Role of Hydro-alcoholic Seed Extract of Trigonella foenum graecum on Bone Structure and Strength in Menopause Induced Osteopenia. Ethiop. J. Health Sci. 2018, 28, 787. [Google Scholar] [CrossRef]
- Kim, H.; Bae, Y.; Park, R.; Choi, S.; Cho, S.; Choi, Y.; Lee, W. Bone-Protecting Effect of Safflower Seeds in Ovariectomized Rats. Calcif. Tissue Res. 2002, 71, 88–94. [Google Scholar] [CrossRef]
- Puzio, I.; Jaśkiewicz, T.; Sagan, A.; Bieńko, M.; Graboś, D. Effects of CLA and Camelina Sativa Seed Oil on Bone Propertoes in Broiler Chickens. Bull. Veter. Inst. Pulawy 2012, 56, 93–97. [Google Scholar] [CrossRef]
- Insights, F.B. Hempseeds Market Size, Share & COVID-19 Impact Analysis, by Form (Whole Hemp Seed, Hulled Hemp Seed, Hempseed Oil, and Hemp Protein Powder), Application (Food & Beverage, Personal Care Products, Industrial Products, and Others), and Regional Forecast, 2020–2027, Fortune Business Insights Market Research Report, 2020, Report ID: FBI103478. Available online: https://www.fortunebusinessinsights.com/hemp-seeds-market-103478 (accessed on 17 September 2022).
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2019, 19, 282–308. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leyva, D.; Pierce, G.N. The cardiac and haemostatic effects of dietary hempseed. Nutr. Metab. 2010, 7, 32. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.; Beyer, M.; Brühl, L.; Matthäus, B. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Boscoboinik, D.; Szewczyk, A.; Hensey, C.; Azzi, A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J. Biol. Chem. 1991, 266, 6188–6194. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Variability in Antinutritional compounds in Hempseed meal of Italian and French varieties. Plant 2013, 1, 25–29. [Google Scholar] [CrossRef]
- Jang, E.; Kim, H.; Jang, S.; Lee, J.; Baeck, S.; In, S.; Kim, E.; Kim, Y.-U.; Han, E. Concentrations of THC, CBD, and CBN in commercial hemp seeds and hempseed oil sold in Korea. Forensic Sci. Int. 2019, 306, 110064. [Google Scholar] [CrossRef]
- Kogan, N.M.; Melamed, E.; Wasserman, E.; Raphael, B.; Breuer, A.; Stok, K.S.; Sondergaard, R.; Escudero, A.V.; Baraghithy, S.; Attar-Namdar, M.; et al. Cannabidiol, a Major Non-Psychotropic Cannabis Constituent Enhances Fracture Healing and Stimulates Lysyl Hydroxylase Activity in Osteoblasts. J. Bone Miner. Res. 2015, 30, 1905–1913. [Google Scholar] [CrossRef]
- Bab, I.; Zimmer, A.; Melamed, E. Cannabinoids and the skeleton: From marijuana to reversal of bone loss. Ann. Med. 2009, 41, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Bab, I.; Zimmer, A. Cannabinoid receptors and the regulation of bone mass. J. Cereb. Blood Flow Metab. 2008, 153, 182–188. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Vít, T.; Skřivanová, E. Hempseed increases gamma-tocopherol in egg yolks and the breaking strength of tibias in laying hens. PLoS ONE 2019, 14, e0217509. [Google Scholar] [CrossRef]
- Domander, R.; A Felder, A.; Doube, M. BoneJ2-refactoring established research software. Wellcome Open Res. 2021, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Sparks, C.A.; Giltner, Z.T.; Blanton, C.A.; Gabaldón, A.M. The Effects of a Synbiotic Diet on Humerus Bone Mineralization and Mechanical Strength in Aging Male Mice. El Río 2022, 4, 3–12. [Google Scholar]
- Sarper, H.; Blanton, C.; DePalma, J.; Melnykov, I.V.; Gabaldón, A.M. Simulated weightlessness and synbiotic diet effects on rat bone mechanical strength. Life Sci. Space Res. 2014, 3, 45–54. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Ammann, P.; Rizzoli, R. Bone strength and its determinants. Osteoporos. Int. 2003, 14, 13–18. [Google Scholar] [CrossRef]
- Fleischer, H.; Vorberg, E.; Thurow, K.; Warkentin, M.; Behrend, D. Determination of Calcium and Phosphor in Bones Using Microwave Digestion and ICP-MS. Imeko Tc19 Symp. 2014, 5, 94–99. [Google Scholar]
- Blanton, C.A.; Barrott, J.J.; Kunz, K.; Bunde, E.; Streff, H.M.; Sparks, C.A.; Williams, D.W.; Gabaldόn, A.M. The Impact of Hempseed Consumption on Bone Parameters and Body Composition in Growing Female C57BL/6 Mice. Int. J. Environ. Res. Public Health 2022, 19, 5839. [Google Scholar] [CrossRef]
- Yamada, M.; Chen, C.; Sugiyama, T.; Kim, W. Effects of Age on Bone Structure Parameters in Laying Hens. Animals 2021, 11, 570. [Google Scholar] [CrossRef]
- Harrison, D.C.; Mellanby, E. Phytic Acid and the Rickets-Producing Action of Cereals. Biochem. J. 1939, 33, 1660–1680. [Google Scholar] [CrossRef]



| Ingredient | Control Diet a | 5% Hempseed Supplemented Diet | 15% Hempseed Supplemented Diet |
|---|---|---|---|
| Casein, High Nitrogen | 200 | 185 | 155 |
| L-Cystine | 3 | 3 | 3 |
| Sucrose b | 100 | 100 | 100 |
| Cornstarch | 397.486 | 395.99 | 392.997 |
| Dyetrose | 132 | 132 | 132 |
| Soybean Oil | 70 | 52 | 16 |
| t-Butylhydroquinone | 0.014 | 0.01 | 0.003 |
| Cellulose | 50 | 34.5 | 3.5 |
| Mineral Mix #210025 c | 35 | 35 | 35 |
| Vitamin Mix #310025 d | 10 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 |
| Hempseed e | 0 | 50 | 150 |
| Total | 1000 | 1000 | 1000 |
| Kilocalories per kg | 3760 | 3814 | 3922 |
| Parameter | Cursors | Procedure |
|---|---|---|
| Stiffness (N/mm) | C–D | Slope between two points |
| Yield Load (N) | D | Load at point |
| Post-yield Displacement (mm) | D–B | Displacement between two points |
| Maximum Load (N) | B | Load at point |
| Work-to-Fracture (N⋅mm) | A–B | Area underneath curve |
| Group | Ct.Th (mm) | Ct.Ar/Tt.Ar (%) | BV/TV (%) | Conn.D (mm−3) | Tb.Th (mm) | Tb.Sp (mm−1) |
|---|---|---|---|---|---|---|
| CON | 0.315 ± 0.0211 | 56.0 ± 1.23 | 35.4 ± 2.43 | 35.0 ± 6.89 | 0.102 ± 0.00644 | 0.167 ± 0.0209 |
| 5-HS | 0.329 ± 0.0278 | 55.4 ± 1.69 | 34.3 ± 1.63 | 40.3 ± 9.87 | 0.096 ± 0.00563 | 0.189 ± 0.0203 |
| 15-HS | 0.345 ± 0.0288 | 56.6 ± 1.68 | 35.7 ± 1.99 | 40.0 ± 8.36 | 0.098 ± 0.00355 | 0.180 ± 0.0137 |
| Group | Stiffness (N/mm) | Yield Load (N) | Post-Yield Displacement (mm) | Work-to-Fracture (N * mm) | Maximum Load (N) |
|---|---|---|---|---|---|
| CON | 67.40 ± 5.427 | 14.27 ± 0.934 | 0.1227 ± 0.02296 | 3.995 ± 0.4750 | 17.83 ± 0.8870 A |
| 5-HS | 68.35 ± 6.657 | 13.69 ± 1.343 | 0.1731 ± 0.05001 | 4.318 ± 1.116 | 17.21 ± 1.251 A,B |
| 15-HS | 54.85 ± 4.395 | 11.63 ± 1.054 | 0.1397 ± 0.02212 | 3.396 ± 0.4353 | 14.16 ± 0.5462 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparks, C.A.; Streff, H.M.; Williams, D.W.; Blanton, C.A.; Gabaldón, A.M. Dietary Hempseed Decreases Femur Maximum Load in a Young Female C57BL/6 Mouse Model but Does Not Influence Bone Mineral Density or Micro-Architecture. Nutrients 2022, 14, 4224. https://doi.org/10.3390/nu14204224
Sparks CA, Streff HM, Williams DW, Blanton CA, Gabaldón AM. Dietary Hempseed Decreases Femur Maximum Load in a Young Female C57BL/6 Mouse Model but Does Not Influence Bone Mineral Density or Micro-Architecture. Nutrients. 2022; 14(20):4224. https://doi.org/10.3390/nu14204224
Chicago/Turabian StyleSparks, Chandler A., Hailey M. Streff, Derrick W. Williams, Cynthia A. Blanton, and Annette M. Gabaldón. 2022. "Dietary Hempseed Decreases Femur Maximum Load in a Young Female C57BL/6 Mouse Model but Does Not Influence Bone Mineral Density or Micro-Architecture" Nutrients 14, no. 20: 4224. https://doi.org/10.3390/nu14204224
APA StyleSparks, C. A., Streff, H. M., Williams, D. W., Blanton, C. A., & Gabaldón, A. M. (2022). Dietary Hempseed Decreases Femur Maximum Load in a Young Female C57BL/6 Mouse Model but Does Not Influence Bone Mineral Density or Micro-Architecture. Nutrients, 14(20), 4224. https://doi.org/10.3390/nu14204224






