A. muciniphila Supplementation in Mice during Pregnancy and Lactation Affects the Maternal Intestinal Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Preparation
2.2. Animals
2.3. Experimental Design
2.4. Histology
2.5. Total RNA Extraction and qPCR Analysis
2.6. Fecal Sample Collection and Genomic DNA Extraction
2.7. Detection of A. muciniphila by qPCR
2.8. Untargeted Metabolomics Analysis
2.9. Statistical Analysis
3. Results
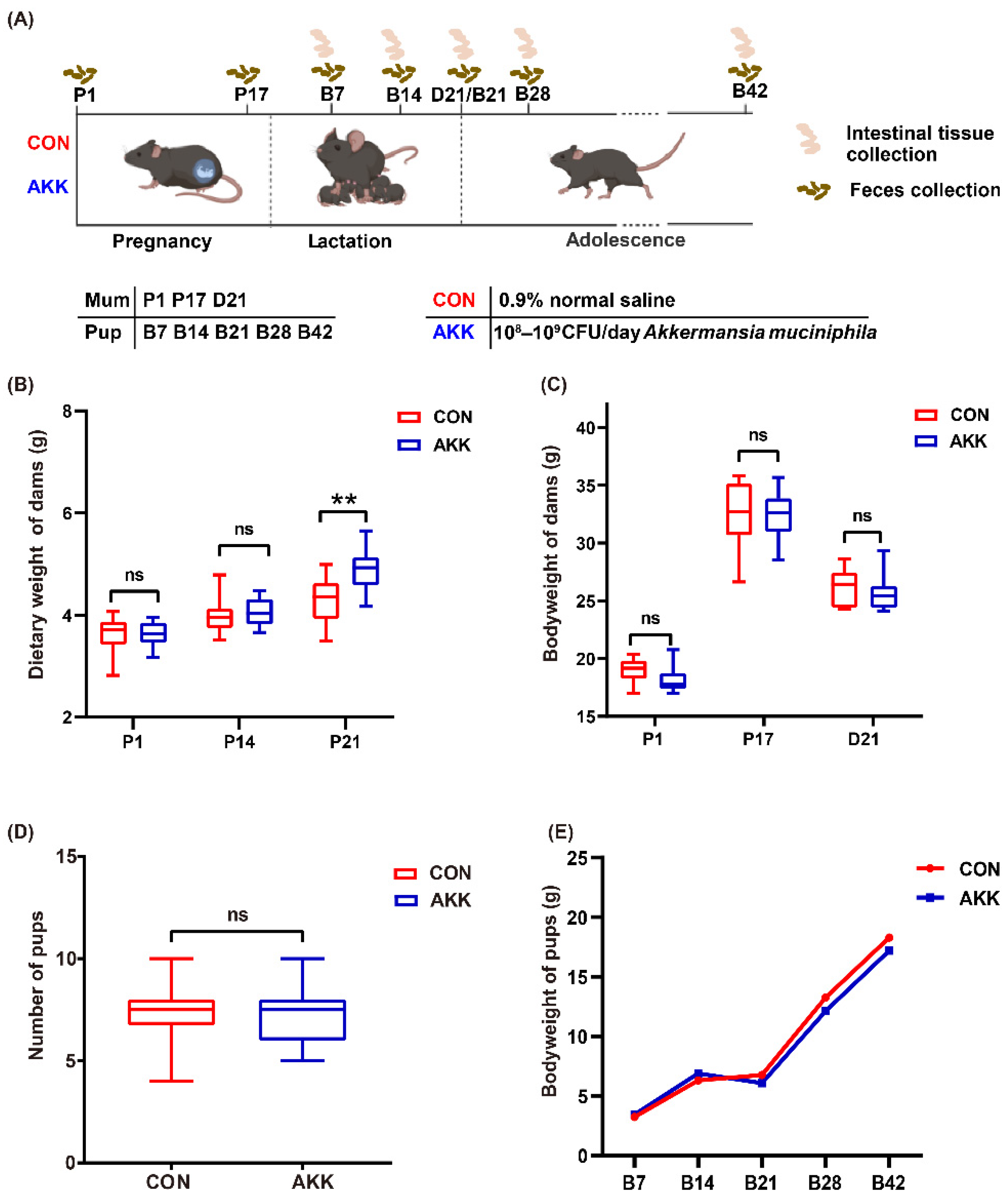
3.1. Effects of A. muciniphila Supplementation on the Basic Physiological Indexes of Mothers and Pups
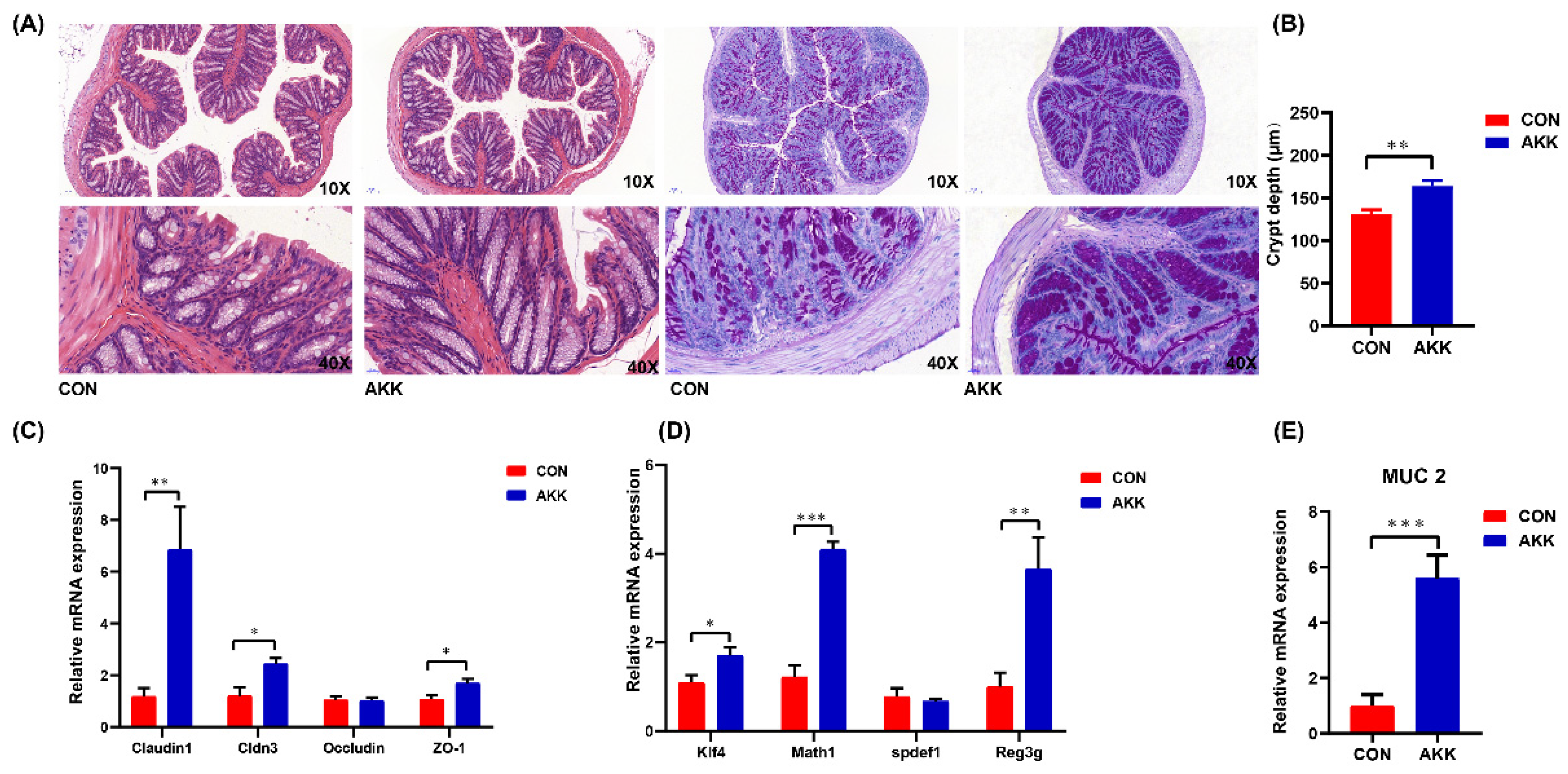
3.2. Effects of A. muciniphila Supplementation on the Intestinal Barrier of Mothers
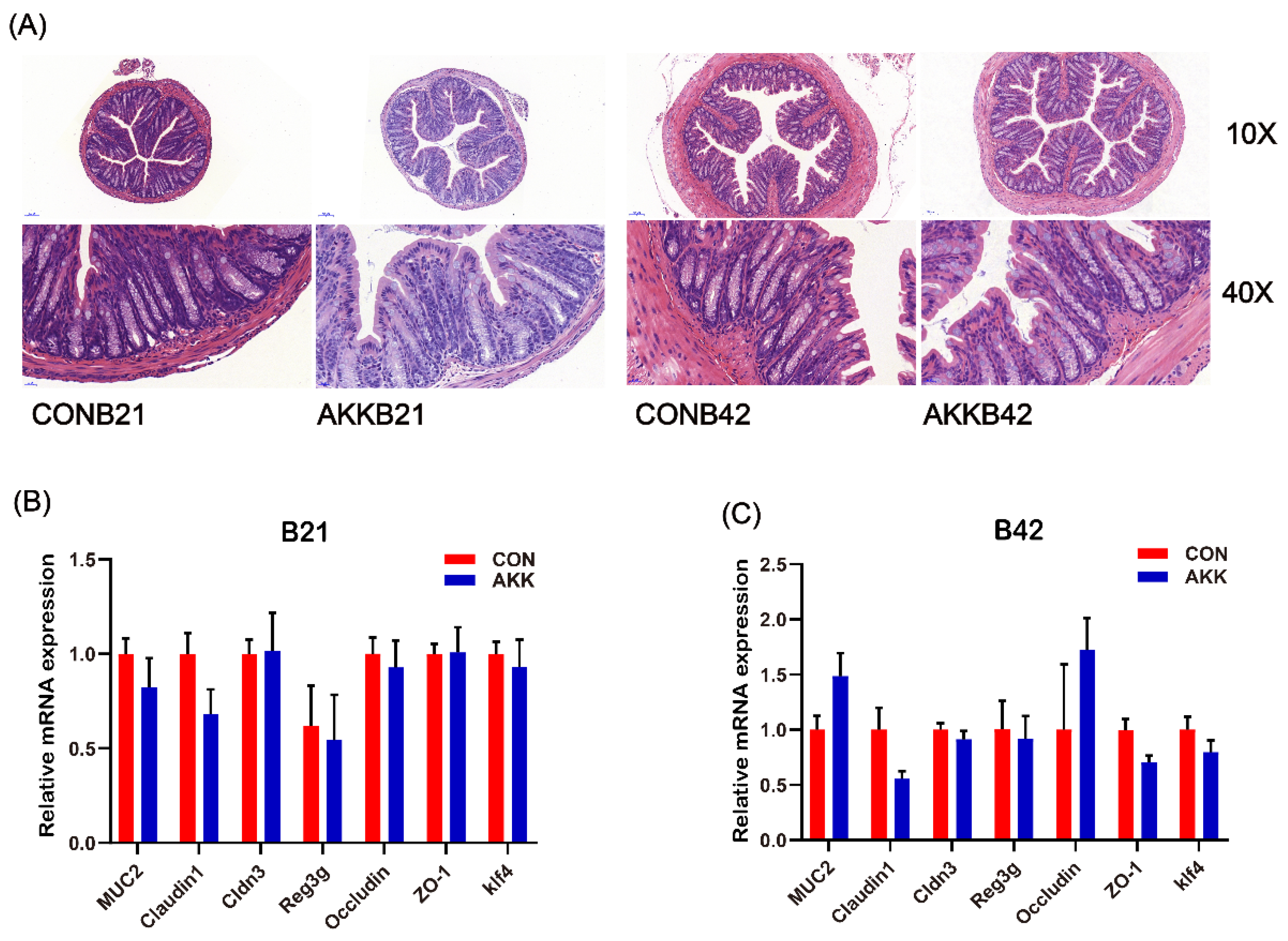
3.3. Maternal A. muciniphila Supplementation Did Not Affect the Intestinal Barrier of Offspring
3.4. Effects of A. muciniphila Supplementation on the Gut Microbiota Composition of Mothers
3.5. Effects of A. muciniphila Supplementation on the Gut Microbiota Composition of Offspring
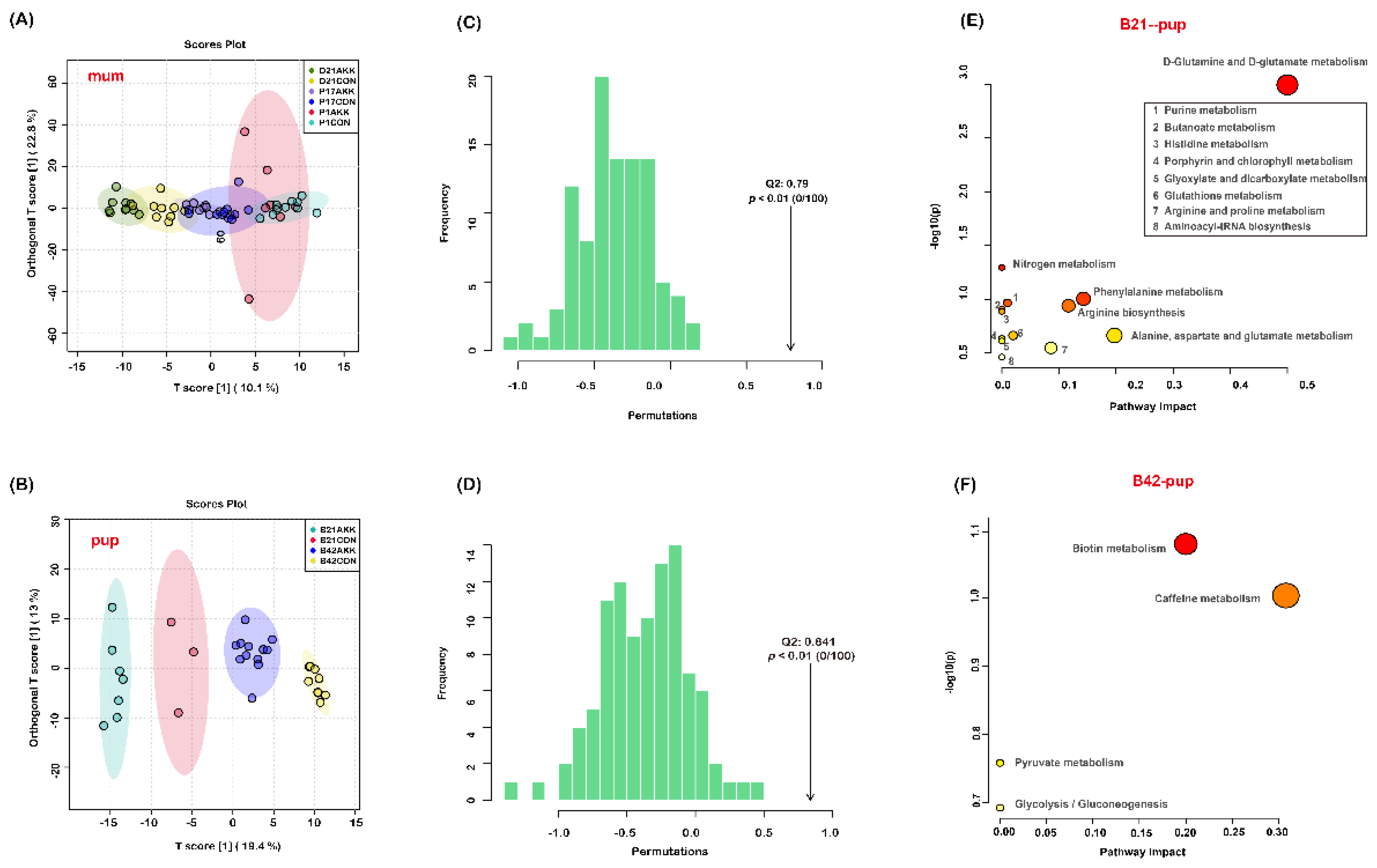
3.6. Fecal Metabolites of Mothers and Pups Are Modified by A. muciniphila Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aversa, Z.; Atkinson, E.J.; Schafer, M.J.; Theiler, R.N.; Rocca, W.A.; Blaser, M.J.; LeBrasseur, N.K. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 2021, 96, 66–77. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Z.; Ma, C.; Zhang, J.; Ma, C.; Zhao, Y.; Wei, H.; Huang, S.; Zhang, H. Exposure to soil environments during earlier life stages is distinguishable in the gut microbiome of adult mice. Gut Microbes 2021, 13, 1830699. [Google Scholar] [CrossRef]
- Wang, S.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Maternal vertical transmission affecting early-life microbiota development. Trends Microbiol. 2020, 28, 28–45. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zi, Y.; Xue, Z.; Xiaokun, G.; Guangyu, H.; Lin, S.; Zhuo, L.; Guixia, W. The effects of gut microbiota on metabolic outcomes in pregnant women and their offspring. Food Funct. 2018, 9, 4537–4547. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, S.L.; O’Hely, M.; Jacka, F.N.; Ponsonby, A.L.; Symeonides, C.; Loughman, A.; Collier, F.; Moreno-Betancur, M.; Sly, P.; Burgner, D.; et al. Maternal prenatal gut microbiota composition predicts child behaviour. EBioMedicine 2021, 68, 103400. [Google Scholar] [CrossRef] [PubMed]
- Vuillermin, P.; O’Hely, M.; Collier, F.; Allen, K.; Tang, M.; Harrison, L.; Carlin, J.; Saffery, R.; Ranganathan, S.; Sly, P.; et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun. 2020, 11, 1452. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, I.; Sharma, M.; Mehta, M.; Badyal, S.; Sharma, V.; Sharma, I.; Singh, H.; Sistla, S. Bioactive compounds and probiotics—A ray of hope in COVID-19 management. Food Sci. Hum. Well 2021, 10, 131–140. [Google Scholar] [CrossRef]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Well 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, W.; Li, Z.; Gao, D.; Gao, Y. Research progress of gut flora in improving human wellness. Food Sci. Hum. Well 2019, 8, 102–105. [Google Scholar] [CrossRef]
- Yao, M.; Qv, L.; Lu, Y.; Wang, B.; Berglund, B.; Li, L. An update on the efficacy and functionality of probiotics for the treatment of non-alcoholic fatty liver disease. Engineering 2021, 7, 679–686. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; Vos, W.D.J.A.; Microbiology, E. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microb. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [Green Version]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.S.; Kim, T.Y.; Lee, S.H.; Kim, S.; Yoo, H.; Kim, E.N.; Kweon, M.-N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872. [Google Scholar] [CrossRef] [Green Version]
- Ellekilde, M.; Krych, L.; Hansen, C.H.F.; Hufeldt, M.R.; Dahl, K.; Hansen, L.H.; Sørensen, S.J.; Vogensen, F.K.; Nielsen, D.S.; Hansen, A.K. Characterization of the gut microbiota in leptin deficient obese mice—Correlation to inflammatory and diabetic parameters. Res. Vet. Sci. 2014, 96, 241–250. [Google Scholar] [CrossRef]
- Hansen, C.H.F.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef] [Green Version]
- Perraudeau, F.; McMurdie, P.; Bullard, J.; Cheng, A.; Cutcliffe, C.; Deo, A.; Eid, J.; Gines, J.; Iyer, M.; Justice, N.; et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: A multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diab. Res. Care 2020, 8, e001319. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; De Vos, W.D.; Salminen, S.J.A.; Microbiology, E. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the Elderly. Appl. Environ. Microb. 2007, 73, 7767. [Google Scholar] [CrossRef] [Green Version]
- Kostopoulos, I.; Elzinga, J.; Ottman, N.; Klievink, J.T.; Belzer, C. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci. Rep. 2020, 10, 14330. [Google Scholar] [CrossRef]
- Ribo, S.; Sánchez-Infantes, D.; Martinez-Guino, L.; García-Mantrana, I.; Ramon-Krauel, M.; Tondo, M.; Arning, E.; Nofrarías, M.; Osorio-Conles, Ó.; Fernández-Pérez, A.; et al. Increasing breast milk betaine modulates Akkermansia abundance in mammalian neonates and improves long-term metabolic health. Sci. Transl. Med. 2021, 13, eabb0322. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, H.; Homayouni-Rad, A.; Mirghafourvand, M.; Mohammad-Alizadeh-Charandabi, S. Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: A randomized controlled clinical trial. Eur. J. Nutr. 2020, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Kallio, S.; Kukkonen, A.K.; Savilahti, E.; Kuitunen, M. Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up. Clin. Exp. Allergy 2019, 49, 506–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmirzaiee, M.A.; Famouri, F.; Moazeni, W.; Hassanzadeh, A.; Hajihashemi, M. The efficacy of the prenatal administration of Lactobacillus reuteri LR92 DSM 26866 on the prevention of infantile colic: A randomized control trial. Eur. J. Pediatr. 2020, 179, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotech. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Demirci, M.; Tokman, H.B.; Uysal, H.K.; Demiryas, S.; Karakullukçu, A.; Saribas, S.; Cokugras, H.; Kocazeybek, B.S. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopath. 2019, 47, 365–371. [Google Scholar] [CrossRef]
- Li, N.; Yan, F.; Wang, N.; Song, Y.; Yue, Y.; Guan, J.; Li, B.; Huo, G. Distinct gut microbiota and metabolite profiles induced by different feeding methods in healthy Chinese infants. Front. Microbiol. 2020, 11, 714. [Google Scholar] [CrossRef]
- Fan, Z.; Ross, R.; Stanton, C.; Hou, B.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus casei CCFM1074 alleviates collagen-induced arthritis in rats via balancing Treg/Th17 and modulating the metabolites and gut microbiota. Front. Immunol. 2021, 12, 680073. [Google Scholar] [CrossRef]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef] [Green Version]
- Paul, H.A.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci. Rep. 2016, 6, 20683. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients. 2021, 13, 423. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237. [Google Scholar] [CrossRef] [Green Version]
- Ganal-Vonarburg, S.; Hornef, M.; Macpherson, A. Microbial—Host molecular exchange and its functional consequences in early mammalian life. Science 2020, 368, 604–607. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, N.; Castell, M.; Guardiola, F.; Pérez-Cano, F.; Cells, M.R.-L.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation impacts maternal and offspring lipid profile, immune system and microbiota. Cells 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, J.H.; Ahn, S.H.; Lee, S.I.; Han, Y.S.; Choi, Y.O.; Lee, S.Y.; Ahn, K.M.; Ji, G.E. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: A double-blind, randomized, placebo-controlled trial. Pediat. Allergy Immun. 2010, 21, e386–e393. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.J.; Ismail, I.H.; Kivivuori, S.; Licciardi, P.V.; Robins-Browne, R.M.; Mah, L.J.; Axelrad, C.; Moore, S.; Donath, S.; Carlin, J.B.; et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: A randomized controlled trial. Allergy 2011, 66, 509–516. [Google Scholar] [CrossRef]
- Navarro-Tapia, E.; Sebastiani, G.; Sailer, S.; Toledano, L.A.; Serra-Delgado, M.; García-Algar, Ó.; Andreu-Fernández, V. Probiotic supplementation during the perinatal and infant period: Effects on gut dysbiosis and disease. Nutrients 2020, 12, 2243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020, 31, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stichelen, O.V.; Rother, K.I.; Hanover, J. Maternal exposure to non-nutritive sweeteners impacts progeny’s metabolism and microbiome. Front. Microbiol. 2019, 10, 1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on intestinal epithelial barrie function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Beiyu, C.; Lixin, Z.; Xin, X.; Xin, W.; Ziming, A.; Li, S.; Hu, Y.Y.; Feng, Q. Liraglutide modulates gut microbiome and attenuates nonalcoholic fatty liver in db/db mice. Life Sci. 2020, 261, 118457. [Google Scholar] [CrossRef]
- Fidélix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef]
- Li, T.; Zhang, T.; Gao, H.; Liu, R.; Gu, M.; Yang, Y.; Cui, T.; Lu, Z.; Yin, C. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. 2021, 41, 101886. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human microbiome and child growth—First 1000 days and beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Dotterud, C.K.; Avershina, E.; Sekelja, M.; Simpson, M.R.; Rudi, K.; Storrø, O.; Johnsen, R.; Øien, T. Does maternal perinatal probiotic supplementation alter the intestinal microbiota of mother and child? J. Pediatr. Gastr. Nutr. 2015, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.; Pronovost, G.; Williams, D.; Coley, E.; Siegler, E.; Qiu, A.; Kazantsev, M.; Wilson, C.; Rendon, T.; Hsiao, E. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.; Rodríguez-Lagunas, M.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation affects breast milk composition. J. Dairy Sci. 2020, 103, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Li, M.; Yu, M.; Wang, X.; Deng, M.; Zhai, X.; Li, R. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota. Front. Endocrinol. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]


| Group | Metabolites | Change 1 | log2(FC) | −log10(p) | |
|---|---|---|---|---|---|
| mum | P1 | 2,4-quinolinediol | − | 5.0125 | 2.5454 |
| P17 | quercetin | − | 3.0601 | 1.7229 | |
| 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-4-one | − | 2.6156 | 2.4021 | ||
| N-({(1S,4S,6S)-4-[2-(4-Acetyl-1-piperazinyl)-2-oxoethyl]-6-isopropyl-3-methyl-2-cyclohexen-1-yl}methyl)-3-fluorobenzamide | −1.2058 | 2.0271 | |||
| 5(Z),8(Z),11(Z)-eicosatrienoic acid methyl ester | − | 1.4851 | 1.3538 | ||
| 3-amino-2-phenyl-2H-pyrazolo[4,3-c]pyridine-4,6-diol | − | 1.4208 | 1.53 | ||
| 2,4-quinolinediol | − | 1.3326 | 1.6166 | ||
| D21 | chalconaringenin | − | 2.2558 | 4.4939 | |
| chrysin | − | 2.2671 | 3.9032 | ||
| 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-4-one | − | 4.0824 | 3.7509 | ||
| kaempferol | − | 4.2064 | 3.6954 | ||
| apigenin | − | 1.9089 | 2.7149 | ||
| quercetin | − | 3.0535 | 2.0839 | ||
| 3-methoxy-5,7,3’,4’-tetrahydroxy-flavone | − | 1.2944 | 2.0068 | ||
| 1-(3-hydroxy-3-methylpent-4-en-1-yl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol | + | −1.6698 | 1.5552 | ||
| N-({(1S,4S,6S)-4-[2-(4-Acetyl-1-piperazinyl)-2-oxoethyl]-6-isopropyl-3-methyl-2-cyclohexen-1-yl}methyl)-3-fluorobenzamide | + | −1.4211 | 1.499 | ||
| pup | B21 | ferulic acid | − | 1.3433 | 2.3336 |
| 2-deoxyadenosine | − | 1.7339 | 2.1811 | ||
| 1,2,3-propanetricarboxylic acid | + | −3.4132 | 1.9936 | ||
| dihomo-γ-linolenic acid ethyl ester | + | −1.1179 | 1.9883 | ||
| 4-dodecylbenzenesulfonic acid | + | −1.1009 | 1.9561 | ||
| N-ethylglycine | + | −1.9219 | 1.9212 | ||
| adenine | − | 1.3701 | 1.8602 | ||
| trigonelline | − | 1.8588 | 1.7411 | ||
| apigenin | − | 1.1448 | 1.6694 | ||
| 13Z,16Z-docosadienoic Acid | + | −1.3788 | 1.5585 | ||
| D-pyroglutamic acid | + | −1.0427 | 1.4999 | ||
| L-glutamic acid | + | −1.6019 | 1.4835 | ||
| isobutyric acid | − | 1.6023 | 1.4738 | ||
| stachydrine | + | −1.4942 | 1.433 | ||
| phenylacetaldehyde | − | 1.2711 | 1.3859 | ||
| 1-[4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4(1H,3H)-dione | − | 1.0677 | 1.3794 | ||
| acamprosate | + | −5.1411 | 1.354 | ||
| L-carnitine | + | −1.4233 | 1.354 | ||
| (1S,19R)-8,10-dioxa-4,17-diazaheptacyclo[15.4.3.01,18 04,19 05,13 07,11 014,19]tetracosa-5(13),6,11,22-tetraen-3-one | + | −1.0226 | 1.3446 | ||
| B42 | acamprosate | − | 4.3948 | 6.7137 | |
| NP-003553 | − | 1.262 | 5.2985 | ||
| NP-015980 | + | −1.8399 | 5.1508 | ||
| L-lactic acid | − | 1.1072 | 4.1074 | ||
| methyl indole-3-acetate | − | 1.3097 | 3.0721 | ||
| tridecylic acid | − | 2.6194 | 2.7511 | ||
| ursolic acid | + | −1.0904 | 2.7069 | ||
| 1-methylxanthine | + | −1.9701 | 2.4263 | ||
| 1-benzyl-3-[(1S,4S)-4-{3,5-dimethyl-1-[4-(2-methyl-2-propanyl)phenyl]-1H-pyrazol-4-yl}-2-cyclopenten-1-yl]urea | + | −1.7189 | 2.3518 | ||
| N-ethylglycine | − | 1.0197 | 2.3183 | ||
| 3-methylxanthine | + | −1.4299 | 1.9015 | ||
| indole-3-lactic acid | + | −1.2901 | 1.8742 | ||
| 5-[({(2R,4S,5R)-5-[1-methyl-3-(2-thienyl)-1H-pyrazol-5-yl]-1-azabicyclo[2.2.2]oct-2-yl}methyl)amino]-5-oxopentanoic acid | + | −3.698 | 1.8261 | ||
| salicylic acid | + | −1.6365 | 1.7769 | ||
| biotin | + | −1.0759 | 1.7525 | ||
| 4-(methylthio)-6-phenyl-2-(3-pyridyl)pyrimidine-5-carbonitrile | + | −1.6808 | 1.6331 | ||
| taurochenodeoxycholic Acid _sodium salt_ | + | −1.1365 | 1.5416 | ||
| (4aS,5R,6S,8aS)-5-[(3E)-5-methoxy-3-methyl-5-oxopent-3-en-1-yl]-5,6,8a-trimethyl-3,4,4a,5,6,7,8,8a-octahydronaphthalene-1-carboxylic acid | + | −1.2552 | 1.5266 | ||
| 4-fluoro-N-{[(1S,4S,6S)-6-isopropyl-3-methyl-4-(2-{[2-(4-morpholinyl)ethyl]amino}-2-oxoethyl)-2-cyclohexen-1-yl]methyl}benzamide | + | −1.0308 | 1.4497 | ||
| 1,2,3-propanetricarboxylic acid | − | 1.0805 | 1.4491 | ||
| cytosine | − | 1.0388 | 1.4034 | ||
| tropine | − | 1.8667 | 1.3217 | ||
| N-desmethyltramadol | + | −1.8015 | 1.3146 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. A. muciniphila Supplementation in Mice during Pregnancy and Lactation Affects the Maternal Intestinal Microenvironment. Nutrients 2022, 14, 390. https://doi.org/10.3390/nu14020390
Qi Y, Yu L, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. A. muciniphila Supplementation in Mice during Pregnancy and Lactation Affects the Maternal Intestinal Microenvironment. Nutrients. 2022; 14(2):390. https://doi.org/10.3390/nu14020390
Chicago/Turabian StyleQi, Yuli, Leilei Yu, Fengwei Tian, Jianxin Zhao, Hao Zhang, Wei Chen, and Qixiao Zhai. 2022. "A. muciniphila Supplementation in Mice during Pregnancy and Lactation Affects the Maternal Intestinal Microenvironment" Nutrients 14, no. 2: 390. https://doi.org/10.3390/nu14020390
APA StyleQi, Y., Yu, L., Tian, F., Zhao, J., Zhang, H., Chen, W., & Zhai, Q. (2022). A. muciniphila Supplementation in Mice during Pregnancy and Lactation Affects the Maternal Intestinal Microenvironment. Nutrients, 14(2), 390. https://doi.org/10.3390/nu14020390






