Abstract
Whole grain foods are rich in nutrients, dietary fibre, a range of antioxidants, and phytochemicals, and may have potential to act in an anti-inflammatory manner, which could help impact chronic disease risk. This systematic literature review aimed to examine the specific effects of whole grains on selected inflammatory markers from human clinical trials in adults. As per the Preferred Reporting Items for Systematic Reviews (PRISMA) protocol, the online databases MEDLINE, Embase, Cochrane, CINAHL, and Scopus were searched from inception through to 31 August 2021. Randomized control trials (RCTs) ≥ 4 weeks in duration, reporting ≥1 of the following: C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor (TNF), were included. A total of 31 RCTs were included, of which 16 studies recruited overweight/obese individuals, 12 had pre-existing conditions, two were in a healthy population, and one study included participants with prostate cancer. Of these 31 RCTs, three included studies with two intervention arms. A total of 32 individual studies measured CRP (10/32 were significant), 18 individual studies measured IL-6 (2/18 were significant), and 13 individual studies measured TNF (5/13 were significant). Most often, the overweight/obese population and those with pre-existing conditions showed significant reductions in inflammatory markers, mainly CRP (34% of studies). Overall, consumption of whole grain foods had a significant effect in reducing at least one inflammatory marker as demonstrated in 12/31 RCTs.
1. Introduction
Whole grains are defined by Food Standards Australia and New Zealand (FSANZ), to be ‘… intact, dehulled, ground, cracked or flaked grains where the components–endosperm, germ and bran are present in substantially the same proportions as they exist in the intact grain’ and includes wholemeal [1]. More recently, a consensus definition of whole grain as a food and as an ingredient was published with the aim of assisting in nutrition education and food labeling, but this also provides useful guidance for research [2]. Foods containing whole grains are both higher in nutrients and dietary fiber, as compared to refined grain alternatives, and in observational studies, diets higher in whole grains positively impact chronic disease, such as type 2 diabetes mellitus [3], cardiovascular disease (CVD) [4], certain cancers [4] including colorectal cancer [5,6,7,8], and other influencing risk factors, such as weight [9], and markers for CVD, such as triglyceride and cholesterol levels [10]. In addition, the nutrient bundle within whole grains contains potential anti-inflammatory properties, which is of importance as elevated levels of inflammatory biomarkers are linked to an increase in chronic disease risk [2,3]. The benefits of whole grain foods, including pseudo grains, quinoa, buckwheat, and amaranth, have been known for several decades, and included in the Australian Dietary Guidelines since 1979 [11]. Chronic disease was responsible for 9 out of 10 deaths in Australia in 2018, and 61% of the total burden of disease in Australians in 2017 [12], indicating the potential importance of improved dietary guidance and dietary patterns. However consumption of whole grain foods continues to remain at a low level, with Australian adults only consuming 21 g/day, less than half of the 48 g daily target intake (DTI) [11,13]. Furthermore, diets low in whole grains have been identified as the second greatest dietary risk factor for mortality in the Global Burden of Disease studies [14], highlighting the importance of dietary patterns.
The anti-inflammatory effects of whole grains can be examined via inflammatory markers, such as C-reactive protein, (CRP), interleukin-6, (IL-6), and tumor necrosis factors (TNF), and can potentially downregulate an inflammatory response [15]. Inflammatory markers change in response to a cascade of internal metabolic processes, where chronic inflammation can lead to chronic disease [15].
There is a growing body of evidence linking whole grain consumption with overall health benefits; however, the specific influence of whole grains on inflammatory markers is conflicting [11,16]. To date, systematic reviews of randomized controlled trials (RCTs) have focused on the consumption of whole grains and their association with individual chronic health diseases, such as CVD or T2D [17]. Others have focused specifically on dietary fiber levels in whole grains and associated effects; however, there is no current summation of the literature focusing solely on the consumption of whole grains and their direct effect on inflammatory markers. Although there are two previously published systematic reviews in this area [17,18], an update was necessary that focused only on adults, with a strict criteria for whole grain to meet the accepted definition and to clarify other discrepancies. This systematic literature review aimed to examine the specific effects of whole grains on inflammatory markers from human clinical trials in adults. The intent was to investigate whether the consumption of whole or pseudo grains, over refined grains, resulted in changes in inflammatory markers, based on results in human subjects in studies ≥ 4 weeks duration.
2. Materials and Methods
This systematic literature review of RCT was performed to assess the effect of whole grain consumption on inflammatory markers following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. This study was registered with the International Prospective Register of Systematic Reviews (CRD: pending).
2.1. Eligibility and Exclusion Criteria
The research question ‘Is there an effect of whole grain consumption on measures of inflammation?’ was developed using the Population, Intervention, Comparator, Outcome (PICO) format (Figure S1). Publications needed to meet the following inclusion criteria: (a) RCT, parallel, or cross-over design; (b) studies conducted on humans aged ≥18 years; (c) studies ≥ 4 weeks in duration; (d) studies with interventions including both whole grain and pseudo grain diets, where whole grains included: cereal grains; wheat; including spelt, emmer, einkorn, Khorasan or kamut, durum, and faro; oats, corn/maize, rice, teff, canary seeds, Job’s Tears, barley, sorghum, rye, millet and triticale, and pseudo-cereal grains; amaranth, buckwheat, quinoa, and wild rice; (d) reporting ≥1 of the following serum inflammatory markers: interleukin-6, (IL-6), C-reactive protein, (CRP), tumor necrosis factor (TNF). Full search terms can be found in Table S2.
The following exclusion criteria were applied; (a) studies conducted on humans < 18 years; (b) study intervention arms not randomized; (c) studies < 4 weeks in duration. Although inflammatory markers were examined by both Jenkins et al. [19] and Kristensen et al. [20], the intervention diet included several foods, not just whole grain foods; therefore, these studies were excluded from the current review.
2.2. Search Strategy
The following online databases were searched: Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL). Available online: https://ovidsp.ovid.com/ (accessed on 13 December 2021), and CINAHL. Available online: https://www.ebsco.com/ (accessed on 13 December 2021), from database inception until 31 August 2021. In addition, reference lists of eligible studies were scanned and PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 13 December 2021).
Was searched manually for any additional studies. The search strategy was designed in Medline and translated for other databases (Table S2). Grey literature, abandoned trials, and any journals published in languages other than English were excluded from the search strategy.
2.3. Study Selection, Data Extraction, and Quality Assessment
Reviewer G.M extracted all citations into EndNote X9, with duplicates removed manually. Reviewer G.M independently double screened all titles and abstracts, with any uncertainty and assistance from S.G. Following title and abstract screening, a full-text screen was completed on the remaining articles by two independent reviewers (S.G. and G.M.). Reviewers met and resolved any discrepancies, with any remaining uncertainty resolved by a third reviewer (A.R.).
A data extraction form was created in a Microsoft® Excel® spreadsheet (Microsoft 365 MSO Version 2109.14430.20306 Redmond, WA, USA) to facilitate the retrieval and storage of relevant data. Extracted data included study design (parallel or cross-over), study duration, participant characteristics, number of participants, control and intervention diet, outcomes measured, and results obtained (baseline, endpoint data, and p-value).
The included studies were reviewed for risk of bias using the Cochrane Risk of Bias tool (Rob2) for RCTs [21]. Reviewer G.M assessed studies to determine if each study had low, some concerns, or high risk of bias. Assessment criteria included risk of bias arising from recruitment of subjects, the randomization process, deviations from the interventions, missing data, measurement of outcome, or selection of the reported result. A second reviewer (S.G.) was consulted over any uncertainties.
2.4. Data Analysis
Tabulation of studies including reported mean ± SD of baseline and endpoint data and statistical significance (p-value) for within-group and between-group intervention changes for each study, and for studies with multiple intervention arms was performed. Within the included studies, outcomes were considered statistically significant when p < 0.05. The outcome measures were maintained as per the study units due to the differences in the various experimental methods used. Studies were then categorized into population groups based on the authors’ description of participants: healthy individuals, overweight or obese individuals, individuals with pre-existing conditions, and others (prostate cancer).
3. Results
3.1. Search Results and Study Selection
The initial search, conducted on 31 August 2021, returned a total of 730 studies. An additional four studies were identified from the reference list of eligible studies and manual searches from PubMed. After the removal of duplicates, 397 were screened for the title and abstract, with a further 312 studies excluded. A full-text review was completed on the remaining 85 records, with 47 removed due to the type of study, study did not have an adult population, or length of the RCT < 4 weeks. The remaining 38 studies were further assessed, with six removed as the control or intervention diet was not whole or refined grains and one measured inflammation in fecal matter, not from blood serum. A remaining total of 31 RCTs met the inclusion criteria and were included in the systematic review (Figure 1).
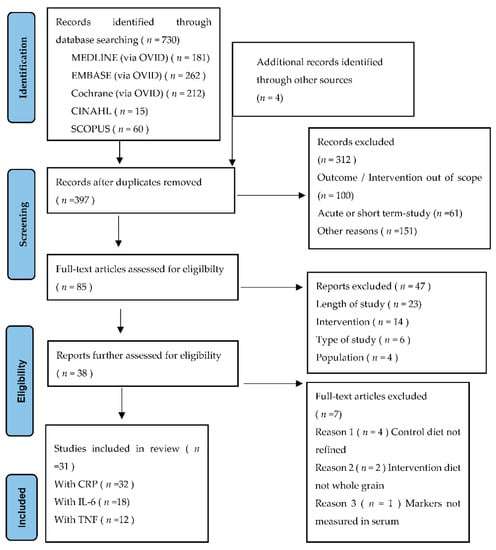
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram for study selection.
3.2. Study Characteristics
Of the 31 studies included in analysis, 16 were parallel RCTs and 15 were crossover trials. Of these studies, three RCTs included two intervention arms, and thus were split into a further three studies [22,23]. Table 1 displays the study characteristics. Two studies comprised whole grain interventions in healthy populations, 16 studies overweight or obese, 12 pre-existing conditions, and one reviewing another disease state: prostate cancer. The studies had a total of 2047 participants, with a mean age of 49.7 (range 20–80 years old) and the mean duration of the study was 12.5 weeks (range 4–24 weeks).

Table 1.
Characteristics of studies examining whole grain consumption and inflammatory markers.
3.3. Risk of Bias
A summary of the within-study risk of bias is shown in Figure 2. The included studies were assessed against the predetermined criteria of the Cochrane RoB2 tool for randomized control and crossover trials [21]. Within Domain 1: Randomization Process, there were five studies with some concerns of bias [24,28,30,31,43], with the remaining studies (n = 26) with a low risk of bias. In Domain 2: Deviations from intended intervention, there was one study with a high risk of bias [22], one with some concern [31], and the remainder with a low risk of bias (n = 29). Three studies had some risk of bias for Domain 3: Missing outcome data, [28,41,45], and the remainder had a low risk of bias (n = 28). Two studies had some risk of bias for both Domain 4: Measurement of the outcome [25,47] and Domain 5: Selection of the reported result [26,31], with the remainder having a low risk of bias (n = 29).
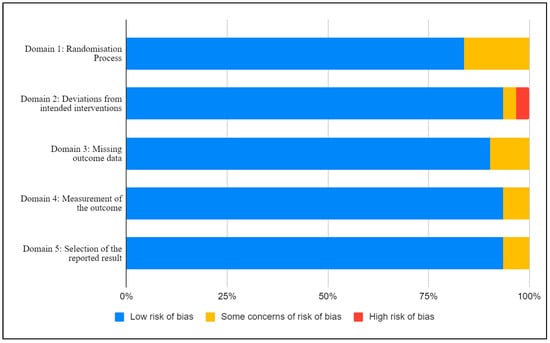
Figure 2.
Risk of bias assessment using the revised Cochrane risk-of-bias (RoB 2).
3.4. Effect of the Intervention on the Outcome
3.4.1. Healthy Individuals
Two studies measured the effect of whole grain consumption on healthy individuals, who had a BMI < 25 and with no pre-existing conditions [23,40]. Within these studies, two measured CRP, while only one measured IL-6 and TNF. No marker for the studies looking at healthy individuals showed any level of statistical significance. The details are displayed in Table 2.

Table 2.
Effect of whole grain consumption on inflammatory markers in healthy individuals between the intervention and control diet.
3.4.2. Overweight or Obese Individuals
Among the 16 studies in the overweight and obese populations (BMI 25–35), two had two intervention arms [22,47], resulting in 18 studies within this category (Table 3). All 18 studies measured CRP levels, with six of these (33%) observing a statistically significant reduction in CRP levels following whole grain consumption [29,30,32,33,38,40]. Nine of the studies measured IL-6 levels, with one observing a statistically significant change in IL-6 levels after consumption of whole grain foods [40]. A further two of the five total studies measuring TNF also observed a statistically significant change in inflammatory marker levels [32,49].

Table 3.
Effect of whole grain consumption on inflammatory markers in overweight and obese individuals.
3.4.3. Individuals with Pre-Existing Conditions
In the 12 studies that reviewed individuals with pre-existing conditions, which included type 2 diabetes [23,35,43,45,50], metabolic syndrome [27,28,31,48], type 2 diabetes and metabolic syndrome [23], acute coronary syndrome [50], and hypercholesterolaemia [42], one study had two intervention arms included in this SLR [23] (Table 4). Of the 11 studies measuring CRP, four observed a statistically significant change [23,31,42,43]. Seven studies measured IL-6 levels, with only one showing a significant change [42]. These seven studies also reviewed TNF levels, with three observing an increase in the level of change between the intervention and the control group, which was statistically significant [28,42,51].

Table 4.
Effect of whole grain consumption on inflammatory markers in individuals with pre-existing conditions.
3.4.4. Individuals with Other Conditions
One study had a population that fit outside of the other population groups: males with prostate cancer [52] (Table 5). This study measured CRP and IL-6 levels and whilst the data was not prepared in accordance with other measures, the study observed no statistical level of significance for either.

Table 5.
Effect of whole grain consumption on inflammatory markers in individuals with other conditions.
4. Discussion
Consumption of whole grains in preference to refined grains is known to have improved health benefits, with the broad range of benefits often attributed solely to the presence of dietary fiber [10,53]; however, other components, phytochemicals, fatty acids, amino acids, vitamins, and minerals are all likely to play a role. This review of 31 RCTs found that consumption of whole grain foods had a moderate effect on reducing inflammatory markers, with five of the possible 15 crossover studies [33,38,40,42,50], and seven of 16 parallel studies demonstrating statistically significant changes [23,29,30,31,32,43,49]. Within the population groups studied, the reduction in markers was most often observed in obese and overweight populations, and among those with pre-existing conditions, compared with studies of healthy populations, although there were only two studies in this category.
Previous systematic reviews and meta analyses, performed by Rahmani et al. [17] and Hajihashemi et al. [18] utilising publications up until 2019, found little evidence of a relationship between whole grain consumption and inflammatory markers. The current review included a total of 13 papers not included in the aforementioned reviews [17,18], six of which were published outside the timeframe utilized by the previous authors [30,31,41,43,44,52], and a further seven were included in the current review due to a variation in the search strategy [23,29,37,39,42,45,51].
While the findings of the current study provide some indication that whole grain consumption leads to a downregulation of inflammation, the wide variety of foods classed as whole grain included in the intervention diets varied between studies, from commercially available whole grain products to a specific dose allocated via food items provided by the research group. Of the 31 studies reviewed, 27 provided the intervention foods; however, the remaining four studies [25,32,36,43] only provided guidelines or instructions of which foods to purchase, adding a significant burden for study participants in sourcing and selecting the correct food types, which is a known issue for consumers [54]. Blind compliance checks are problematic and alkylresorcinol levels were only utilized by Harris Jackson et al. [28]; however, this test is only relevant for whole grain wheat and rye [55,56]. Despite this limitation, such biomarkers have been suggested in research to help support dietary assessment of consumption [56].
Only three of the 31 studies noted that subjects were instructed to maintain weight for the duration of the study [27,29,42], and only one study controlled for weight in their analysis [50], with all others showing a slight decrease in weight or no data mentioned. In addition, only eight studies recorded or mentioned physical activity or exercise, with six asked to maintain [23,25,27,30,33,41], one asked to record any exercise [32], and one asked to refrain completely [29]. A change in weight either through diet or exercise could be a possible confounder, as it becomes difficult to isolate the changes in inflammatory status as a result of the consumption of whole grain or as a result of the weight (fat) loss [57]. Despite the focus of papers based on the overweight and obese population, only 16 of 30 RCTs measured body fat mass [22,24,25,27,30,31,34,35,37,38,40,41,45,46,48,49], with no consistency in the method or type of body fat measured between studies, making comparisons between studies difficult. Furthermore, the more favorable results within studies of overweight populations are likely due to higher inflammatory marker levels at baseline in comparison to healthy populations. This finding is of particular importance as dietary interventions that result in a reduction in inflammation are important due to the link with reduced risk of chronic diseases [58].
As inflammation is known to increase with age [59] and the average age of the participants was 50 years (20–80 years), future studies could look at potential differences in age groups, or alternatively study a larger population sample segmenting by age, health status, or gender. This would enable the identification of population groups where the diet prescription may be most efficacious.
Chronic disease remains one of the largest cost contributors to the global burden of disease, with overweight contributing 8.7% of the annual cost of the total burden of disease in Australia in 2019 [12]. On a population level, swapping from refined grains to whole grains has the possibility of reducing the risk of chronic disease, in turn lowering the costs related to the burden of disease. A recent nutrition economics analysis found that a swap to whole grain from refined grain foods could provide significant healthcare cost savings for cardiovascular disease, type 2 diabetes, and cancer, particularly colorectal cancer, for the Australian population [60,61].
Further studies investigating the relationship between consumption of whole grain foods over comparable refined grain products and the influence on inflammatory markers are needed to confirm the presence and strength of the relationship. Studies with standardized diets where the single focus of the dietary intervention was whole grain foods compared with refined grain foods would help to narrow the possibility that the intervention diet was responsible for the change in the inflammatory response. Previous research has emphasized the need to accurately assess and record the whole grain content of foods in participant diets, with a minimum DTI of 48 g of whole grain, rather than using the weight of the whole grain food to allow for a more accurate dose assessment [56]. Products in the Australian market can claim a whole grain content from as little as 8 g per manufacturer serve or 25% whole grain and these may be consumed alongside products that are 100% whole grain, such as oats or brown rice. The recently proposed global definition for whole grains as an ingredient and as a whole grain food provides further guidance for research to assist with comparison between studies [2]. Studies also need to consider that the health outcomes from various whole grain food products may not be homogeneous, with potential differences between types of whole grains, for example, wheat versus rye versus oats versus brown rice; differing proportions of dietary fiber, and within that, soluble to insoluble fiber content; and also consideration of other components, such as beta-glucan. This has been discussed in a previous systematic review regarding cardiovascular risk factors, where whole grain oats were found to be more effective than other grains in reducing cholesterol, and brown rice was more effective in reducing triglycerides.
A strength of this analysis was the study design, clarifying the discrepancies in previously published systematic reviews. For example, the careful inclusion of only adult RCTs, and the removal of quasi-experimental studies including only those utilizing blood measures of cytokines (not faecal measures) and those with test diets that included whole grain rather than the fiber component from whole grain sources. The collection of data from the differing population groups enabled categorization and comparison between study population types, highlighting differences between healthy and unhealthy population groups, a potential consideration for future research.
5. Conclusions
With obesity rates continuing to grow in Australia and globally, coupled with the link to a higher risk of chronic disease, dietary interventions that investigate simple food changes, such as exchanging refined grain for whole grain, are of particular interest. This study further contributes to increasing current knowledge, pointing to future research considerations, particularly the need to conduct research with individual whole grain food types, discern potential differences, accurately account for the dose of whole grain, and measure compliance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14020374/s1, Table S1: PICO Framework, Table S2: Search Terms, Figures S1 and S2: Results of Risk of Bias Assessment.
Author Contributions
Conceptualization, G.M., S.G. and A.R.; methodology, G.M. and S.G.; formal analysis; writing—original draft preparation, G.M.; writing—review and editing, G.M., S.G. and A.R.; supervision, S.G. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding however was supported by the Grains & Legumes Nutrition Council, a not-for-profit charity.
Conflicts of Interest
S.G. was employed by the Grains & Legumes Nutrition Council, a not-for-profit charity at the time of data collection. G.M. and A.R. declare no conflict of interest.
References
- Food Standards Australia New Zealand (FSANZ) Wholegrain Definition. Available online: https://www.foodstandards.gov.au/consumer/nutrition/wholegrain/Pages/default.aspx (accessed on 25 August 2021).
- Van der Kamp, J.-W.; Jones, J.M.; Miller, K.B.; Ross, A.B.; Seal, C.J.; Tan, B.; Beck, E.J. Consensus, global definitions of whole grain as a food ingredient and of whole-grain foods presented on behalf of the whole grain initiative. Nutrients 2022, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; de Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Kissock, K.R.; Neale, E.P.; Beck, E.J. Whole grain food definition effects on determining associations of whole grain intake and body weight changes: A systematic review. Adv. Nutr. 2020, 12, 693–707. [Google Scholar] [CrossRef]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The effect of replacing refined grains with whole grains on cardiovascular risk factors: A systematic review and meta-analysis of randomized controlled trials with GRADE clinical recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883. [Google Scholar] [CrossRef]
- Curtain, F.; Grafenauer, S. Historical and global perspectives on grains and whole grains within dietary guidelines. Cereal Foods World 2020, 65, 1–7. [Google Scholar]
- Australian Institute of Health and Welfare. Exploring the Definition of Chronic Conditions for Collective Monitoring in Australia; Australian Institute of Health and Welfare: Darlinghurst, Australia, 2021. Available online: https://www.aihw.gov.au/reports/chronic-disease/exploring-the-definition-of-chronic-conditions/summary (accessed on 25 August 2021)ISBN 978-1-76054-803-2.
- Galea, L.; Beck, E.; Probst, Y.; Cashman, C. Whole grain intake of Australians estimated from a cross-sectional analysis of dietary intake data from the 2011–13 Australian Health Survey. Public Health Nutr. 2017, 20, 2166–2172. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Browning, L.M.; Krebs, J.D.; Jebb, S.A. Discrimination ratio analysis of inflammatory markers: Implications for the study of inflammation in chronic disease. Metabolism 2004, 53, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Idehen, E.; Zhao, Y.; Chu, Y. Emerging science on whole grain intake and inflammation. Nutr Rev. 2020, 78 (Suppl. 1), 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Sadeghi, O.; Sadeghian, M.; Sadeghi, N.; Larijani, B.; Esmaillzadeh, A. The effect of whole-grain intake on biomarkers of subclinical inflammation: A comprehensive meta-analysis of randomized controlled trials. Adv. Nutr. 2020, 11, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, P.; Haghighatdoost, F. Effects of whole-grain consumption on selected biomarkers of systematic inflammation: A Systematic review and meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2019, 38, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Faulkner, D.A.; Wong, J.M.; de Souza, R.; Emam, A.; Parker, T.L.; Vidgen, E.; Lapsley, K.G.; et al. Effects of a dietary portfolio of cholesterol-lowering foods vs. lovastatin on serum lipids and C-reactive protein. JAMA 2003, 290, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, M.; Toubro, S.; Jensen, M.G.; Ross, A.B.; Riboldi, G.; Petronio, M.; Bugel, S.; Tetens, I.; Astrup, A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J. Nutr. 2012, 142, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, I.A.; Moore, C.; Chatfield, M.; Richardson, D.P.; Ashby, P.; Kuznesof, S.A.; Jebb, S.A.; Seal, C.J. Markers of cardiovascular risk are not changed by increased whole-grain intake: The WHOLEheart study, a randomised, controlled dietary intervention. Br. J. Nutr. 2010, 104, 125–134. [Google Scholar] [CrossRef]
- Ma, X.; Gu, J.; Zhang, Z.; Jing, L.; Xu, M.; Dai, X.; Jiang, Y.; Bao, L.; Cai, X.; Ding, Y.; et al. Effects of Avena nuda L. on metabolic control and cardiovascular disease risk among Chinese patients with diabetes and meeting metabolic syndrome criteria: Secondary analysis of a randomized clinical trial. Eur. J. Clin. Nutr. 2013, 67, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Ampatzoglou, A.; Williams, C.L.; Atwal, K.K.; Maidens, C.M.; Ross, A.B.; Thielecke, F.; Jonnalagadda, S.S.; Kennedy, O.B.; Yaqoob, P. Effects of increased wholegrain consumption on immune and inflammatory markers in healthy low habitual wholegrain consumers. Eur. J. Nutr. 2016, 55, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Tengblad, S.; Karlstrom, B.; Kamal-Eldin, A.; Landberg, R.; Basu, S.; Aman, P.; Vessby, B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J. Nutr. 2007, 137, 1401–1407. [Google Scholar] [CrossRef]
- Connolly, M.L.; Tzounis, X.; Tuohy, K.M.; Lovegrove, J.A. Hypocholesterolemic and prebiotic effects of a whole-grain oat-based granola breakfast cereal in a cardio-metabolic “at risk” population. Front. Microbiol. 2016, 7, 1675. [Google Scholar] [CrossRef] [Green Version]
- Giacco, R.; Lappi, J.; Costabile, G.; Kolehmainen, M.; Schwab, U.; Landberg, R.; Uusitupa, M.; Poutanen, K.; Pacini, G.; Rivellese, A.A.; et al. Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: A randomised controlled two-centre intervention study. Clin. Nutr. 2013, 32, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Harris Jackson, K.; West, S.G.; Vanden Heuvel, J.P.; Jonnalagadda, S.S.; Ross, A.B.; Hill, A.M.; Grieger, J.A.; Lemieux, S.K.; Kris-Etherton, P.M. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: A randomized controlled-feeding trial. Am. J. Clin. Nutr. 2014, 100, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Hoevenaars, F.P.M.; Esser, D.; Schutte, S.; Priebe, M.G.; Vonk, R.J.; van den Brink, W.J.; van der Kamp, J.W.; Stroeve, J.H.M.; Afman, L.A.; Wopereis, S. Whole grain wheat consumption affects postprandial inflammatory response in a randomized controlled trial in overweight and obese adults with mild hypercholesterolemia in the graandioos study. J. Nutr. 2019, 149, 2133–2144. [Google Scholar] [CrossRef]
- Iversen, K.N.; Carlsson, F.; Andersson, A.; Michaelsson, K.; Langton, M.; Riserus, U.; Hellstrom, P.M.; Landberg, R. A hypocaloric diet rich in high fiber rye foods causes greater reduction in body weight and body fat than a diet rich in refined wheat: A parallel randomized controlled trial in adults with overweight and obesity (the RyeWeight study). Clin. Nutr. ESPEN 2021, 45, 155–169. [Google Scholar] [CrossRef]
- Joo, N.; Han, S.; Kim, K.; Kim, B.; Park, S.; Yeum, K. Black rice with giant embryo ameliorates serum C-reactive protein in adults with metabolic syndrome. J. Clin. Biochem. Nutr. 2020, 67, 344–348. [Google Scholar] [CrossRef]
- Katcher, H.I.; Legro, R.S.; Kunselman, A.R.; Gillies, P.J.; Demers, L.M.; Bagshaw, D.M.; Kris-Etherton, P.M. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 79–90. [Google Scholar] [CrossRef]
- Kazemzadeh, M.; Safavi, S.M.; Nematollahi, S.; Nourieh, Z. Effect of brown rice consumption on inflammatory marker and cardiovascular risk factors among overweight and obese non-menopausal female adults. Int. J. Prev. Med. 2014, 5, 478–488. [Google Scholar] [PubMed]
- Kirwan, J.P.; Malin, S.K.; Scelsi, A.R.; Kullman, E.L.; Navaneethan, S.D.; Pagadala, M.R.; Haus, J.M.; Filion, J.; Godin, J.P.; Kochhar, S.; et al. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: A randomized controlled trial. J. Nutr. 2016, 146, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Morino, K.; Nishio, Y.; Ishikado, A.; Arima, H.; Nakao, K.; Nakagawa, F.; Nikami, F.; Sekine, O.; Nemoto, K.I.; et al. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: A randomized controlled trial. PLoS ONE 2017, 12, e0179869. [Google Scholar] [CrossRef]
- Kopf, J.C.; Suhr, M.J.; Clarke, J.; Eyun, S.I.; Riethoven, J.J.M.; Ramer-Tait, A.E.; Rose, D.J. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: A randomized controlled trial. Nutr. J. 2018, 17, 72. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lietz, G.; Bal, W.; Watson, A.; Morfey, B.; Seal, C. Effects of quinoa (Chenopodium quinoa Willd.) consumption on markers of CVD risk. Nutrients 2018, 10, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, V.S.; Sudha, V.; Wedick, N.M.; RamyaBai, M.; Vijayalakshmi, P.; Lakshmipriya, N.; Gayathri, R.; Kokila, A.; Jones, C.; Hong, B.; et al. Substituting brown rice for white rice on diabetes risk factors in India: A randomised controlled trial. Br. J. Nutr. 2019, 121, 1389–1397. [Google Scholar] [CrossRef]
- Meng, H.; Matthan, N.R.; Fried, S.K.; Berciano, S.; Walker, M.E.; Galluccio, J.M.; Lichtenstein, A.H. Effect of Dietary Carbohydrate Type on Serum Cardiometabolic Risk Indicators and Adipose Tissue Inflammatory Markers. J Clin. Endocrinol. Metab. 2018, 103, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Munch Roager, H.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrugger, S.; Maerkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frokiaer, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.L.; Tarkhan, A.; Shojaie, A.; Randolph, T.W.; Gu, H.; Djukovic, D.; Osterbauer, K.J.; Hullar, M.A.; Kratz, M.; Neuhouser, M.L.; et al. Plasma metabolomics profiles suggest beneficial effects of a low-glycemic load dietary pattern on inflammation and energy metabolism. Am. J. Clin. Nutr. 2019, 110, 984–992. [Google Scholar] [CrossRef]
- Pavadhgul, P.; Bumrungpert, A.; Harjani, Y.; Kurilich, A. Oat porridge consumption alleviates markers of inflammation and oxidative stress in hypercholesterolemic adults. Asia Pac. J. Clin. Nutr. 2019, 28, 260–265. [Google Scholar]
- Pavithran, N.; Kumar, H.; Menon, A.S.; Pillai, G.K.; Sundaram, K.R.; Ojo, O. South Indian cuisine with low glycemic index ingredients reduces cardiovascular risk factors in subjects with type 2 diabetes. Int. J. Environ. Res. Public Health 2020, 17, 6232. [Google Scholar] [CrossRef]
- Pourshahidi, L.K.; Caballero, E.; Osses, A.; Hyland, B.W.; Ternan, N.G.; Gill, C.I.R. Modest improvement in CVD risk markers in older adults following quinoa (Chenopodium quinoa Willd.) consumption: A randomized-controlled crossover study with a novel food product. Eur. J. Nutr. 2020, 59, 3313–3323. [Google Scholar] [CrossRef]
- Saglam, D.; Saka, M.; Sayaca, N. The effect of consumption of low glycemic index, high fat content bread on anthropometric measurement and cardiometabolic risk factors in women with type 2 diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2019, 39, 166–172. [Google Scholar] [CrossRef]
- Schutte, S.; Esser, D.; Hoevenaars, F.P.M.; Hooiveld, G.J.E.J.; Priebe, M.G.; Vonk, R.J.; Wopereis, S.; Afman, L.A. A 12-wk whole-grain wheat intervention protects against hepatic fat: The Graandioos study, a randomized trial in overweight subjects. Am. J. Clin. Nutr. 2018, 108, 1264–1274. [Google Scholar] [CrossRef] [Green Version]
- Tighe, P.; Duthie, G.; Vaughan, N.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Mutch, W.; Wahle, K.; Horgan, G.; Thies, F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 92, 733–740. [Google Scholar] [CrossRef]
- Vetrani, C.; Costabile, G.; Luongo, D.; Naviglio, D.; Rivellese, A.A.; Riccardi, G.; Giacco, R. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition 2016, 32, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittaker, A.; Sofi, F.; Luisi, M.L.; Rafanelli, E.; Fiorillo, C.; Becatti, M.; Abbate, R.; Casini, A.; Gensini, G.F.; Benedettelli, S. An organic khorasan wheat-based replacement diet improves risk profile of patients with acute coronary syndrome: A randomized crossover trial. Nutrients 2015, 7, 3401–3415. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, A.; Dinu, M.; Cesari, F.; Gori, A.M.; Fiorillo, C.; Becatti, M.; Casini, A.; Marcucci, R.; Benedettelli, S.; Sofi, F. A khorasan wheat-based replacement diet improves risk profile of patients with type 2 diabetes mellitus (T2DM): A randomized crossover trial. Eur. J. Nutr. 2017, 56, 1191–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamaratskaia, G.; Mhd Omar, N.A.; Brunius, C.; Hallmans, G.; Johansson, J.E.; Andersson, S.O.; Larsson, A.; Aman, P.; Landberg, R. Consumption of whole grain/bran rye instead of refined wheat decrease concentrations of TNF-R2, e-selectin, and endostatin in an exploratory study in men with prostate cancer. Clin. Nutr. 2020, 39, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Koh-Banerjee, P.; Hu, F.B.; Franz, M.; Sampson, L.; Grønbæk, M.; Rimm, E.B. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 2004, 80, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Beck, E.; Hughes, J.; Grafenauer, S. Whole grains and consumer understanding: Investigating consumers’ identification, knowledge and attitudes to whole grains. Nutrients 2020, 12, 2170. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Kamal-Eldin, A.; Andersson, A.; Vessby, B.; Åman, P. Alkylresorcinols as biomarkers of whole-grain wheat and rye intake: Plasma concentration and intake estimated from dietary records. Am. J. Clin. Nutr. 2008, 87, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.B.; Kristensen, M.; Seal, C.J.; Jacques, P.; McKeown, N.M. Recommendations for reporting whole-grain intake in observational and intervention studies. Am. J. Clin. Nutr. 2015, 101, 903–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galland, L. Diet and Inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of whole grain foods and risk of type 2 diabetes: Results from three prospective cohort studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef] [PubMed]
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Zielenkiewicz, M.; Paczek, L. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch. Immunol. Ther. Exp. 2016, 64, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, M.M.H.; Hughes, J.; Grafenauer, S. Whole grain intakes are associated with healthcare cost savings following reductions in risk of colorectal cancer and total cancer mortality in Australia: A cost-of-illness model. Nutrients 2021, 13, 2982. [Google Scholar] [CrossRef]
- Abdullah, M.M.H.; Hughes, J.; Grafenauer, S. Healthcare cost savings associated with increased whole grain consumption among Australian adults. Nutrients 2021, 13, 1855. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).