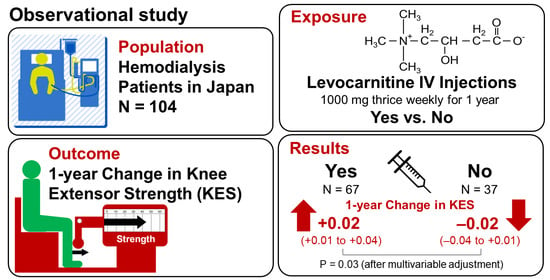
Association between Levocarnitine Treatment and the Change in Knee Extensor Strength in Patients Undergoing Hemodialysis: A Post-Hoc Analysis of the Osaka Dialysis Complication Study (ODCS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Treatment with Levocarnitine
2.4. Measurement of Muscle Strength
2.5. Other Variables
2.6. Statistical Analysis
3. Results
3.1. Selection of Study Participants
3.2. Clinical Characteristics of the Participants in 2014
3.3. Changes in Knee Extensor Strength during the Period before Levocarnitine Treatment
3.4. Changes in Knee Extensor Strength during the Period of Levocarnitine Treatment
3.5. Independent Association of Treatment with Levocarnitine with the Change in Knee Extensor Strength
3.6. Independent Association of Treatment with Levocarnitine with the Change in Handgrip Strength
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kutner, N.G.; Zhang, R.; Huang, Y.; Painter, P. Gait Speed and Mortality, Hospitalization, and Functional Status Change Among Hemodialysis Patients: A US Renal Data System Special Study. Am. J. Kidney Dis. 2015, 66, 297–304. [Google Scholar] [CrossRef]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Barany, P.; Heimburger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gong, D.; Jia, F.; Xu, B.; Liu, Z. Sarcopenia in patients undergoing maintenance hemodialysis: Incidence rate, risk factors and its effect on survival risk. Ren. Fail. 2016, 38, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nishide, K.; Okuno, S.; Shoji, T.; Emoto, M.; Tsuda, A.; Nakatani, S.; Imanishi, Y.; Ishimura, E.; Yamakawa, T.; et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Kittiskulnam, P.; Chertow, G.M.; Carrero, J.J.; Delgado, C.; Kaysen, G.A.; Johansen, K.L. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017, 92, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Hortegal, E.V.F.; Alves, J.; Santos, E.J.F.; Nunes, L.C.R.; Galvao, J.C.; Nunes, R.F.; Lula, D.A.; Carvalho, S.C.R.; Franca, A.; Santos, E.M.D.; et al. Sarcopenia and inflammation in patients undergoing hemodialysis. Nutr. Hosp. 2020, 37, 855–862. [Google Scholar] [CrossRef]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Carrero, J.J.; von Walden, F.; Ikizler, T.A.; Nader, G.A. Muscle wasting in end-stage renal disease promulgates premature death: Established, emerging and potential novel treatment strategies. Nephrol. Dial. Transplant. 2016, 31, 1070–1077. [Google Scholar] [CrossRef]
- Bremer, J. Carnitine--metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef]
- Matera, M.; Bellinghieri, G.; Costantino, G.; Santoro, D.; Calvani, M.; Savica, V. History of L-carnitine: Implications for renal disease. J. Ren. Nutr. 2003, 13, 2–14. [Google Scholar] [CrossRef]
- Evans, A.M.; Fornasini, G. Pharmacokinetics of L-carnitine. Clin. Pharmacokinet. 2003, 42, 941–967. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef]
- Choi, J.W.; Ohn, J.H.; Jung, H.S.; Park, Y.J.; Jang, H.C.; Chung, S.S.; Park, K.S. Carnitine induces autophagy and restores high-fat diet-induced mitochondrial dysfunction. Metabolism 2018, 78, 43–51. [Google Scholar] [CrossRef]
- Debska-Slizien, A.; Kawecka, A.; Wojnarowski, K.; Zadrony, D.; Kunicka, D.; Krol, E.; Lysiak-Szydlowska, W.; Rutkowski, B. Carnitine content in different muscles of patients receiving maintenance hemodialysis. J. Ren. Nutr. 2007, 17, 275–281. [Google Scholar] [CrossRef]
- Bellinghieri, G.; Santoro, D.; Calvani, M.; Mallamace, A.; Savica, V. Carnitine and hemodialysis. Am. J. Kidney Dis. 2003, 41, S116–S122. [Google Scholar] [CrossRef]
- Ahmad, S.; Robertson, H.T.; Golper, T.A.; Wolfson, M.; Kurtin, P.; Katz, L.A.; Hirschberg, R.; Nicora, R.; Ashbrook, D.W.; Kopple, J.D. Multicenter trial of L-carnitine in maintenance hemodialysis patients. II. Clinical and biochemical effects. Kidney Int. 1990, 38, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Koziol, B.J.; Shapiro, J.I.; Brass, E.P. Carnitine metabolism during exercise in patients on chronic hemodialysis. Kidney Int. 1992, 41, 1613–1619. [Google Scholar] [CrossRef][Green Version]
- Brass, E.P.; Adler, S.; Sietsema, K.E.; Hiatt, W.R.; Orlando, A.M.; Amato, A.; Investigators, C. Intravenous L-carnitine increases plasma carnitine, reduces fatigue, and may preserve exercise capacity in hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 1018–1028. [Google Scholar] [CrossRef]
- Bellinghieri, G.; Savica, V.; Mallamace, A.; Di Stefano, C.; Consolo, F.; Spagnoli, L.G.; Villaschi, S.; Palmieri, G.; Corsi, M.; Maccari, F. Correlation between increased serum and tissue L-carnitine levels and improved muscle symptoms in hemodialyzed patients. Am. J. Clin. Nutr. 1983, 38, 523–531. [Google Scholar] [CrossRef]
- Sakurauchi, Y.; Matsumoto, Y.; Shinzato, T.; Takai, I.; Nakamura, Y.; Sato, M.; Nakai, S.; Miwa, M.; Morita, H.; Miwa, T.; et al. Effects of L-carnitine supplementation on muscular symptoms in hemodialyzed patients. Am. J. Kidney Dis. 1998, 32, 258–264. [Google Scholar] [CrossRef]
- Lynch, K.E.; Feldman, H.I.; Berlin, J.A.; Flory, J.; Rowan, C.G.; Brunelli, S.M. Effects of L-carnitine on dialysis-related hypotension and muscle cramps: A meta-analysis. Am. J. Kidney Dis. 2008, 52, 962–971. [Google Scholar] [CrossRef]
- Giovenali, P.; Fenocchio, D.; Montanari, G.; Cancellotti, C.; D’Iddio, S.; Buoncristiani, U.; Pelagaggia, M.; Ribacchi, R. Selective trophic effect of L-carnitine in type I and IIa skeletal muscle fibers. Kidney Int. 1994, 46, 1616–1619. [Google Scholar] [CrossRef]
- Maruyama, T.; Maruyama, N.; Higuchi, T.; Nagura, C.; Takashima, H.; Kitai, M.; Utsunomiya, K.; Tei, R.; Furukawa, T.; Yamazaki, T.; et al. Efficacy of L-carnitine supplementation for improving lean body mass and physical function in patients on hemodialysis: A randomized controlled trial. Eur. J. Clin. Nutr. 2019, 73, 293–301. [Google Scholar] [CrossRef]
- Yano, J.; Kaida, Y.; Maeda, T.; Hashida, R.; Tonan, T.; Nagata, S.; Hazama, T.; Nakayama, Y.; Ito, S.; Kurokawa, Y.; et al. l-carnitine supplementation vs cycle ergometer exercise for physical activity and muscle status in hemodialysis patients: A randomized clinical trial. Ther. Apher. Dial. 2021, 25, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, T.; Avlund, K.; Suominen, H.; Schroll, M.; Frändin, K.; Pertti, E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin. Exp. Res. 2002, 14, 10–15. [Google Scholar] [PubMed]
- Shoji, T.; Fujii, H.; Mori, K.; Nakatani, S.; Nagata, Y.; Morioka, T.; Inaba, M.; Emoto, M. Associations of cardiovascular disease and blood pressure with cognition in hemodialysis patients: The Osaka Dialysis Complication Study. Nephrol. Dial. Transplant. 2021, gfab247. [Google Scholar] [CrossRef] [PubMed]
- Casciani, C.; Caruso, U.; Cravotto, E.; Corsi, M.; Maccari, F. Beneficial effects of L-carnitine in post-dialysis syndrome. Curr. Ther. Res. 1982, 32, 116–127. [Google Scholar]
- Matsufuji, S.; Shoji, T.; Yano, Y.; Tsujimoto, Y.; Kishimoto, H.; Tabata, T.; Emoto, M.; Inaba, M. Effect of chair stand exercise on activity of daily living: A randomized controlled trial in hemodialysis patients. J. Ren. Nutr. 2015, 25, 17–24. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Matsunaga, A.; Wang, G.; Yamamoto, S.; Kutsuna, T.; Ishii, A.; Abe, Y.; Yoneki, K.; Yoshida, A.; Takahira, N. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys. Ther. 2014, 94, 947–956. [Google Scholar] [CrossRef]
- Vogt, B.P.; Borges, M.C.C.; Goes, C.R.; Caramori, J.C.T. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin. Nutr. 2016, 35, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Frih, B.; Jaafar, H.; Mkacher, W.; Ben Salah, Z.; Hammami, M.; Frih, A. The Effect of Interdialytic Combined Resistance and Aerobic Exercise Training on Health Related Outcomes in Chronic Hemodialysis Patients: The Tunisian Randomized Controlled Study. Front. Physiol. 2017, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B209–B217. [Google Scholar] [CrossRef]
- Frontera, W.R.; Reid, K.F.; Phillips, E.M.; Krivickas, L.S.; Hughes, V.A.; Roubenoff, R.; Fielding, R.A. Muscle fiber size and function in elderly humans: A longitudinal study. J. Appl. Physiol. 2008, 105, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kiguchi, D.; Ninomiya, T.; Hirooka, M.; Abe, M.; Matsuura, B.; Hiasa, Y.; Michitaka, K. Can L-carnitine supplementation and exercise improve muscle complications in patients with liver cirrhosis who receive branched-chain amino acid supplementation? Eur. J. Gastroenterol. Hepatol. 2019, 31, 878–884. [Google Scholar] [CrossRef]
- Kono, K.; Nishida, Y.; Moriyama, Y.; Yabe, H.; Taoka, M.; Sato, T. Investigation of factors affecting the six-minute walk test results in hemodialysis patients. Ther. Apher. Dial. 2014, 18, 623–627. [Google Scholar] [CrossRef]
- Brevetti, G.; Chiariello, M.; Ferulano, G.; Policicchio, A.; Nevola, E.; Rossini, A.; Attisano, T.; Ambrosio, G.; Siliprandi, N.; Angelini, C. Increases in walking distance in patients with peripheral vascular disease treated with L-carnitine: A double-blind, cross-over study. Circulation 1988, 77, 767–773. [Google Scholar] [CrossRef]
- Vasiljevski, E.R.; Burns, J.; Bray, P.; Donlevy, G.; Mudge, A.J.; Jones, K.J.; Summers, M.A.; Biggin, A.; Munns, C.F.; McKay, M.J.; et al. L-carnitine supplementation for muscle weakness and fatigue in children with neurofibromatosis type 1: A Phase 2a clinical trial. Am. J. Med. Genet. A 2021, 185, 2976–2985. [Google Scholar] [CrossRef]
- Hey, P.; Gow, P.; Testro, A.G.; Apostolov, R.; Chapman, B.; Sinclair, M. Nutraceuticals for the treatment of sarcopenia in chronic liver disease. Clin. Nutr. ESPEN 2021, 41, 13–22. [Google Scholar] [CrossRef]
- Stefan, M.; Sharp, M.; Gheith, R.; Lowery, R.; Ottinger, C.; Wilson, J.; Durkee, S.; Bellamine, A. L-Carnitine Tartrate Supplementation for 5 Weeks Improves Exercise Recovery in Men and Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 3432. [Google Scholar] [CrossRef] [PubMed]



| Total Participants (N = 104) | Carnitine Group (N = 67) | Non-Carnitine Group (N = 37) | p Value | |
|---|---|---|---|---|
| Age (year) | 65 (58–71) | 64 (56–71) | 65 (58–72) | 0.66 |
| Female sex [N (%)] | 35 (33.6%) | 25 (37.3%) | 10 (27.0%) | 0.28 |
| Duration of hemodialysis (year) | 10 (4–17) | 11 (5–20) | 7 (4–14) | 0.14 |
| Diabetes mellitus [N (%)] | 29, (27.9%) | 22 (32.8%) | 7 (18.9%) | 0.12 |
| Prior cardiovascular disease [N (%)] | 22 (21.1%) | 16 (23.9%) | 6 (16.2%) | 0.35 |
| Body weight (kg) | 56.0 (50.0–63.1) | 55.5 (50.0–63.0) | 57.4 (49.3–64.0) | 0.51 |
| Body mass index (kg/m2) | 21.3 (19.3–23.0) | 21.0 (19.2–22.6) | 21.3 (18.9–23.6) | 0.77 |
| Hemoglobin (g/dL) | 10.9 (10.4–11.6) | 10.8 (10.4–11.5) | 11.2 (10.5–11.6) | 0.39 |
| Serum albumin (g/dL) | 3.9 (3.8–4.1) | 3.9 (3.8–4.1) | 3.9 (3.7–4.1) | 0.59 |
| C-reactive protein (mg/dL) | 0.10 (0.10–0.19) | 0.10 (0.10–0.19) | 0.10 (0.10–0.22) | 0.71 |
| Total carnitine (μmol/L) | 37.3 (31.5–46.8) | 36.5 (31.3–45.7) | 76.1 (50.4–122.0) | 0.004 |
| Free carnitine (μmol/L) | 20.7 (16.0–24.9) | 20.4 (15.8–23.1) | 44.2 (31.4–72.2) | 0.001 |
| Acyl carnitine (μmol/L) | 17.1 (14.2–21.5) | 17.0 (14.3–20.1) | 31.9 (19.0–49.8) | 0.02 |
| Acy/free carnitine ratio | 0.86 (0.71–1.02) | 0.87 (0.73–1.03) | 0.61 (0.56–0.81) | 0.03 |
| Handgrip strength (kg) | 25.4 (19.7–31.9) | 23.9 (18.7–30.4) | 27.3 (23.8–33.4) | 0.01 |
| Knee extensor strength (kgf/kg) | 0.43 (0.36–0.49) | 0.41 (0.33–0.48) | 0.44 (0.38–0.56) | 0.09 |
| Exposure Variables | Outcome Variables | |||||
|---|---|---|---|---|---|---|
| Change in Knee Extensor Strength from 2013 to 2014 | Change in Knee Extensor Strength from 2014 to 2015 | |||||
| Coefficient (95% CI) | p Value | Std. Coefficient | Coefficient (95% CI) | p Value | Std. Coefficient | |
| Age (year) | 0.0004 (−0.003 to 0.004) | 0.81 | 0.03 | −0.001 (−0.003 to 0.002) | 0.06 | −0.19 |
| Sex (female = 0, male = 1) | −0.01 (−0.05 to 0.02) | 0.48 | −0.07 | −0.01 (−0.024 to 0.008) | 0.31 | −0.10 |
| Duration of hemodialysis (year) | 0.002 (−0.002 to 0.007) | 0.23 | 0.13 | −0.001 (−0.003 to 0.001) | 0.32 | −0.10 |
| Diabetes mellitus (yes = 1, no = 0) | 0.01 (−0.03 to 0.05) | 0.53 | 0.07 | −0.006 (−0.024 to 0.011) | 0.49 | −0.07 |
| Prior cardiovascular disease (yes = 1, no = 0) | −0.004 (−0.05 to 0.04) | 0.86 | −0.02 | 0.02 (−0.003 to 0.03) | 0.10 | 0.15 |
| Knee extensor strength at the beginning of the period (kgf/kg) | –0.44 (−0.71 to −0.17) | 0.002 | −0.35 | −0.19 (−0.32 to −0.07) | 0.003 | −0.31 |
| Group (Carnitine = 1, Non-carnitine = 0) | −0.01 (−0.05 to 0.02) | 0.43 | −0.08 | 0.02 (0.002 to 0.03) | 0.03 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsufuji, S.; Shoji, T.; Lee, S.; Yamaguchi, M.; Nishimura, M.; Tsujimoto, Y.; Nakatani, S.; Morioka, T.; Mori, K.; Emoto, M. Association between Levocarnitine Treatment and the Change in Knee Extensor Strength in Patients Undergoing Hemodialysis: A Post-Hoc Analysis of the Osaka Dialysis Complication Study (ODCS). Nutrients 2022, 14, 343. https://doi.org/10.3390/nu14020343
Matsufuji S, Shoji T, Lee S, Yamaguchi M, Nishimura M, Tsujimoto Y, Nakatani S, Morioka T, Mori K, Emoto M. Association between Levocarnitine Treatment and the Change in Knee Extensor Strength in Patients Undergoing Hemodialysis: A Post-Hoc Analysis of the Osaka Dialysis Complication Study (ODCS). Nutrients. 2022; 14(2):343. https://doi.org/10.3390/nu14020343
Chicago/Turabian StyleMatsufuji, Shota, Tetsuo Shoji, Suhye Lee, Masao Yamaguchi, Mari Nishimura, Yoshihiro Tsujimoto, Shinya Nakatani, Tomoaki Morioka, Katsuhito Mori, and Masanori Emoto. 2022. "Association between Levocarnitine Treatment and the Change in Knee Extensor Strength in Patients Undergoing Hemodialysis: A Post-Hoc Analysis of the Osaka Dialysis Complication Study (ODCS)" Nutrients 14, no. 2: 343. https://doi.org/10.3390/nu14020343
APA StyleMatsufuji, S., Shoji, T., Lee, S., Yamaguchi, M., Nishimura, M., Tsujimoto, Y., Nakatani, S., Morioka, T., Mori, K., & Emoto, M. (2022). Association between Levocarnitine Treatment and the Change in Knee Extensor Strength in Patients Undergoing Hemodialysis: A Post-Hoc Analysis of the Osaka Dialysis Complication Study (ODCS). Nutrients, 14(2), 343. https://doi.org/10.3390/nu14020343