Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Study Procedures
2.4. Fecal Sampling and Analysis
2.5. Statistical Analysis
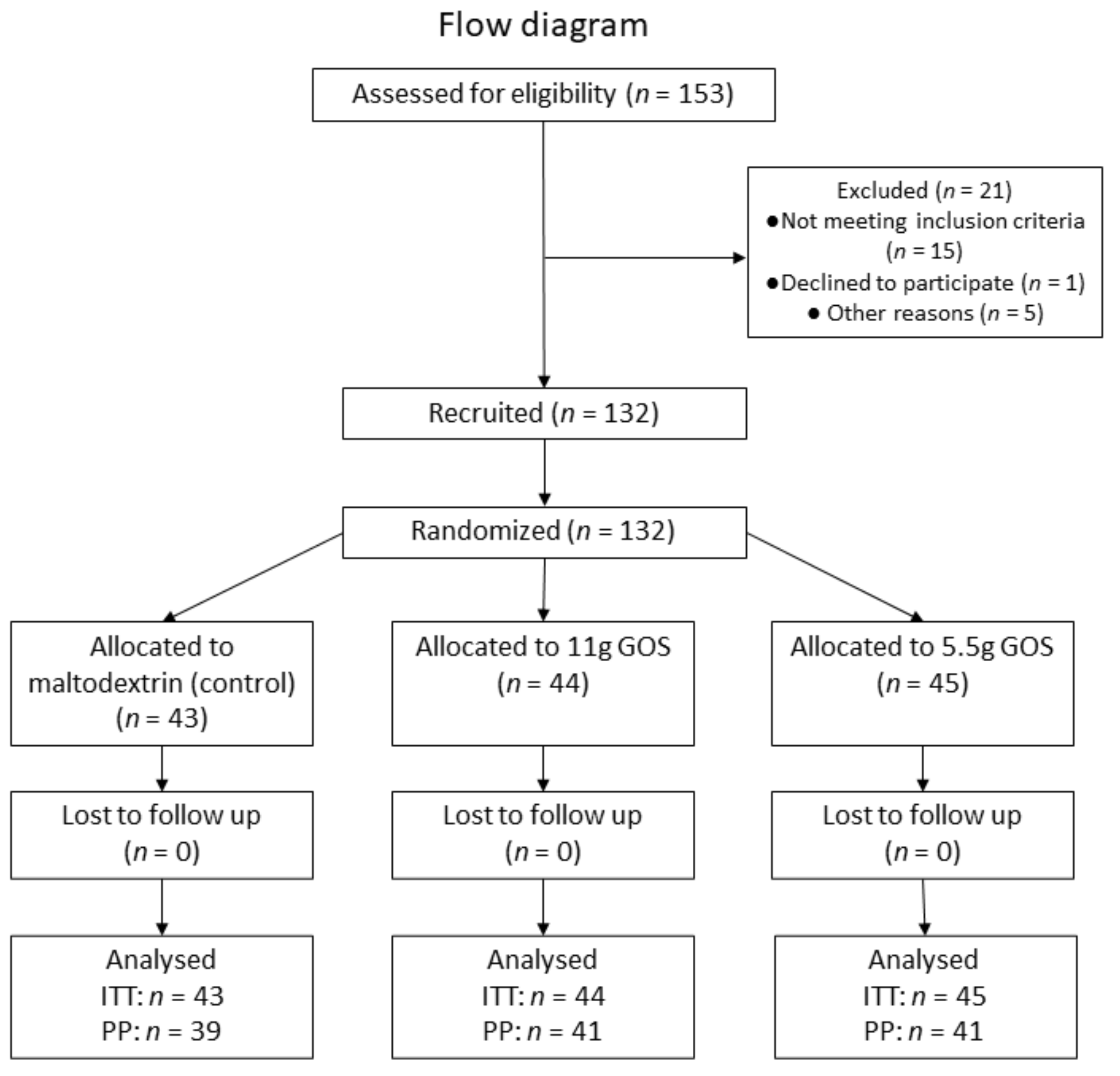
3. Results
3.1. Baseline Characteristics
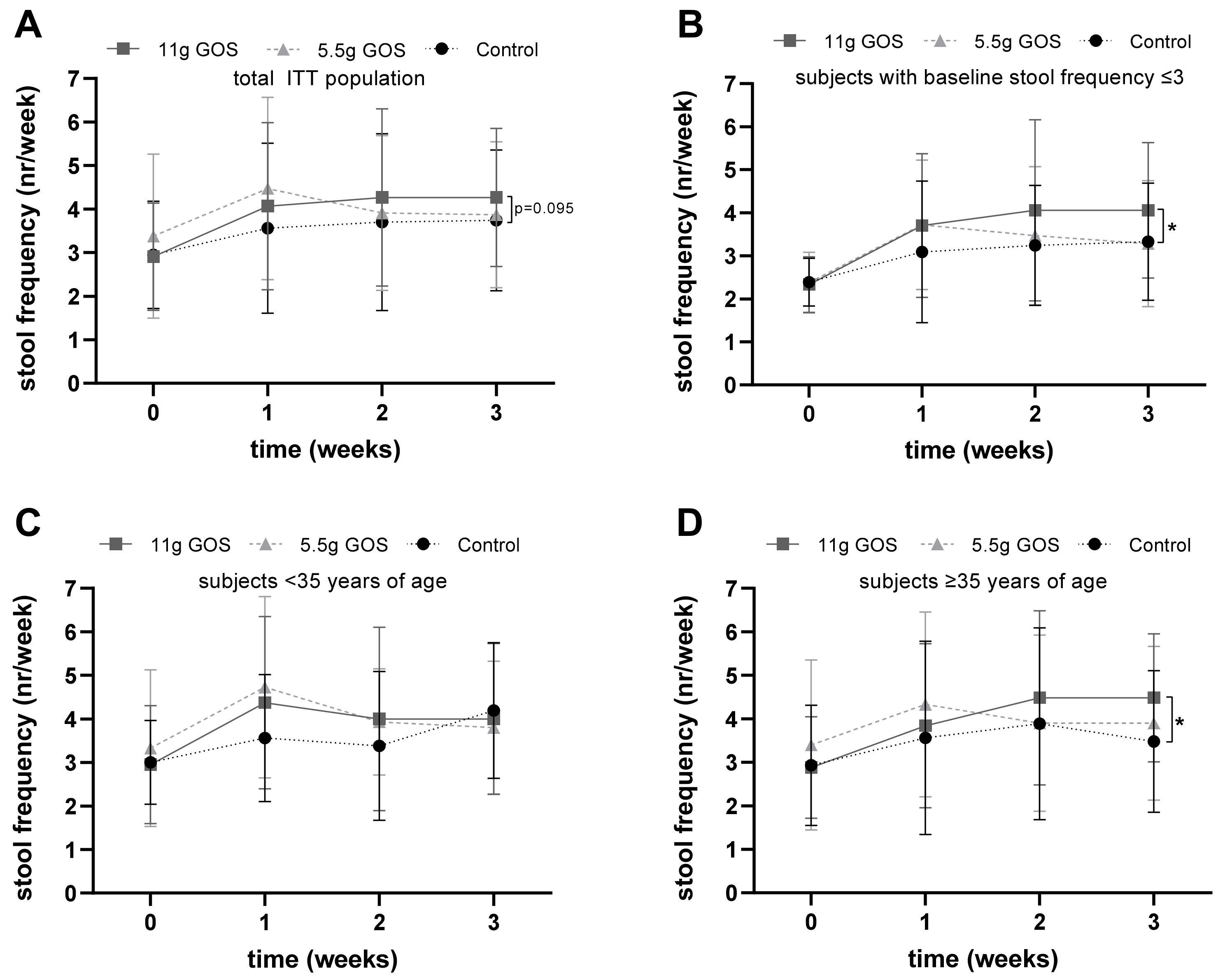
3.2. Stool Characteristics
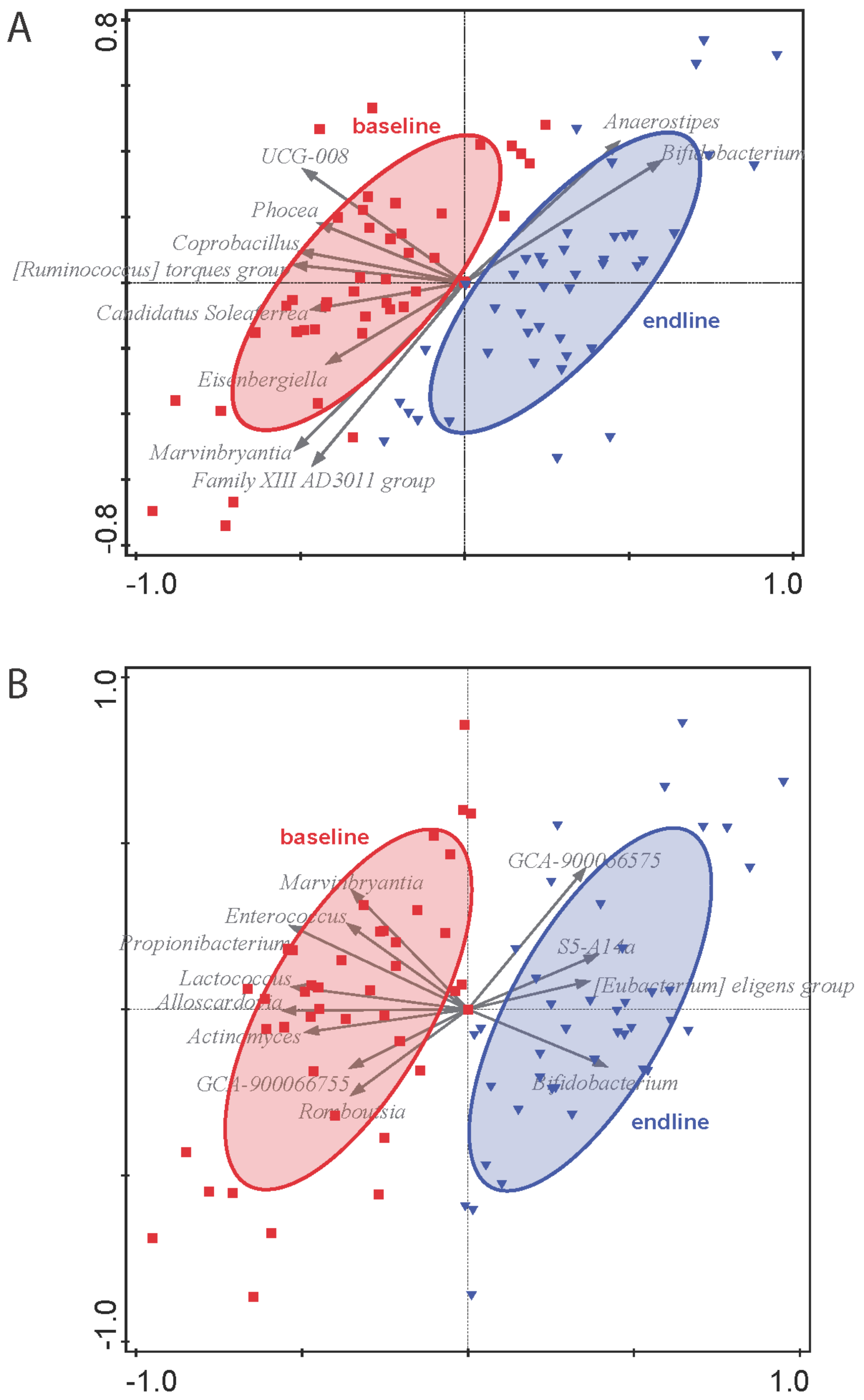
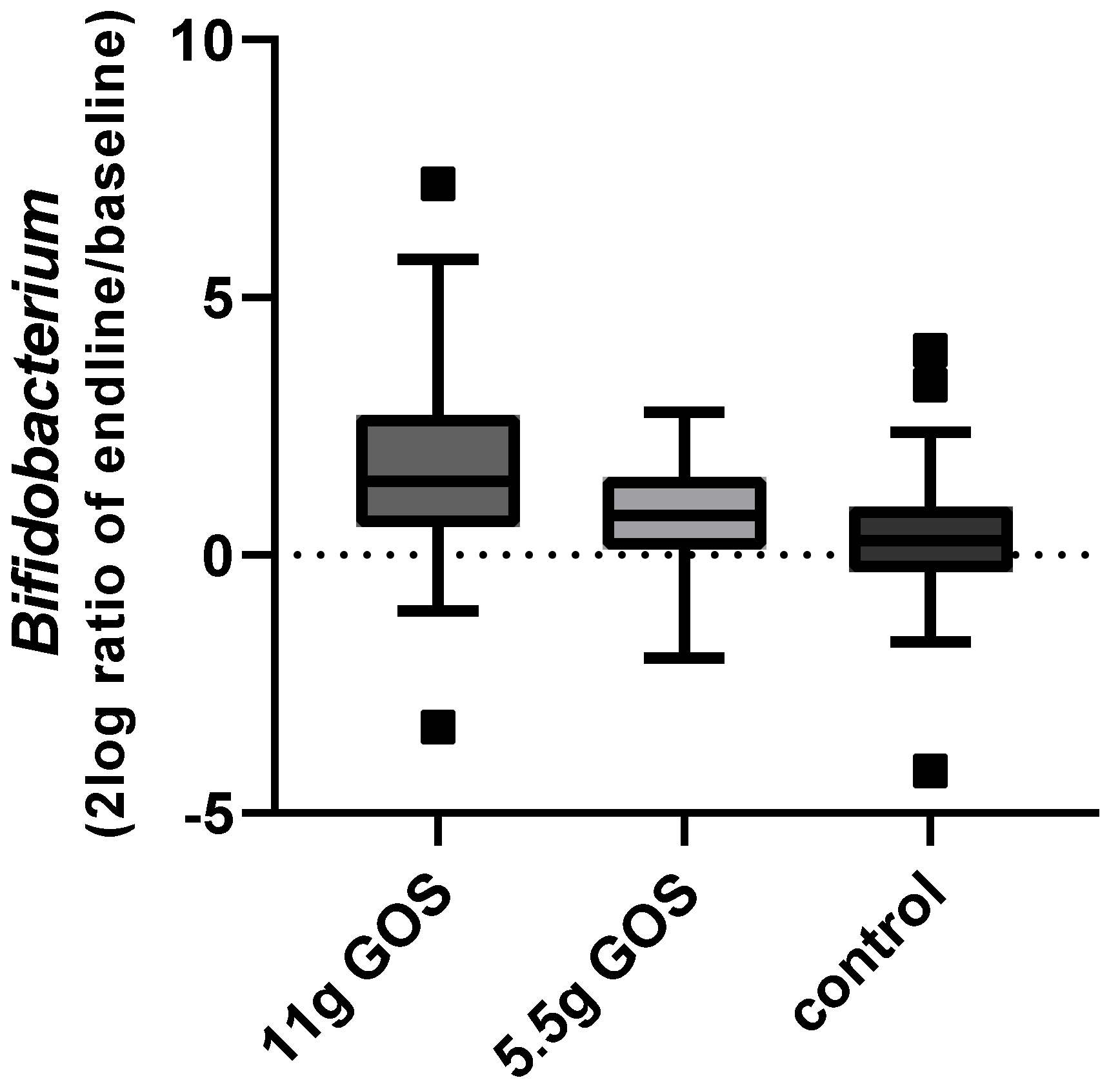
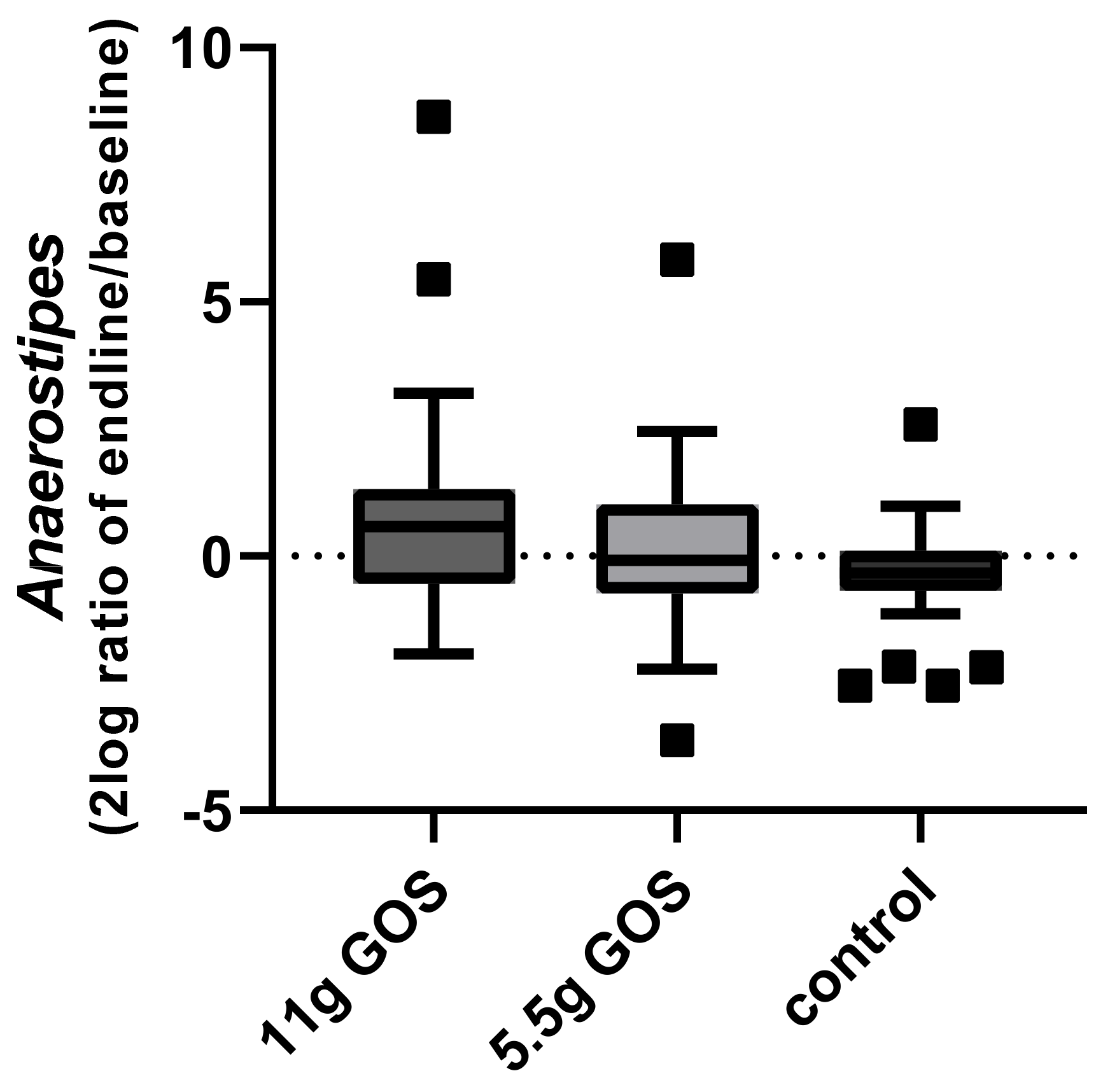
3.3. Fecal Microbiota
4. Discussion
Strengths and Limitations of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serra, J.; Pohl, D.; Azpiroz, F.; Chiarioni, G.; Ducrotté, P.; Gourcerol, G.; Hungin, A.P.S.; Layer, P.; Mendive, J.-M.; Pfeifer, J.; et al. European Society of Neurogastroenterology and Motility Guidelines on Functional Constipation in Adults. Neurogastroenterol. Motil. 2020, 32, e13762. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Rattanakovit, K.; Patcharatrakul, T. Diagnosis and Management of Chronic Constipation in Adults. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Jani, B.; Marsicano, E. Constipation: Evaluation and Management. Mo. Med. 2018, 115, 236–240. [Google Scholar]
- Higgins, P.D.R.; Johanson, J.F. Epidemiology of Constipation in North America: A Systematic Review. Am. J. Gastroenterol. 2004, 99, 750–759. [Google Scholar] [CrossRef]
- Sanchez, M.I.P.; Bercik, P. Epidemiology and Burden of Chronic Constipation. Can. J. Gastroenterol. 2011, 25, 11B–15B. [Google Scholar] [CrossRef]
- Belsey, J.; Greenfield, S.; Candy, D.; Geraint, M. Systematic Review: Impact of Constipation on Quality of Life in Adults and Children. Aliment. Pharmacol. Ther. 2010, 31, 938–949. [Google Scholar] [CrossRef] [Green Version]
- Tack, J.; Müller-Lissner, S.; Stanghellini, V.; Boeckxstaens, G.; Kamm, M.A.; Simren, M.; Galmiche, J.-P.; Fried, M. Diagnosis and Treatment of Chronic Constipation—A European Perspective. Neurogastroenterol. Motil. 2011, 23, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Wald, A.; Scarpignato, C.; Mueller-Lissner, S.; Kamm, M.A.; Hinkel, U.; Helfrich, I.; Schuijt, C.; Mandel, K.G. A Multinational Survey of Prevalence and Patterns of Laxative Use among Adults with Self-Defined Constipation. Aliment. Pharmacol. Ther. 2008, 28, 917–930. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.T.; Poorsaadati, S.; Radkani, B.; Forootan, M. Psychological Disorders in Patients with Chronic Constipation. Gastroenterol. Hepatol. Bed Bench 2011, 4, 159–163. [Google Scholar]
- Yu, T.; Zheng, Y.-P.; Tan, J.-C.; Xiong, W.-J.; Wang, Y.; Lin, L. Effects of Prebiotics and Synbiotics on Functional Constipation. Am. J. Med. Sci. 2017, 353, 282–292. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef]
- Watson, D.; O’Connell Motherway, M.; Schoterman, M.H.C.; van Neerven, R.J.J.; Nauta, A.; van Sinderen, D. Selective Carbohydrate Utilization by Lactobacilli and Bifidobacteria. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K. Probiotic Bifidobacterium Strains and Galactooligosaccharides Improve Intestinal Barrier Function in Obese Adults but Show No Synergism When Used Together as Synbiotics. Microbiome 2018, 6, 1–16. [Google Scholar] [CrossRef]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in Irritable Bowel Syndrome and Other Functional Bowel Disorders in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef]
- Teuri, U.; Korpela, R. Galacto-Oligosaccharides Relieve Constipation in Elderly People. Ann. Nutr. Metab. 1998, 42, 319–327. [Google Scholar] [CrossRef]
- Sairanen, U.; Piirainen, L.; Nevala, R.; Korpela, R. Yoghurt Containing Galacto-Oligosaccharides, Prunes and Linseed Reduces the Severity of Mild Constipation in Elderly Subjects. Eur. J. Clin. Nutr. 2007, 61, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Ladirat, S.E.; Schoterman, M.H.C.; Rahaoui, H.; Mars, M.; Schuren, F.H.J.; Gruppen, H.; Nauta, A.; Schols, H.A. Exploring the Effects of Galacto-Oligosaccharides on the Gut Microbiota of Healthy Adults Receiving Amoxicillin Treatment. Br. J. Nutr. 2014, 11, 536–546. [Google Scholar] [CrossRef]
- Surakka, A.; Kajander, K.; Rajilić-Stojanović, M.; Karjalainen, H.; Hatakka, K.; Vapaatalo, H.; Zoetendal, E.G.; de Vos, W.M.; Korpela, R.; Tynkkynen, S. Yoghurt Containing Galactooligosaccharides Facilitates Defecation among Elderly Subjects and Selectively Increases the Number of Bifidobacteria. Int. J. Probiot. Prebiot. 2009, 4, 65–74. [Google Scholar]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalif, I.L.; Quigley, E.M.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the Colonic Flora and Intestinal Permeability and Evidence of Immune Activation in Chronic Constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional Dysbiosis within the Gut Microbiota of Patients with Constipated-Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients with Chronic Constipation. Gastroenterology 2016, 150, 367–379.e1. [Google Scholar] [CrossRef] [Green Version]
- Walton, G.E.; van den Heuvel, E.G.H.M.; Kosters, M.H.W.; Rastall, R.A.; Tuohy, K.M.; Gibson, G.R. A Randomised Crossover Study Investigating the Effects of Galacto-Oligosaccharides on the Faecal Microbiota in Men and Women over 50 Years of Age. Br. J. Nutr. 2012, 107, 1466–1475. [Google Scholar] [CrossRef]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A Constipation Scoring System to Simplify Evaluation and Management of Constipated Patients. Dis. Colon Rectum 1996, 39, 681–685. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Gommers, L.M.M.; Ederveen, T.H.A.; van der Wijst, J.; Overmars-Bos, C.; Kortman, G.A.M.; Boekhorst, J.; Bindels, R.J.M.; de Baaij, J.H.F.; Hoenderop, J.G.J. Low Gut Microbiota Diversity and Dietary Magnesium Intake Are Associated with the Development of PPI-Induced Hypomagnesemia. FASEB J. 2019, 33, 11235–11246. [Google Scholar] [CrossRef] [Green Version]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic Galacto-Oligosaccharides Mitigate the Adverse Effects of Iron Fortification on the Gut Microbiome: A Randomised Controlled Study in Kenyan Infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.A.; Kjellström, L.; Nyhlin, H.; Talley, N.J.; Agréus, L. Assessment of Normal Bowel Habits in the General Adult Population: The Popcol Study. Scand. J. Gastroenterol. 2010, 45, 556–566. [Google Scholar] [CrossRef]
- McCrea, G.L.; Miaskowski, C.; Stotts, N.A.; Macera, L.; Paul, S.M.; Varma, M.G. Gender Differences in Self-Reported Constipation Characteristics, Symptoms, and Bowel and Dietary Habits among Patients Attending a Specialty Clinic for Constipation. Gend. Med. 2009, 6, 259–271. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Sanchez-Carrillo, S.; Ciordia, S.; Mena, M.C.; Méndez-García, C.; Rojo, D.; Bargiela, R.; Zubeldia-Varela, E.; Martínez-Martínez, M.; Barbas, C.; et al. Functional Microbiome Deficits Associated with Ageing: Chronological Age Threshold. Aging Cell 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; de Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Grimm, V.; Westermann, C.; Riedel, C.U. Bifidobacteria-Host Interactions-An Update on Colonisation Factors. BioMed Res. Int. 2014, 2014, 960826. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.I.; Martiny, J.B.H. Evolutionary Relationships among Bifidobacteria and Their Hosts and Environments. BMC Genom. 2020, 21, 26. [Google Scholar] [CrossRef] [Green Version]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial Degradation of Complex Carbohydrates in the Gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Kant, R.; Rasinkangas, P.; Satokari, R.; Pietilä, T.E.; Palva, A. Genome Sequence of the Butyrate-Producing Anaerobic Bacterium Anaerostipes Hadrus PEL 85. Genome Announc. 2016, 3, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Ai, D.; Pan, H.; Li, X.; Gao, Y.; Liu, G.; Xia, L.C. Identifying Gut Microbiota Associated with Colorectal Cancer Using a Zero-Inflated Lognormal Model. Front. Microbiol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Lyra, A.; Rinttilä, T.; Nikkilä, J.; Krogius-Kurikka, L.; Kajander, K.; Malinen, E.; Mättö, J.; Mäkelä, L.; Palva, A. Diarrhoea-Predominant Irritable Bowel Syndrome Distinguishable by 16S RRNA Gene Phylotype Quantification. World J. Gastroenterol. 2009, 15, 5936–5945. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic Inulin-Type Fructans Induce Specific Changes in the Human Gut Microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [Green Version]
- Yarullina, D.R.; Shafigullin, M.U.; Sakulin, K.A.; Arzamastseva, A.A.; Shaidullov, I.F.; Markelova, M.I.; Grigoryeva, T.V.; Karpukhin, O.Y.; Sitdikova, G.F. Characterization of Gut Contractility and Microbiota in Patients with Severe Chronic Constipation. PLoS ONE 2020, 15, e0235985. [Google Scholar] [CrossRef]
- Wilms, E.; An, R.; Smolinska, A.; Stevens, Y.; Weseler, A.R.; Elizalde, M.; Drittij, M.-J.; Ioannou, A.; van Schooten, F.J.; Smidt, H.; et al. Galacto-Oligosaccharides Supplementation in Prefrail Older and Healthy Adults Increased Faecal Bifidobacteria, but Did Not Impact Immune Function and Oxidative Stress. Clin. Nutr. 2021, 40, 3019–3031. [Google Scholar] [CrossRef]
- Sakata, T. Pitfalls in Short-Chain Fatty Acid Research: A Methodological Review. Anim. Sci. J. 2019, 90, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, D.; Basseri, R.J.; Makhani, M.D.; Chong, K.; Chang, C.; Pimentel, M. Methane on Breath Testing Is Associated with Constipation: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2011, 56, 1612–1618. [Google Scholar] [CrossRef]
- Chatterjee, S.; Park, S.; Low, K.; Kong, Y.; Pimentel, M. The Degree of Breath Methane Production in IBS Correlates with the Severity of Constipation. Am. J. Gastroenterol. 2007, 102, 837–841. [Google Scholar] [CrossRef]
- Shah, E.; Pimentel, M. Placebo Effect in Clinical Trial Design for Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2014, 20, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Moayyedi, P. Meta-Analysis: Factors Affecting Placebo Response Rate in the Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2010, 32, 144–158. [Google Scholar] [CrossRef] [Green Version]

| Control | 11 g GOS * | 5.5 g GOS * | |
|---|---|---|---|
| Amount (g) | 15.1 | 15.1 | 7.5 |
| Energy (kcal) | 58.3 | 32.2 | 22.0 |
| Fat (g) | 0 | 0 | 0 |
| Carbohydrates (g) | 14.5 | 14.6 | 7.3 |
| Maltodextrin (g) | 13.6 | - | - |
| Galacto-oligosaccharides, (g) (≥DP2) ** | 0 | 10.4 | 5.2 |
| Lactose (g) | 0.9 | 3.5 | 1.7 |
| Glucose (g) | 0.6 | 0.3 | |
| Galactose (g) | 0.06 | 0.03 | |
| Protein (g) | <0.1 | 0 | 0 |
| Dietary fiber (g) (≥DP3) * | 0 | 7.2 | 3.6 |
| Salt (g) | <0.005 | 0 | 0 |
| Control | 11 g GOS | 5.5 g GOS | |
|---|---|---|---|
| n | 43 | 44 | 45 |
| Gender (% female) | 93.0% | 90.9% | 97.8% |
| Age (years) | 38.8 ± 13.3 | 37.3 ± 11.5 | 38.9 ± 11.8 |
| BMI (kg/m2) | 24.4 ± 2.2 | 24.2 ± 2.8 | 24.8 ± 2.8 |
| Weight (kg) | 70.8 ± 6.9 | 70.5 ± 9.8 | 72.0 ± 10.8 |
| Waist circumference | 86.5 ± 7.9 | 84.1 ± 9.6 | 86.8 ± 8.9 |
| Hip circumference | 103.3 ± 4.7 | 102.0 ± 6.8 | 103.4 ± 7.2 |
| Fiber intake category (% low) | 58.1% | 59.1% | 60.0% |
| Stool frequency (bowel movements/week) | 3.0 ± 1.2 | 2.9 ± 1.2 | 3.4 ± 1.9 |
| Stool consistency (BSS score) | 2.7 ± 1.3 | 2.7 ± 1.2 | 2.8 ± 1.3 |
| 11 g GOS | 5.5 g GOS | Control | p-Value 11 g GOS vs. Control | p-Value 5.5 g GOS vs. Control | |
|---|---|---|---|---|---|
| n | 43 | 45 | 42 | ||
| Baseline | 2.7 ± 1.2 | 2.8 ± 1.3 | 2.7 ± 1.3 | 0.791 | 0.666 |
| Week 1 | 3.3 ± 1.4 | 2.8 ± 1.1 | 3.1 ± 1.4 | 0.395 | 0.315 |
| Week 2 | 3.0 ± 1.2 | 2.9 ± 1.2 | 2.7 ± 1.1 | 0.133 | 0.411 |
| Week 3 | 3.1 ± 1.1 | 2.8 ± 1.3 | 2.9 ± 1.3 | 0.371 | 0.621 |
| 11 g GOS | 5.5 g GOS | Control | p-Value | |
|---|---|---|---|---|
| Initial responders | 80.5% (n = 33) a | 65.9% (n = 27) a,b | 48.7% (n = 19) b | 0.012 |
| Consistent responders | 63.4% (n = 26) a | 43.9% (n = 18) a,b | 35.9% (n = 14) b | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoemaker, M.H.; Hageman, J.H.J.; ten Haaf, D.; Hartog, A.; Scholtens, P.A.M.J.; Boekhorst, J.; Nauta, A.; Bos, R. Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial. Nutrients 2022, 14, 309. https://doi.org/10.3390/nu14020309
Schoemaker MH, Hageman JHJ, ten Haaf D, Hartog A, Scholtens PAMJ, Boekhorst J, Nauta A, Bos R. Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial. Nutrients. 2022; 14(2):309. https://doi.org/10.3390/nu14020309
Chicago/Turabian StyleSchoemaker, Marieke H., Jeske H. J. Hageman, Dominique ten Haaf, Anita Hartog, Petra A. M. J. Scholtens, Jos Boekhorst, Arjen Nauta, and Rolf Bos. 2022. "Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial" Nutrients 14, no. 2: 309. https://doi.org/10.3390/nu14020309
APA StyleSchoemaker, M. H., Hageman, J. H. J., ten Haaf, D., Hartog, A., Scholtens, P. A. M. J., Boekhorst, J., Nauta, A., & Bos, R. (2022). Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial. Nutrients, 14(2), 309. https://doi.org/10.3390/nu14020309






