Prescription, Compliance, and Burden Associated with Salt-Restricted Diets in Heart Failure Patients: Results from the French National OFICSel Observatory
Abstract
:1. Introduction
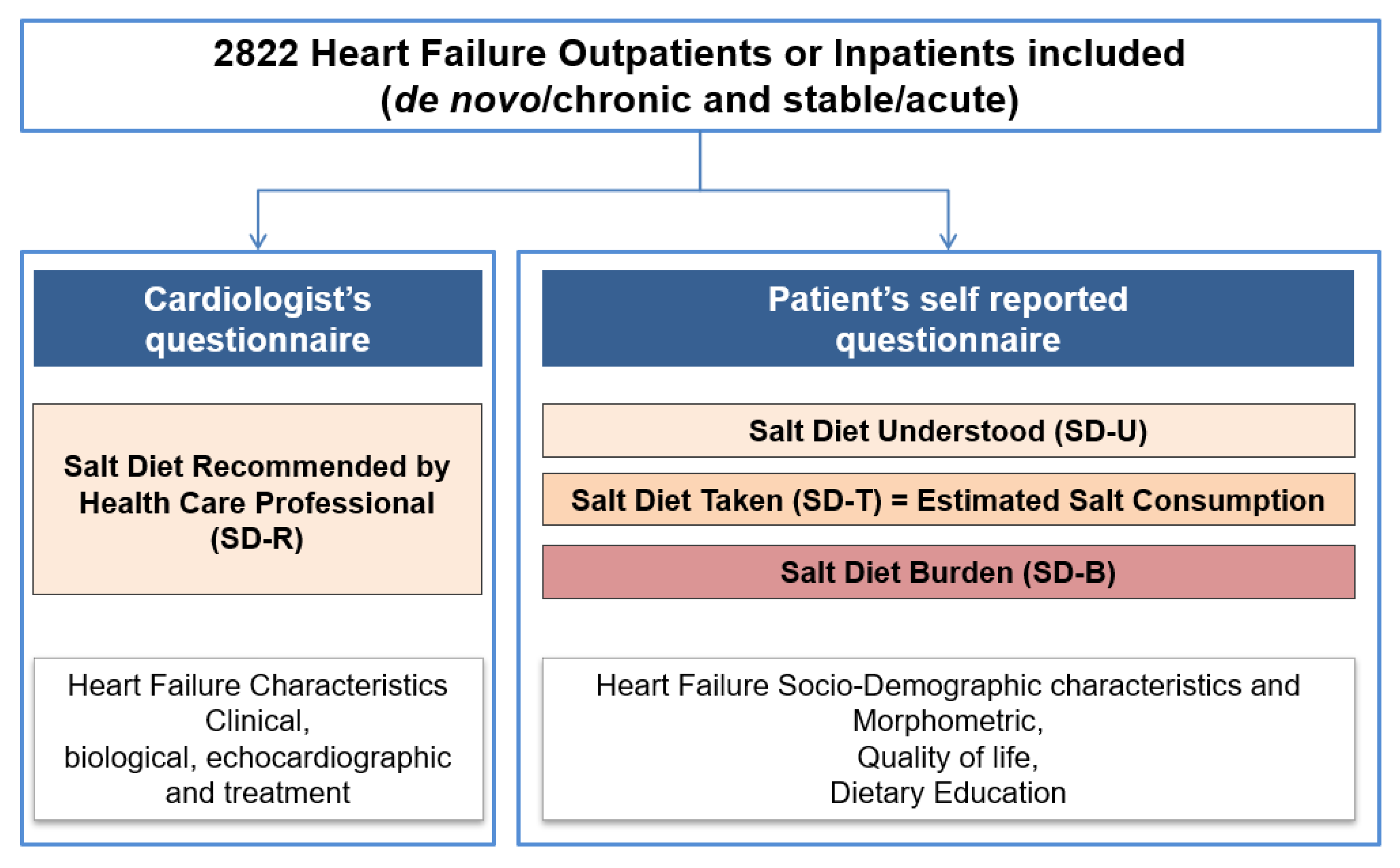
2. Method
2.1. Study Design
2.2. Data Collection
2.3. Study Objectives and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Heart Failure Patients
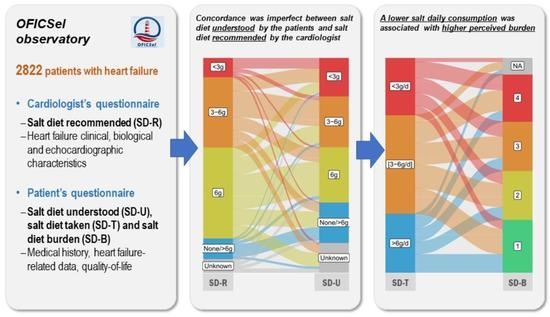
3.2. Diets Recommended (SD-R) and Understood (SD-U)
3.3. Estimation of Salt Consumption (SD-T) and Dietary Compliance
3.4. Burden (SD-B) and Quality of Life Associated with Adopting a Low-Salt Diet
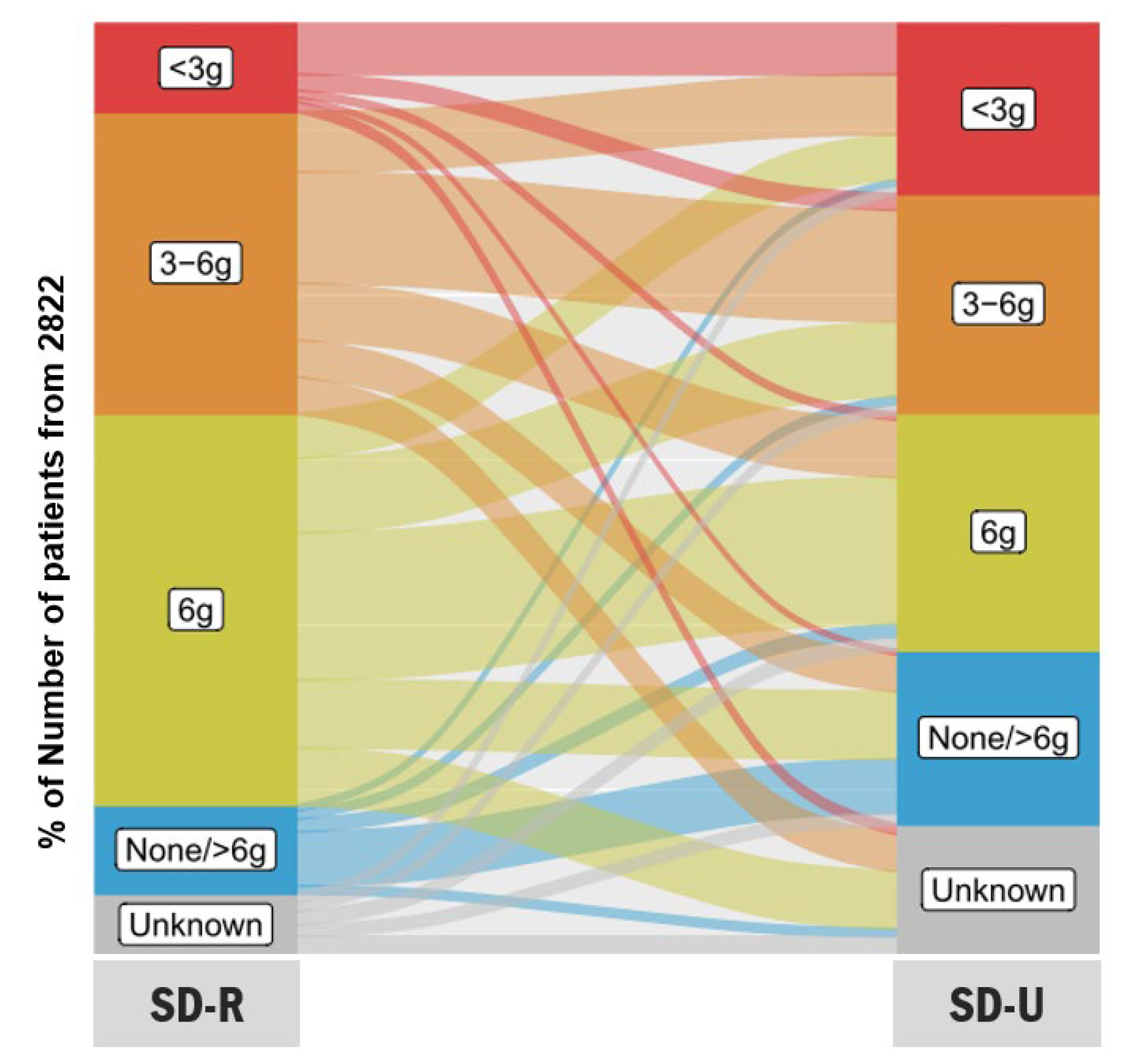
3.5. Concordance between Salt Diet Recommended (SD-R) and Salt Diet Understood (SD-U)
3.6. Univariate and Multivariate Analyses Identifying Determinants of Daily Estimated Salt Consumption (SD-T)
3.7. Univariate and Multivariate Analysis Identifying Determinants of Patients’ Burden Associated with Salt Diet Understood (SD-U), According to BIRD Scores
3.8. Relatedness of the Daily Estimated Salt Consumption (SD-T) with Perceived Burden Stratifying by Compliance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Anker, S.D.; Alhabib, K.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Colin-Ramirez, E.; Ezekowitz, J. Salt in the diet in patients with heart failure. Curr. Opin. Cardiol. 2016, 31, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Estep, J.D. Economic impact of chronic heart failure management in today’s cost-conscious environment. Card. Electrophysiol. Clin. 2019, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Shah, N.D.; Shi, Q.; Morlan, B.; VanHouten, H.; Long, K.H.; Roger, V.L. Lifetime costs of medical care after heart failure diagnosis. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, E.C.; Arcand, J.; Woo, E.; Brum, M.; Morgan, K.; Christopher, W.; Velázquez, L.; Sharifzad, A.; Feeney, S.; Ezekowitz, J.A. Design and region-specific adaptation of the dietary intervention used in the sodium-hf trial: A multicentre study. CJC Open 2019, 2, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Spineti, P.P.D.M. Evaluating sodium restriction in heart failure. Arq. Bras. Cardiol. 2019, 112, 171–172. [Google Scholar] [CrossRef]
- Khan, M.S.; Jones, D.W.; Butler, J. Salt, no salt, or less salt for patients with heart failure? Am. J. Med. 2020, 133, 32–38. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 comprehensive update of the canadian cardiovascular society guidelines for the management of heart failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, G.; Di Pasquale, P.; Licata, G.; Torres, D.; Giammanco, M.; Fasullo, S.; Mezzero, M.; Paterna, S. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J. Card. Fail. 2009, 15, 864–873. [Google Scholar] [CrossRef]
- Paterna, S.; Parrinello, G.; Cannizzaro, S.; Fasullo, S.; Torres, D.; Sarullo, F.; Di Pasquale, P. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am. J. Cardiol. 2009, 103, 93–102. [Google Scholar] [CrossRef]
- Paterna, S.; Gaspare, P.; Fasullo, S.; Sarullo, F.; Di Pasquale, P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: Is sodium an old enemy or a new friend? Clin. Sci. 2008, 114, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Georgiopoulou, V.V.; Kalogeropoulos, A.; Dunbar, S.B.; Reilly, C.; Sands, J.M.; Fonarow, G.; Jessup, M.; Gheorghiade, M.; Yancy, C.; et al. Dietary sodium intake in heart failure. Circulation 2012, 126, 479–485. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Atherton, J.J.; Sindone, A.; De Pasquale, C.; Driscoll, A.; MacDonald, P.S.; Hopper, I.; Kistler, P.; Briffa, T.; Wong, J.; Abhayaratna, W.; et al. National heart foundation of australia and cardiac society of australia and new zealand: Guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. 2018, 27, 1123–1208. [Google Scholar] [CrossRef] [Green Version]
- Audureau, E.; Guellich, A.; Guéry, E.; Canouï-Poitrine, F.; Benedyga, V.; Duchossoir, H.; Taieb, C.; Damy, T. Development and validation of a new tool to assess burden of dietary sodium restriction in patients with chronic heart failure: The BIRD questionnaire. Nutrients 2018, 10, 1453. [Google Scholar] [CrossRef] [Green Version]
- De Tejada, M.G.-S.; Bilbao, A.; Ansola, L.; Quirós, R.; García-Perez, L.; Navarro, G.; Escobar, A. Responsiveness and minimal clinically important difference of the Minnesota living with heart failure questionnaire. Heal. Qual. Life Outcomes 2019, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Duchossoir, H.B.; Audureau, E.; Taieb, C. Un nouvel outil diététique pour évaluer les apports sodés d’un patient. Inf. Diét. 2016, 2, 15–20. [Google Scholar]
- Naveiro-Rilo, J.C.; Diez-Juárez, D.M.; Blanco, A.R.; Rebollo-Gutiérrez, F.; Rodríguez-Martínez, A.; Rodriguez-Garcia, M.A. Validation of the Minnesota living with heart failure questionnaire in primary care. Rev. Esp. Cardiol. 2010, 63, 1419–1427. [Google Scholar] [CrossRef]
- Van Der Wal, M.H.; Jaarsma, T.; Van Veldhuisen, D.J. Non-compliance in patients with heart failure; how can we manage it? Eur. J. Heart Fail. 2005, 7, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Marti, C.N.; Georgiopoulou, V.V.; Giamouzis, G.; Cole, R.T.; Deka, A.; Tang, W.W.; Dunbar, S.B.; Smith, A.L.; Kalogeropoulos, A.P.; Butler, J. Patient-reported selective adherence to heart failure self-care recommendations: A prospective cohort study: The atlanta cardiomyopathy consortium. Congest. Heart Fail. 2012, 19, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, S.; Tsuchihashi-Makaya, M.; Kayane, T.; Yamada, M.; Wakabayashi, R.; Kato, N.P.; Yazawa, M. Health literacy is independently associated with self-care behavior in patients with heart failure. Patient Educ. Couns. 2016, 99, 1026–1032. [Google Scholar] [CrossRef]
- Lennie, T.A.; Moser, D.K.; Chung, M.L. Insight into differences in dietary sodium adherence between men and women with heart failure. J. Cardiovasc. Nurs. 2020, 35, 131–136. [Google Scholar] [CrossRef]
- Dunbar, S.B.; Clark, P.C.; Quinn, C.; Gary, R.A.; Kaslow, N.J. Family influences on heart failure self-care and outcomes. J. Cardiovasc. Nurs. 2008, 23, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Dunbar, S.B.; Clark, P.C.; Deaton, C.; Smith, A.L.; De, A.K.; O’Brien, M.C. Family education and support interventions in heart failure. Nurs. Res. 2005, 54, 158–166. [Google Scholar] [CrossRef]
- Clark, A.; Spaling, M.; Harkness, K.; Spiers, J.; Strachan, P.H.; Thompson, D.; Currie, K. Determinants of effective heart failure self-care: A systematic review of patients’ and caregivers’ perceptions. Heart 2014, 100, 716–721. [Google Scholar] [CrossRef]
- Kuehneman, T.; Saulsbury, D.; Splett, P.; Chapman, D.B. Demonstrating the impact of nutrition intervention in a heart failure program. J. Am. Diet. Assoc. 2002, 102, 1790–1794. [Google Scholar] [CrossRef]
- West, A.J.; Miller, N.H.; Parker, K.M.; Senneca, D.; Ghandour, G.; Clark, M.; Greenwald, G.; Heller, R.S.; Fowler, M.B.; DeBusk, R.F. A Comprehensive management system for heart failure improves clinical outcomes and reduces medical resource utilization. Am. J. Cardiol. 1997, 79, 58–63. [Google Scholar] [CrossRef]
- Sevilla-Cazes, J.; Ahmad, F.S.; Bowles, K.H.; Jaskowiak, A.; Gallagher, T.; Goldberg, L.R.; Kangovi, S.; Alexander, M.; Riegel, B.; Barg, F.; et al. Heart failure home management challenges and reasons for readmission: A qualitative study to understand the patient’s perspective. J. Gen. Intern. Med. 2018, 33, 1700–1707. [Google Scholar] [CrossRef] [Green Version]
- Colin-Ramirez, E.; Arcand, J.; Ezekowitz, J.A. Estimates of dietary sodium consumption in patients with chronic heart failure. J. Card. Fail. 2015, 21, 981–988. [Google Scholar] [CrossRef]
- Damman, K.; Ter Maaten, J.M.; Coster, J.E.; Krikken, J.A.; Van Deursen, V.M.; Krijnen, H.K.; Hofman, M.; Nieuwland, W.; Van Veldhuisen, D.J.; Voors, A.A.; et al. Clinical importance of urinary sodium excretion in acute heart failure. Eur. J. Heart Fail. 2020, 22, 1438–1447. [Google Scholar] [CrossRef]
- Evangelista, L.S.; Berg, J.; Dracup, K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart Lung 2001, 30, 294–301. [Google Scholar] [CrossRef] [Green Version]

| Variables | All Patients | |
|---|---|---|
| Patients with Data | Estimate | |
| Demographic data | ||
| Age, years | 2729 | 67.4 (±13.8) |
| Sex | 2818 | |
| Female | 840 (29.8) | |
| Male | 1978 (70.2) | |
| Living environment | 2540 | |
| Urban | 1726 (68.0) | |
| Rural | 814 (32.0) | |
| Living situation | 2779 | |
| Couple | 1401 (50.4) | |
| Family | 502 (18.1) | |
| Retirement home/community | 43 (1.5) | |
| Alone | 833 (30.0) | |
| Heart failure history | ||
| Type of HF, | 2607 | |
| De novo (<3 months) | 427 (16.4) | |
| Chronic | 2180 (83.6) | |
| Current HF, stable vs. acute | 2577 | |
| Stable | 1788 (69.4) | |
| Acute | 789 (30.6) | |
| Last acute HF episode (months) | 2424 | |
| <3 | 1068 (44.1) | |
| 3–12 | 535 (22.1) | |
| >12 | 821 (33.9) | |
| Type of cardiopathy | 2639 | |
| Ischemic | 1162 (44.0) | |
| Non-ischemic | 1262 (47.8) | |
| Valvular | 215 (8.1) | |
| Cardiovascular risk factors | ||
| Current smoker | 2822 | 321 (11.4) |
| Number of cigarettes/day | 272 | 10.0 (5.0; 15.0) |
| Hypercholesterolemia | 2822 | 1072 (38.0) |
| Hypertension | 2822 | 1578 (55.9) |
| Obesity | 2822 | 584 (20.7) |
| Diabetes | 2822 | 816 (28.9) |
| Family history of coronary disease | 2822 | 230 (8.15) |
| Dialysis | 2822 | 17 (0.6) |
| Sleep apnoea syndrome | 2822 | 231 (8.2) |
| Patients with chronic obstructive pulmonary disease | 2822 | 199 (7.1) |
| Clinical and biological variables | ||
| NYHA class (physician), n (%) | 2530 | |
| I | 344 (13.6) | |
| II | 1215 (48.0) | |
| III | 786 (31.1) | |
| IV | 185 (7.3) | |
| Self-reported symptoms, n (%) | 2541 | |
| Asymptomatic | 413 (16.3) | |
| Mild exercise symptoms not limiting daily life | 880 (34.6) | |
| Symptoms limiting daily life and/or orthopnoea | 1248 (49.1) | |
| Weight loss within the last 6 months (kg) | 1255 | 7.0 (±5.7) |
| BMI (kg/m2) | 2688 | 27.1 (±5.9) |
| Systolic blood pressure (mmHg) | 2688 | 120.2 (±20.7) |
| Diastolic blood pressure (mmHg) | 2710 | 70.5 (±12.4) |
| Heart rate (bpm) | 2607 | 73.0 (±16.4) |
| Sinus rhythm | 2822 | 1742 (61.7) |
| QRS width (ms) | 772 | 115.6 (±33.5) |
| LVEF (%) | 2680 | 38.7 (±13.7) |
| NT-proBNP levels (pg/mL) | 1739 | 1811 (703; 4384) |
| BNP levels (pg/mL) | 828 | 438 (177; 885) |
| NT-proBNP and BNP quartiles combined | 2448 | |
| Q1 | 615 (25.1) | |
| Q2 | 605 (24.7) | |
| Q3 | 609 (24.9) | |
| Q4 | 619 (25.3) | |
| Creatinine level (µmol/L) | 2677 | 99.0 (177; 885) |
| Haemoglobin level (g/L) | 2581 | 12.9 (11.6; 14.2) |
| Patients with implantable cardioverter defibrillator | 2822 | 725 (25.7) |
| Variables | All Patients | |
|---|---|---|
| Patients with Data | Estimate | |
| Patients with therapeutic education programme | 2822 | 657 (23.3) |
| Patient’s frequency of weighing | 2750 | |
| Daily | 554 (20.1) | |
| Weekly | 1014 (36.9) | |
| Monthly | 709 (25.8) | |
| Never | 473 (17.2) | |
| Diet recommended to the patients * | 2822 | |
| Low-salt diet | 2618 (92.8) | |
| Water restriction | 402 (14.2) | |
| Diabetic diet (carbohydrate-controlled diet) | 768 (27.2) | |
| Low-fat diet | 1090 (38.6) | |
| Healthcare professional recommending low-salt diet * | 2618 | |
| General practitioner | 561 (21.4) | |
| Cardiologist | 1541 (58.9) | |
| Dietician | 626 (23.9) | |
| Nurse | 148 (5.7) | |
| Salt diet recommended (SD-R) by healthcare professional (g/day) | 2822 | |
| <3 | 267 (9.5) | |
| 3≥ salt <6 | 915 (32.4) | |
| 6 | 1186 (42.0) | |
| >6 | 269 (9.5) | |
| Unknown | 185 (6.6) | |
| Salt diet understood (SD-U) by patient (g/day) | 2822 | |
| <3 | 516 (18.3) | |
| 3≥ salt <6 | 664 (23.5) | |
| 6 | 719 (25.5) | |
| >6 | 528 (18.7) | |
| Unknown | 395 (14.0) | |
| Estimated salt consumption (g/day) | 2822 | 4.4 (2.8; 6.2) |
| <3 | 753 (26.7) | |
| 3≥ salt <6 | 1291 (45.7) | |
| >6 | 778 (27.6) | |
| Patients compliance with salt diet understood versus estimated salt consumption | 2822 | |
| Compliant | 933 (33.1) | |
| Overcompliant | 969 (34.3) | |
| Undercompliant | 525 (18.6) | |
| Unknown | 395 (14.0) | |
| BIRD score (maximum score = 48) | 2602 | 8.1 (±8.8) |
| BIRD score for the 12 items | ||
| On account of my diet, I am not living as I would like, because … | ||
| … every meal is difficult for me | 2665 | 0.7 (±1.0) |
| … having a meal away from home is complicated | 2664 | 0.9 (±1.2) |
| … grocery shopping is complicated | 2677 | 0.7 (±1.1) |
| … it results in additional expenses | 2618 | 0.6 (±1.0) |
| … I have the impression of being a bother or a burden to those preparing my meals | 2671 | 0.5 (±0.9) |
| … it makes relationships or activities with friends or family difficult | 2640 | 0.5 (±1.0) |
| … it makes my leisure activities difficult (favourite pastimes, sports) | 2671 | 0.8 (±1.2) |
| … it prevents me from travelling, going on vacation | 2626 | 0.8 (±1.3) |
| … it makes me feel tired, weary, or I lack energy | 2648 | 1.1 (±1.2) |
| … it is difficult to manage in my workplace/professional activity | 2632 | 0.4 (±0.9) |
| … it depresses me | 2624 | 0.6 (±1.0) |
| … it aggravates my health | 2611 | 0.5 (±0.9) |
| MLHFQ score (maximum score = 105) | 2200 | 35.4 (±24.5) |
| Physical subscale (maximum score = 40) | 2505 | 16.7 (±11.7) |
| Emotional subscale (maximum score = 25) | 2589 | 7.7 (±6.6) |
| Salt Diet Recommended (SD-R) by Healthcare Professional (g/Day), n (%) | ||||||
|---|---|---|---|---|---|---|
| Unknown (n = 185) | None/>6 (n = 269) | 6 (n = 1186) | 3–6 (n = 915) | <3 (n = 267) | ||
| Salt diet understood (SD-U) by patient (g/day), n (%) | Unknown (n = 395) | 55 (29.7) | 24 (8.9) | 174 (14.7) | 112 (12.2) | 30 (11.2) |
| None/>6 (n = 528) | 35 (18.9) | 163 (60.6) | 205 (17.3) | 111 (12.1) | 14 (5.2) | |
| 6 (n = 719) | 41 (22.2) | 39 (14.5) | 445 (37.5) | 173 (18.9) | 21 (7.9) | |
| 3–6 (n = 664) | 27 (14.6) | 25 (9.3) | 226 (19.1) | 337 (36.8) | 49 (18.4) | |
| <3 (n = 516) | 27 (14.6) | 18 (6.7) | 136 (11.5) | 182 (19.9) | 153 (57.3) | |
| Determinants of Daily Estimated Salt Consumption (SD-T) | Estimated Salt Consumption (g/Day) | Unadjusted Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Raw Mean (SD) | Unadjusted Beta (95% CI) | p-Value | Adjusted Beta (95% CI) | p-Value | |
| Sex, female vs. male | |||||
| No | 4.89 (2.52) | 0 (ref) | <0.0001 | 0 (ref) | <0.0001 |
| Yes | 4.17 (2.08) | −0.72 (−0.91; −0.52) | −0.65 (−0.86; −0.43) | ||
| Living environment: urban vs. rural | |||||
| Rural | 4.57 (2.30) | 0 (ref) | 0.050 | 0 (ref) | 0.035 |
| Urban | 4.77 (2.46) | 0.20 (0.001; 0.40) | 0.23 (0.02; 0.44) | ||
| Living situation | |||||
| Couple | 4.64 (2.33) | 0 (ref) | 0.017 | 0 (ref) | 0.001 |
| Family | 4.93 (2.51) | 0.29 (0.04; 0.54) | −0.01 (−0.28; 0.26) | ||
| Retirement home/community | 3.89 (2.22) | −0.74 (−1.48; −0.01) | −1.54 (−2.34; −0.74) | ||
| Alone | 4.67 (2.50) | 0.04 (−0.17; 0.24) | −0.24 (−0.47; −0.01) | ||
| Chronic vs. de novo HF | |||||
| De novo | 5.11 (2.54) | 0 (ref) | <0.0001 | 0 (ref) | <0.0001 |
| Chronic | 4.60 (2.39) | −0.51 (−0.76; −0.26) | −0.47 (−0.73; −0.21) | ||
| Acute vs. stable HF | |||||
| Stable | 4.61 (2.32) | 0 (ref) | 0.009 | 0 (ref) | 0.002 |
| Acute | 4.88 (2.61) | 0.27 (0.07; 0.47) | 0.34 (0.12; 0.57) | ||
| Current smoker | |||||
| No | 4.56 (2.34) | 0 (ref) | <0.0001 | 0 (ref) | <0.0001 |
| Yes | 5.58 (2.79) | 1.02 (0.74; 1.30) | 0.63 (0.33; 0.94) | ||
| NT-proBNP and BNP quartiles combined | |||||
| Q1 | 4.90 (2.54) | 0 (ref) | <0.0001 | 0 (ref) | <0.0001 |
| Q2 | 4.79 (2.39) | −0.10 (−0.37; 0.16) | −0.22 (−0.50; 0.06) | ||
| Q3 | 4.50 (2.39) | −0.40 (−0.67; −0.13) | −0.44 (−0.72; −0.16) | ||
| Q4 | 4.28 (2.28) | −0.62 (−0.89; −0.35) | −0.67 (−0.96; −0.38) | ||
| Cardiologist | |||||
| No | 5.15 (2.43) | 0 (ref) | <0.0001 | 0 (ref) | 0.022 |
| Yes | 4.28 (2.34) | −0.87 (−1.05; −0.69) | −0.27 (−0.50; −0.04) | ||
| Salt diet understood (SD-U) by patient (g/day) | |||||
| Unknown | 4.79 (2.58) | 0 (ref) | <0.0001 | 0 (ref) | <0.0001 |
| None or >6 g/day | 5.94 (2.20) | 1.15 (0.85; 1.45) | 0.87 (0.51; 1.24) | ||
| 6 g/day | 5.04 (2.27) | 0.25 (−0.03; 0.53) | 0.36 (0.03; 0.69) | ||
| 3–6 g/day | 4.16 (2.16) | −0.63 (−0.92; −0.35) | −0.48 (−0.82; −0.14) | ||
| <3 g/day | 3.44 (2.25) | −1.35 (−1.65; −1.05) | −1.16 (−1.51; −0.80) | ||
| Patients’ frequency of weighing | |||||
| None | 5.39 (2.54) | 0 (ref) | <0.0001 | 0 (ref) | <0.0001 |
| Daily | 3.86 (2.16) | −1.53 (−1.82; −1.24) | −1.19 (−1.52; −0.86) | ||
| Weekly | 4.46 (2.25) | −0.93 (−1.19; −0.68) | −0.79 (−1.08; −0.50) | ||
| Monthly | 5.19 (2.46) | −0.19 (−0.47; 0.08) | −0.35 (−0.66; −0.05) | ||
| Patients with therapeutic education programme | |||||
| No | 4.82 (2.42) | 0 (ref) | <0.0001 | 0 (ref) | 0.004 |
| Yes | 4.21 (2.34) | −0.61 (−0.82; −0.40) | −0.35 (−0.59; −0.11) | ||
| BIRD Score | Lowest to Medium Burden | Highest Burden | Unadjusted Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| Q1 to Q3 | Q4 | |||||
| N | 1983 | 619 | ||||
| Factors Assessed | Raw Estimate | Raw Estimate | Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
| Age, years, mean (SD) | 67.2 (13.5) | 65.8 (14.5) | 0.99 (0.99; 1.00) | 0.028 | 0.98 (0.97; 0.99) | <0.0001 |
| Sex, females vs. males, n (%) | 535 (27.0) | 219 (35.4) | 1.48 (1.22; 1.79) | <0.0001 | 1.65 (1.28; 2.13) | <0.0001 |
| Living environment urban vs. rural, n (%) | 1183 (65.7) | 421 (74.5) | 1.52 (1.23; 1.89) | <0.0001 | 1.64 (1.26; 2.12) | <0.0001 |
| Acute vs. stable HF, n (%) | 466 (25.7) | 255 (45.3) | 2.40 (1.97; 2.92) | <0.0001 | 1.52 (1.16; 2.00) | 0.003 |
| Diabetes, n (%) | 521 (26.3) | 235 (38.0) | 1.72 (1.42; 2.08) | <0.0001 | 1.72 (1.35; 2.20) | <0.0001 |
| Chronic obstructive pulmonary disease, n (%) | 113 (5.7) | 74 (12.0) | 2.25 (1.65; 3.06) | <0.0001 | 1.57 (1.05; 2.33) | 0.026 |
| NYHA class, n (%) | <0.0001 | <0.0001 | ||||
| I | 279 (15.8) | 46 (8.1) | 1 (ref) | 1 (ref) | ||
| II | 904 (51.1) | 215 (38.1) | 1.44 (1.02; 2.04) | 1.24 (0.81; 1.91) | ||
| III | 478 (27.0) | 246 (43.5) | 3.12 (2.20; 4.42) | 2.52 (1.60; 3.97) | ||
| IV | 109 (6.2) | 58 (10.3) | 3.23 (2.07; 5.04) | 2.49 (1.42; 4.39) | ||
| LVEF, %, mean (SD) | 39.2 (13.7) | 36.0 (13.2) | 0.98 (0.98; 0.99) | <0.0001 | 0.98 (0.97; 0.99) | <0.0001 |
| Haemoglobin level, g/L, mean (SD) | 13.0 (1.9) | 12.5 (1.9) | 0.87 (0.83; 0.91) | <0.0001 | 0.92 (0.86; 0.98) | 0.011 |
| Salt diet recommended (SD-R) by healthcare professional (g/day) | <0.0001 | NS | ||||
| None or >6 | 205 (10.3) | 32 (5.2) | 1 (ref) | - | ||
| 6 | 837 (42.2) | 262 (42.3) | 2.01 (1.35; 2.98) | - | ||
| 3–6 | 645 (32.5) | 206 (33.3) | 2.05 (1.37; 3.07) | - | ||
| <3 | 163 (8.2) | 89 (14.4) | 3.50 (2.22; 5.50) | - | ||
| Unknown | 133 (6.7) | 30 (4.8) | 1.45 (0.84; 2.49) | - | ||
| Salt diet understood (SD-U) by patient (g/day) | <0.0001 | 0.006 | ||||
| None or >6 | 402 (20.3) | 62 (10.0) | 1 (ref) | 1 (ref) | ||
| 6 | 500 (25.2) | 179 (28.9) | 2.32 (1.69; 3.19) | 2.14 (1.40; 3.26) | ||
| 3–6 | 499 (25.2) | 141 (22.8) | 1.83 (1.32; 2.54) | 1.88 (1.21; 2.94) | ||
| <3 | 338 (17.0) | 156 (25.2) | 2.99 (2.16; 4.15) | 2.19 (1.39; 3.46) | ||
| Unknown | 244 (12.3) | 81 (13.1) | 2.15 (1.49; 3.11) | 2.12 (1.32; 3.41) | ||
| Estimated salt consumption (g/day) | <0.0001 | 0.027 | ||||
| >7 | 333 (16.8) | 102 (16.5) | 1.53 (1.13; 2.08) | 1.38 (0.92; 2.05) | ||
| 5–7 | 514 (25.9) | 103 (16.6) | 1 (ref) | 1 (ref) | ||
| 3–5 | 646 (32.6) | 207 (33.4) | 1.60 (1.23; 2.08) | 1.50 (1.07; 2.09) | ||
| <3 | 490 (24.7) | 207 (33.4) | 2.11 (1.61; 2.75) | 1.72 (1.20; 2.45) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damy, T.; Benedyga, V.; Pezel, T.; Berthelot, E.; Gauthier, J.; Habib, G.; Iliou, M.-C.; Aupetit, J.-F.; Baudry, G.; De Groote, P.; et al. Prescription, Compliance, and Burden Associated with Salt-Restricted Diets in Heart Failure Patients: Results from the French National OFICSel Observatory. Nutrients 2022, 14, 308. https://doi.org/10.3390/nu14020308
Damy T, Benedyga V, Pezel T, Berthelot E, Gauthier J, Habib G, Iliou M-C, Aupetit J-F, Baudry G, De Groote P, et al. Prescription, Compliance, and Burden Associated with Salt-Restricted Diets in Heart Failure Patients: Results from the French National OFICSel Observatory. Nutrients. 2022; 14(2):308. https://doi.org/10.3390/nu14020308
Chicago/Turabian StyleDamy, Thibaud, Véronique Benedyga, Théo Pezel, Emmanuelle Berthelot, Jacques Gauthier, Gilbert Habib, Marie-Christine Iliou, Jean-François Aupetit, Guillaume Baudry, Pascal De Groote, and et al. 2022. "Prescription, Compliance, and Burden Associated with Salt-Restricted Diets in Heart Failure Patients: Results from the French National OFICSel Observatory" Nutrients 14, no. 2: 308. https://doi.org/10.3390/nu14020308
APA StyleDamy, T., Benedyga, V., Pezel, T., Berthelot, E., Gauthier, J., Habib, G., Iliou, M.-C., Aupetit, J.-F., Baudry, G., De Groote, P., Logeart, D., Chaufourier, L., Ciobotaru, V., Pousset, F., Beauvais, F., Bauer, F., Zores, F., Lairez, O., Richard, K., ... Audureau, E. (2022). Prescription, Compliance, and Burden Associated with Salt-Restricted Diets in Heart Failure Patients: Results from the French National OFICSel Observatory. Nutrients, 14(2), 308. https://doi.org/10.3390/nu14020308