Biological Role of Vitamin K—With Particular Emphasis on Cardiovascular and Renal Aspects
Abstract
:1. Introduction
2. Biological Role of VK
3. Vitamin K, Cardiovascular Risk and Vascular Calcification
4. Vitamin K and Chronic Kidney Disease
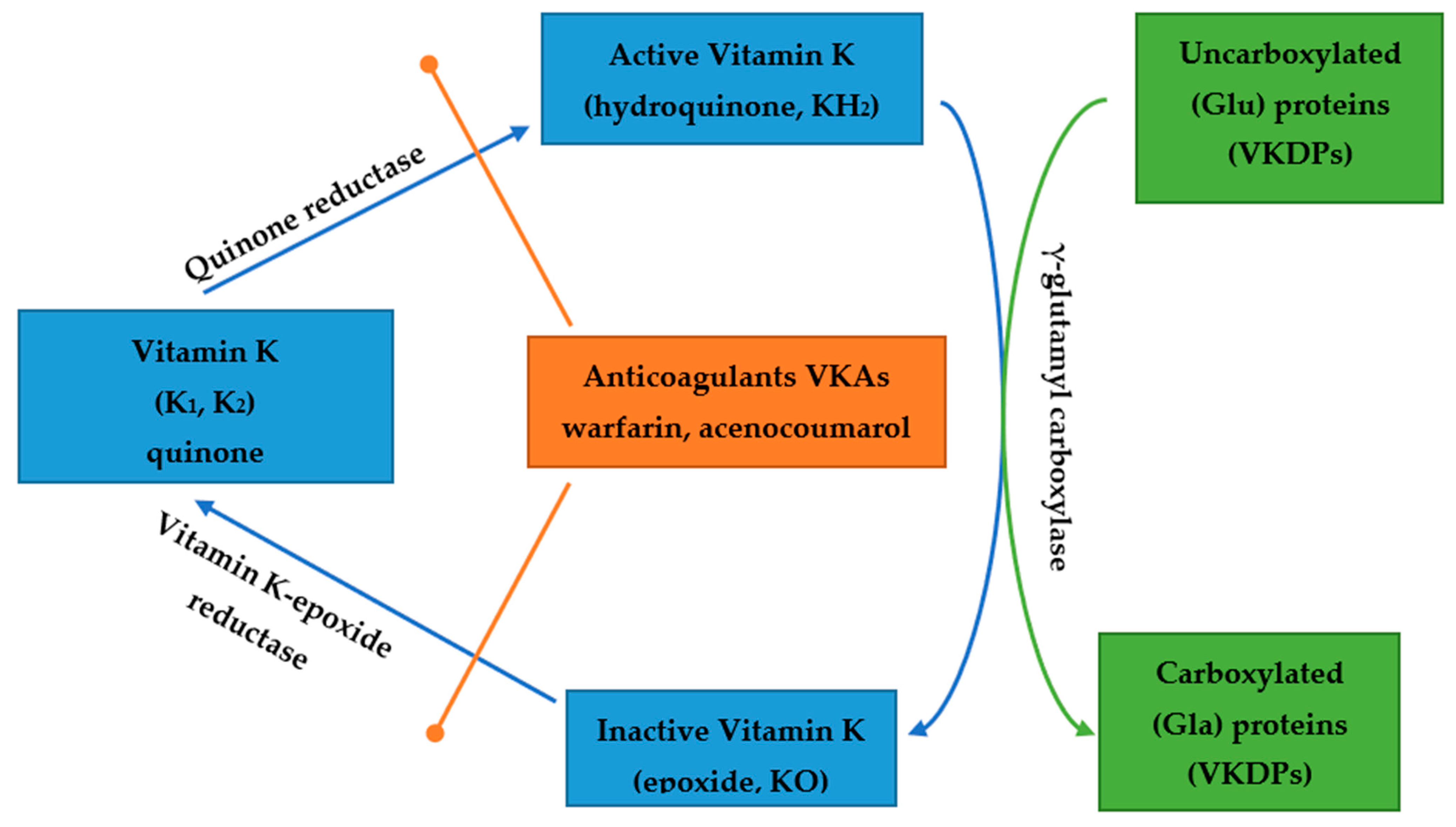
5. Vitamin K and Anticoagulant Therapy
6. Vitamin K Supplementation
7. Conclusions and Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AACS | abdominal aorta calcification score |
| AC | aortic calcifications |
| aBMD | areal bone mineral density |
| AF | atrial fibrillation |
| AS | arterial stiffness |
| AVC | aortic valve calcification |
| BMP-2 | bone morphogenetic protein-2 |
| CAC | coronary artery calcification |
| cfPWV | carotid-femoral pulse wave velocity |
| CHD | coronary heart disease |
| CI | Confidence Interval |
| CIMT | carotid intimal medial thickness |
| CKD cMGP | chronic kidney disease carboxylated matrix Gla protein |
| CUA | calcific uremic arteriolopathy |
| CVD | cardiovascular disease |
| dp-cMGP | dephosphorylated-carboxylated MGP |
| dp-ucMGP | dephosphorylated-uncarboxylated MGP |
| EPCs | endothelial progenitor cells |
| ESRD | end-stage renal disease |
| EVA | early vascular ageing |
| Gla Glu HR | γ-carboxyglutamate glutamate hazard ratio |
| MGP | Matrix Gla Protein |
| MK-4 | menaquinone-4 |
| NOACs | novel oral anticoagulants |
| NRF2 | erythroid 2–related factor 2 |
| OC | osteocalcin |
| PIVKAII | hepatic protein induced by vitamin K absence-II |
| PD | peritoneal dialysis |
| PTH | parathyroid hormone |
| p-ucMGP | phosphorylated ucMGP |
| RCTs | randomized controlled trials |
| sHR | sub-hazard ratio |
| t-ucMGP | total uncarboxylated Matrix Gla Protein |
| ucMGP | uncarboxylated Matrix Gla Protein |
| ucOC | undercarboxylated osteocalcin |
| VC | vascular calcification |
| VD | vitamin D |
| VK | vitamin K |
| VK1 | vitamin K1 |
| VK2 | vitamin K2 |
| VKA | Vitamin K Antagonist |
| VKDP(s) | VK-dependent protein(s) |
| VKOR | vitamin K epoxide reductase |
| VS | vascular stiffness |
| VSMC | vascular smooth muscle cells |
References
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. The health benefits of vitamin K. Open Heart 2015, 2, e000300. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.-S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, J.; Duan, L.; Li, S. Role of emerging vitamin K-dependent proteins: Growth arrest-specific protein 6, Gla-rich protein and periostin (Review). Int. J. Mol. Med. 2021, 47, 2. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, B.N.; Jerin, S.S.; Vinod, K.V.; Yaseen, M.; Jiss, P.J.; Alex, D.; Prolay, P.; Sayantan, G. Chronic Kidney Diseases: Role of Vitamin-K and Vitamin-D. CMRO 2021, 4, 852–866. [Google Scholar]
- Palmer, C.A.; Blekkenhorst, L.C.; Lewis, J.R.; Ward, N.C.; Schultz, C.J.; Jonathan, M.; Hodgson, J.M.; Croft, K.D.; Sim, M. Quantifying dietary vitamin K and its link to cardiovascular health: A narrative review. Food Funct. 2020, 11, 2826–2837. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.B.; Stinghen, A.E.M.; Massy, Z.A. Vitamin K role in mineral and bone disorder of chronic kidney disease. Clin. Chim. Acta 2020, 502, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Galassi, A.; Ciceri, P.; Messa, P.; Nigwekar, S. Vitamin K in Chronic Kidney Disease. Nutrients 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; An, W.S. Supplementary nutrients for prevention of vascular calcification in patients with chronic kidney disease. Korean J. Intern. Med. 2019, 34, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Cosmai, M.; Evenepoel, P.; Nickolas, T.L.; Cheung, A.M.; Aghi, A.; Tripepi, G.; Plebani, M.; Iervasi, G.; Vettor, R.; et al. Vitamin K and Kidney Transplantation. Nutrients 2020, 12, 2717. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, U.; Stasiak, U.; Wasilewska, A.; Przybylski, D.; Marchelek-Myśliwiec, M.; Nowosiad-Magda, M. Multi-faceted function of vitamin K with particular consideration of vitamin K2—Literature review. Pomeranian J. Life Sci. 2020, 66, 39–44. [Google Scholar] [CrossRef]
- Silaghi, C.N.; Ilyés, T.; Filip, V.P.; Farcas, M.; van Ballegooijen, A.J.; Crăciun, A.M. Vitamin K Dependent Proteins in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Chen, J.; Duan, L.; Li, S. Vitamin K-dependent proteins involved in bone and cardiovascular health. Mol. Med. Rep. 2018, 18, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mladĕnka, P.; Macáková, K.; Kujovská Krčmová, L.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L.; on behalf of the OEMONOM Researchers and Collaborators. Vitamin K—Sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2021, nuab061. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-J.; Komai, M.; Shirakawa, H. Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review. Nutrients 2020, 12, 2485. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.J.; Shirakawa, H.; Hirahara, K.; Sone, H.; Kamiyama, S.; Komai, M. Menaquinone-4 Amplified Glucose-Stimulated Insulin Secretion in Isolated Mouse Pancreatic Islets and INS-1 Rat Insulinoma Cells. Int. J. Mol. Sci. 2019, 20, 1995. [Google Scholar] [CrossRef] [Green Version]
- Stefanos Roumeliotis, S.; Evangelia Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Vassilios Liakopoulos, V. Vascular Calcifification in Chronic Kidney Disease: The Role of Vitamin K- Dependent Matrix Gla Protein. Front. Med. 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Puzantian, H.; Akers, S.R.; Oldland, G.; Javaid, K.; Miller, R.; Ge, Y.; Ansari, B.; Lee, J.; Suri, A.; Hasmath, Z.; et al. Circulating Dephospho-Uncarboxylated Matrix Gla-Protein Is Associated with Kidney Dysfunction and Arterial Stiffness. Am. J. Hypertens. 2018, 31, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Sardana, M.; Vasim, I.; Varakantam, S.; Kewan, U.; Tariq, A.; Koppula, M.R.; Syed, A.A.; Beraun, M.; Drummen, N.E.A.; Vermeer, C.; et al. Inactive Matrix Gla-Protein and Arterial Stiffness in Type 2 Diabetes Mellitus. Am. J. Hypertens. 2016, 30, 196–201. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.J.; van der Meer, I.M.; Hofman, A.; Witteman, J.C.M. Dietary Intake of Menaquinone Is Associated with a Reduced Risk of Coronary Heart Disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef]
- Gast, G.C.M.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.J.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.M.; van der Schouw, Y.T. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metabol. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Haugsgjer, T.R.; Egeland, G.M.; Nygård, O.K.; Vinknes, K.J.; Sulo, G.; Lysne, V.; Igland, J.; Tell, G.S. Association of dietary vitamin K and risk of coronary heart disease in middle-age adults: The Hordaland Health Study Cohort. BMJ Open 2020, 10, e035953. [Google Scholar] [CrossRef] [PubMed]
- Caluwé, R.; Verbeke, F.; De Vriese, A.S. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol. Dial. Transplant. 2020, 35, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.; Reinartz, S.; Kaesler, N.; Krüger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower Progress of Aortic Valve Calcification with Vitamin K Supplementation: Results from a Prospective Interventional Proof-of-Concept Study. Circulation 2017, 135, 2081–2084. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K status and cardiovascular events or mortality: A meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 549–553. [Google Scholar] [CrossRef]
- Chen, H.; Sheng, L.; Zhang, Y.; Cao, A.; Lai, Y.; Kunutsor, S.; Jiang, L.; Pan, A. Association of vitamin K with cardiovascular events and all-cause mortality: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Giuseppe Cianciolo, G.; Podestà, M.A.; Ciceri, P.; Galassi, A.; Gasperoni, L.; La Manna, G. Current Therapy in CKD Patients Can Affect Vitamin K Status. Nutrients 2020, 12, 1609. [Google Scholar] [CrossRef]
- Turner, M.E.; Adams, M.A.; Holden, R.M. The Vitamin K Metabolome in Chronic Kidney Disease. Nutrients 2018, 10, 1076. [Google Scholar] [CrossRef] [Green Version]
- Giangola, M.D.; Yang, W.L.; Rajayer, S.R.; Kuncewitch, M.; Molmenti, E.; Nicastro, J.; Coppa, G.F.; Wang, P. Growth arrest-specific protein 6 protects against renal ischemia-reperfusion injury. J. Surg. Res. 2015, 199, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Gluba-Brzózka, A.; Michalska-Kasiczak, M.; Franczyk, B.; Nocuń, M.; Toth, P.; Banach, M.; Rysz, J. Markers of increased atherosclerotic risk in patients with chronic kidney disease: A preliminary study. Lipids Health Dis. 2016, 15, 22. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Li, L.; Erlandsson, H.; Jaminon, A.M.G.; Qureshi, A.R.; Ripsweden, J.; Torkel, B.; Brismar, T.B.; Witasp, A.; Heimbürger, O.; et al. Functional vitamin K insufficiency, vascular calcification and mortality in advanced chronic kidney disease: A cohort study. PLoS ONE 2021, 16, e0247623. [Google Scholar] [CrossRef]
- Nagai, K.; Arai, H.; Yanagita, M.; Matsubara, T.; Kanamori, H.; Nakano, T.; Iehara, N.; Fukatsu, A.; Kita, T.; Doi, T. Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J. Biol. Chem. 2003, 278, 18229–18234. [Google Scholar] [CrossRef] [Green Version]
- Hung, Y.J.; Lee, C.H.; Chu, N.F.; Shieh, Y.S. Plasma protein growth arrest-specific 6 levels are associated with altered glucose tolerance, inflammation, and endothelial dysfunction. Diabetes Care. 2010, 35, 1840–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, J.; Ge, L.; Shan, J.; Zhang, C.; Liu, J. Growth arrest-specific protein 6 (Gas6) as a noninvasive biomarker for early detection of diabetic nephropathy. Clin. Exp. Hypertens. 2017, 39, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Olivier, B.; Vemeer, C.; Theuwissen, E.; Magdeleyns, E.; Auber, C.E.; Brazier, M.; Mentaverri, R.; Hartemann, A.; Massy, Z.A. Vascular calcification in patients with type 2 diabetes: The involvement of matrix Gla protein. Cardiovasc. Diabetol. 2014, 13, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Leivaditis, K.; Kantartzi, K.; Panagoutsos, S.; Liakopoulos, V. The Association of dp-ucMGP with Cardiovascular Morbidity and Decreased Renal Function in Diabetic Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 6035. [Google Scholar] [CrossRef]
- Riphagen, I.J.; Keyzer, C.A.; Drummen, N.E.A.; de Borst, M.H.; Beulens, J.W.J.; Gansevoort, R.T.; Geleijnse, J.M.; Muskiet, F.A.J.; Navis, G.; Visser, S.T.; et al. Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study. Nutrients 2017, 9, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Schurgers, L.J.; Paul, G.; Shiels, P.G.; Stenvinkel, P. Early vascular ageing in chronic kidney disease: Impact of inflammation, vitamin K, senescence and genomic damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef] [Green Version]
- Aoun, M.; Makki, M.; Azar, H.; Hiam Matta, H.; Chelala, D.N. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: Risk factorsand response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017, 18, 191. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Brandenburg, V.M. K-alcification Protection in Dialysis Patients: The Underestimated Phenomenon of Vitamin K Deficiency. J. Am. Soc. Nephrol. 2017, 28, 1667–1668. [Google Scholar] [CrossRef]
- Ketteler, M.; Schanz, M.; Schricker, S. Vitamin K: Should we supplement to protect the kidneys and the heart? Nephrol. Dial. Transplant. 2021, 36, 2196–2198. [Google Scholar] [CrossRef]
- Grzejszczak, P.; Kurnatowska, I. Role of Vitamin K in CKD: Is Its Supplementation Advisable in CKD Patients? Kidney Blood Press. Res. 2021, 46, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Bloch, D.B.; Nazarian, R.M.; Vermeer, C.; Booth, S.L.; Xu, D.; Thadhani, R.I.; Malhotra, R. Vitamin K–Dependent Carboxylation of Matrix Gla Protein Influences the Risk of Calciphylaxis. J. Am. Soc. Nephrol. 2017, 28, 1717–1722. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Lu, C.-L.; Zheng, C.-M.; Chen, R.-M.; Lin, Y.-F.; Liu, W.-C.; Yen, T.-H.; Chen, R.; Lu, K.-C. Emerging Role of Vitamins D and K in Modulating Uremic Vascular Calcification: The Aspect of Passive Calcification. Nutrients 2019, 11, 152. [Google Scholar] [CrossRef] [Green Version]
- van Ballegooijen, A.J.; Joline, W.J.; Beulens, J.W.J.; Keyzer, C.A.; Navis, G.J.; Berger, S.P.; Martin, H.; de Borst, M.H.; Vervloet, M.G.; Bakker, S.J.L. Joint association of vitamins D and K status with long-term outcomes in stable kidney transplant recipients. Nephrol. Dial. Transplant. 2020, 35, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Poor Vitamin K Status Is Associated with Low Bone Mineral Density and Increased Fracture Risk in End-Stage Renal Disease. J. Bone Miner. Res. 2019, 34, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Cranenburg, E.C.M.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.J.; Dalmeijer, G.W.; Westerhuis, R.; Elke, J.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikstrøm, S.; Lentz, K.A.; Hansen, D.; Rasmussen, L.M.; Jakobsen, J.; Hansen, H.P.; Andersen, J.R. Causes of Vitamin K Deficiency in Patients on Hemodialysis. Nutrients 2020, 12, 2513. [Google Scholar] [CrossRef]
- Fusaro, M.; Noale, M.; Valentina Viola, V.; Galli, F.; Tripepi, G.; Vajente, N.; Plebani, M.; Zaninotto, M.; Giuseppe Guglielmi, G.; Miotto, D.; et al. Vitamin K, Vertebral Fractures, Vascular Calcifications, and Mortality: VItamin K Italian (VIKI) Dialysis Study. J. Bone Miner. Res. 2012, 27, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Olleros Rodríguez, C.; Díaz Curie, M. Vitamin K and Bone Health: A Review on the Effects of Vitamin K Deficiency and Supplementation and the Effect of Non-Vitamin K Antagonist Oral Anticoagulants on Different Bone Parameters. Hindawi J. Osteopor. 2019, 2069176. [Google Scholar] [CrossRef]
- Posch, F.; Ay, C.; Herbert Stöger, H.; Kreutz, R.; Beyer-Westendorf, J. Exposure to vitamin k antagonists and kidney function decline in patients with atrial fibrillation and chronic kidney disease. Res. Pract. Thromb. Haemost. 2019, 3, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Voskamp, P.W.M.; Dekker, F.W.; Rookmaaker, M.B.; Verhaar, M.C.; Jan, W.; Bos, W.J.W.; van Diepen, M.; Ocak, G. Vitamin K antagonist use and renal function in pre-dialysis patients. Clin. Epidemiol. 2018, 10, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.-C.; Zhou, L.-Y.; Shen, L.; Zhang, C.; Pu, J.; Lin, H.-W.; Liu, X.-Y. Non-vitamin K Antagonist Oral Anticoagulants vs. Warfarin at Risk of Fractures: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vriese, A.S.; Caluwé, R.; Pyfferoen, L.; De Bacquer, D.; De Boeck, K.; Delanote, J.; De Surgeloose, D.; Van Hoenacker, P.; Van Vlem, B.; Verbeke, F. Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: The Valkyrie Study. JASN 2020, 31, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Lucenteforte, E.; Bettiol, A.; Lombardi, N.; Mugelli, A.; Vannacci, A. Risk of bone fractures among users of oral anticoagulants: An administrative database cohort study. Eur. J. Intern. Med. 2017, 44, e30–e31. [Google Scholar] [CrossRef] [PubMed]
- Fiordellisi, W.; Schweizer, M. A Systematic Review and Meta-analysis of the Association between Vitamin K Antagonist Use and Fracture. J. Gen. Intern. Med. 2018, 34, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, M.; Stein, A.; Stein, G. How to Manage Functional Vitamin K Deficiency in CKD. Am. J. Kidney Dis. 2012, 60, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3–5. Pol. Arch. Med. Wewn. 2015, 125, 631–640. [Google Scholar] [CrossRef]
- Cockayne, S.; Adamson, J.; Lanham-New, S.; Shearer, M.J.; Gilbody, S.; Torgerson, D.J. Vitamin K and the Prevention of Fractures. Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch. Intern. Med. 2006, 166, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Weber, P.; Willett, W.C.; Rockett, H.; Booth, S.L.; Colditz, G.A. Vitamin K intake and hip fractures in women: A prospective study. Am. J. Clin. Nutr. 1999, 69, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Witham, M.D.; Lees, J.S.; White, M.; Band, M.; Bell, S.; Chantler, D.J.; Ford, I.; Fulton, R.L.; Kennedy, G.; Littleford, R.C.; et al. Vitamin K Supplementation to Improve Vascular Stiffness in CKD: The K4Kidneys Randomized Controlled Trial. JASN 2020, 31, 2434–2445. [Google Scholar] [CrossRef]
- Keyzer, C.A.; Vermeer, C.; Joosten, M.M.; Knapen, M.H.J.; Drummen, N.E.A.; Navis, G.; Bakker, S.J.L.; de Borst, M.H. Vitamin K Status and Mortality After Kidney Transplantation: A Cohort Study. Am. J. Kidney Dis. 2015, 65, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.S.; Rankin, A.J.; Keith, A.; Gillis, K.A.; Zhu, L.Y.; Mangion, K.; Rutherford, E.; Roditi, G.H.; Witham, M.D.; Chantler, D.; et al. The ViKTORIES trial: A randomized, double-blind, placebo-controlled trial of vitamin K supplementation to improve vascular health in kidney transplant recipients. Am. J. Transplant. 2021, 21, 3356–3368. [Google Scholar] [CrossRef] [PubMed]
- Mosa, M.F.I.; Harfoosh, A.K. Role of Vitamin K Therapy in Prevention of Vascular Calcification in Chronic Kidney Disease. EJMED 2020, 2. [Google Scholar] [CrossRef]
- Lees, J.S.; Chapman, F.A.; Witham, M.D.; Jardine, A.G.; Mark, P.B. Vitamin K status, supplementation and vascular disease: A systematic review and meta-analysis. Heart 2019, 105, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.G.; Hariri, E.; Daaboul, Y.; Korjian, S.; El Alam, A.; Protogerou, A.D.; Kilany, H.; Karam, A.; Stephan, A.; Bahous, A.A. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients-a single-arm, single-center clinical trial. J. Am. Soc. Hypertens. 2017, 11, 589–597. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year, [Ref.] | Vitamin K Type, Dosage and Follow-Up | Type of Patients | Outcomes |
|---|---|---|---|
| Geleijnse et al. 2004 [19] |
| Women and men aged ≥55 years without MI n = 4807 | VK1—no association with incidents of CHD mortality, all-cause mortality and aortic calcification VK2—reduction of CHD mortality and inverse relation to all-cause mortality and severe aortic calcification |
| Gast et al. 2009 [20] | Mean VK1 intake 211.7 ± 100.3 µg/d Mean VK2 intake 29 ± 12.8 µg/d 8.1 ± 1.6 years | Postmenopausal women n = 16,057 | Inverse association between VK2 intake and risk of CHD; no significant relationship for VK1 intake |
| Haugsgjerd et al. [21] | VK1 intake median 48 µg/d/1000 kcal VK2 intake median 7 µg/d/1000 kcal 11 years | Men and women aged 46–49 years n = 2987 | No association between VK1 and CHD Higher intake of VK2 is related with lower risk of CHD (p = 0.03) |
| Brandenburg et al. 2017 [23] | VK1 2 mg/d n = 38 PL n = 34 for 12 months | patients with asymptomatic or mildly symptomatic AVC n = 72 | Lower progression of AVC by 12% (p = 0.03) after VK1 vs. PL ↓ plasma dp-ucMGP by 45% (p < 0.001) in the VK1 group; |
| Kurnatowska et al. 2015 [57] | VK2 90 µg/d + Vit. D 10 µg/d n = 29 or Vit. D 10 µg/d alone n = 13 for 270 ± 12 days | non-dialyzed patients with CKD in stages 3–5 n = 42 | VK2 + VitD—lower increase of CCA-IMT (p = 0.005) compared to VitD alone VK2 + VitD: ↓ dp-ucMGP (p = 0.02), OC (p = 0.04) and OPG (p = 0.02) levels |
| Witham et al. [60] | VK2 400 µg/d or PL for 1 year | patients with CKD in stages 3b or 4 n = 159 | No effect on carotid-femoral PWV (primary outcome), AI, BP, B-type natriuretic peptide and physical function (secondary outcomes) |
| Aoun et al. 2017 [38] | VK2 (menaquinone-7) 360 μg/d for 4 weeks | hemodialysis adult patients n = 50 | ↓ dp-ucMGP plasma levels (p = 0.01) |
| Lees et al. 2021 [62] | VK3 (menadiol diphosphate) 5 mg/d n = 45 or PL n = 45 thrice weekly for 12 months | kidney transplant recipients n = 90 | No impact on vascular stiffness and vascular calcifications |
| Mosa et al. 2020 [63] | VK1 10 mg after each dialysis for 1 year n = 20 or No VK1 n = 20 | adult patients with ESRD regularly hemodialysed n = 40 | ↑ in MGP levels (p < 0.05) in VK1 group VK1—no significant changes in CIMT and AACS (no significant progression) No VK group—↑ CIMT (p < 0.005) and ↑ AACS (p < 0.005) (significant progression) |
| Mansour et al. 2017 [65] | VK2 360 μg/d for 8 weeks | renal transplant recipients n = 60 | a 14.2% reduction in mean cfPWV (p < 0.001) ↓ dp-ucMGP by 55.1% with a ↓ in the prevalence of subclinical deficiency by 40% (p = 0.001) improvement in AS related independently with the ↓ dp-ucMGP (p = 0.014) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, A.; Koziarska-Rościszewska, M.; Rysz, J.; Stępień, M. Biological Role of Vitamin K—With Particular Emphasis on Cardiovascular and Renal Aspects. Nutrients 2022, 14, 262. https://doi.org/10.3390/nu14020262
Stępień A, Koziarska-Rościszewska M, Rysz J, Stępień M. Biological Role of Vitamin K—With Particular Emphasis on Cardiovascular and Renal Aspects. Nutrients. 2022; 14(2):262. https://doi.org/10.3390/nu14020262
Chicago/Turabian StyleStępień, Anna, Małgorzata Koziarska-Rościszewska, Jacek Rysz, and Mariusz Stępień. 2022. "Biological Role of Vitamin K—With Particular Emphasis on Cardiovascular and Renal Aspects" Nutrients 14, no. 2: 262. https://doi.org/10.3390/nu14020262
APA StyleStępień, A., Koziarska-Rościszewska, M., Rysz, J., & Stępień, M. (2022). Biological Role of Vitamin K—With Particular Emphasis on Cardiovascular and Renal Aspects. Nutrients, 14(2), 262. https://doi.org/10.3390/nu14020262







