Priming with Retinoic Acid, an Active Metabolite of Vitamin A, Increases Vitamin A Uptake in the Small Intestine of Neonatal Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Oral Dose Preparation
2.3. Kinetic Studies
2.4. VA Mass Quantification
2.5. 3H Tracer Determination
2.6. Kinetic Data Calculations
2.7. Statistical Analysis
3. Results
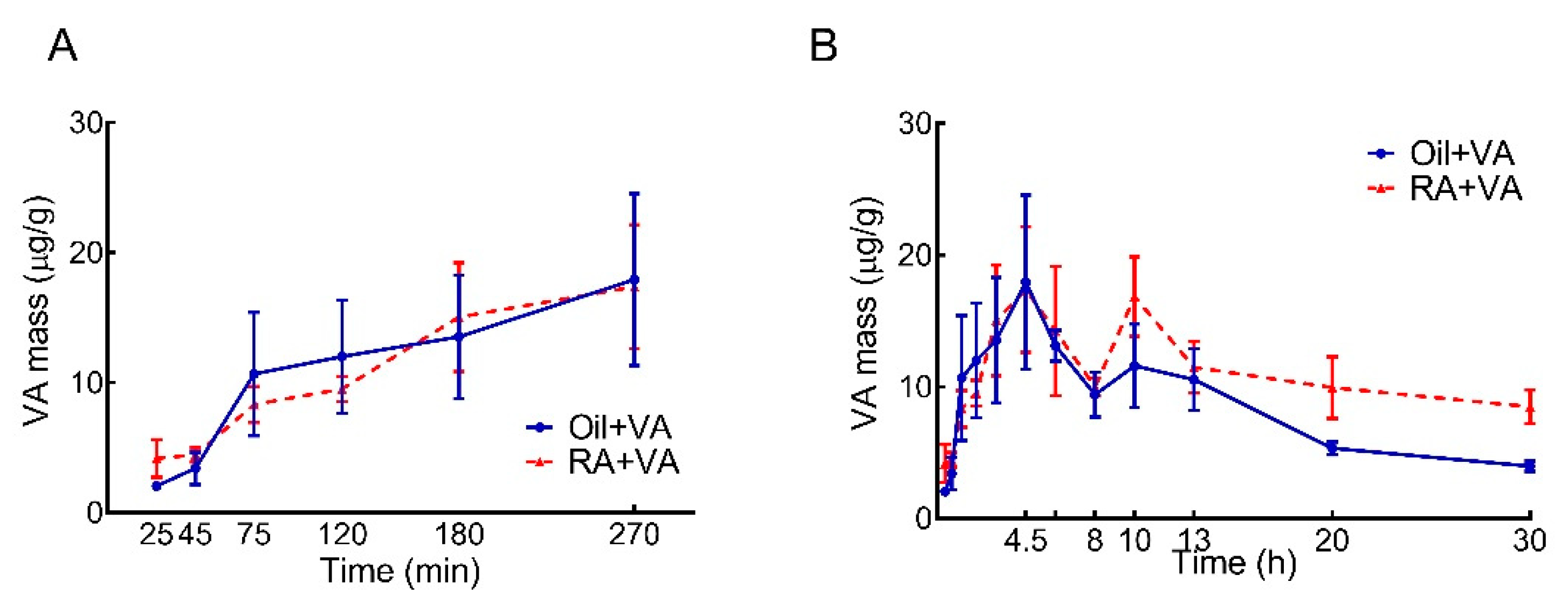
3.1. RA Pretreatment Stimulated VA Accumulation in the Intestine of Neonatal Rats
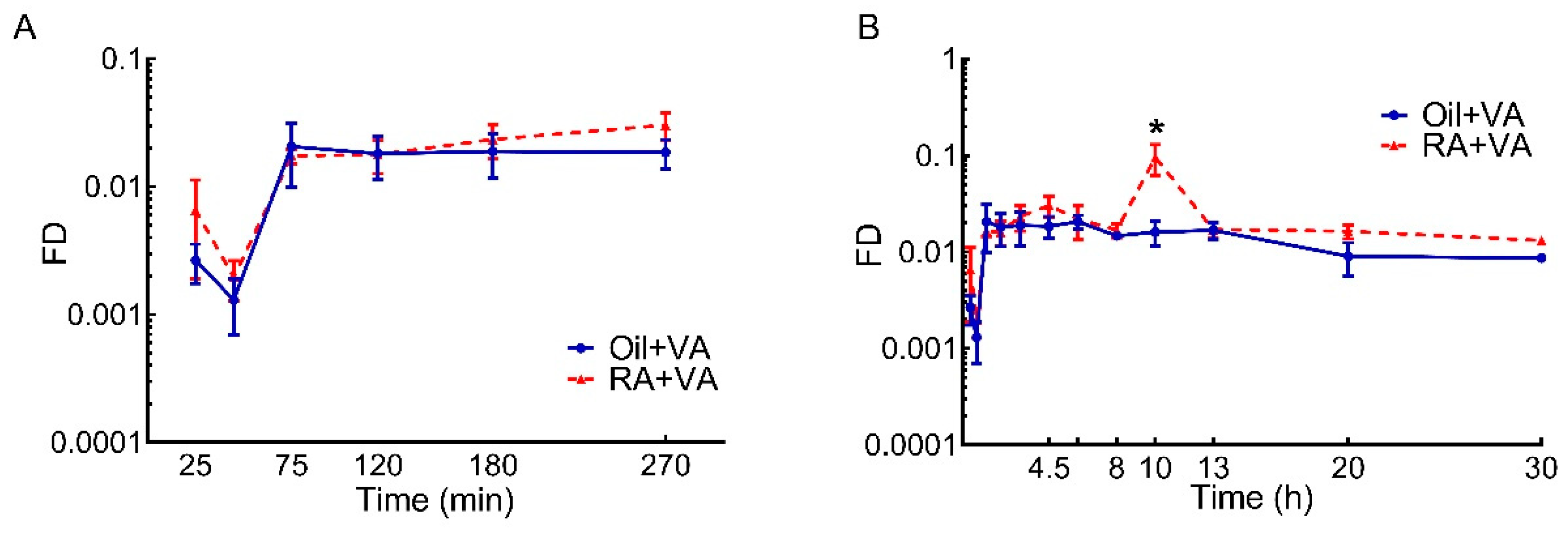
3.2. RA Stimulated the Uptake of Newly Ingested 3H Labeled VA
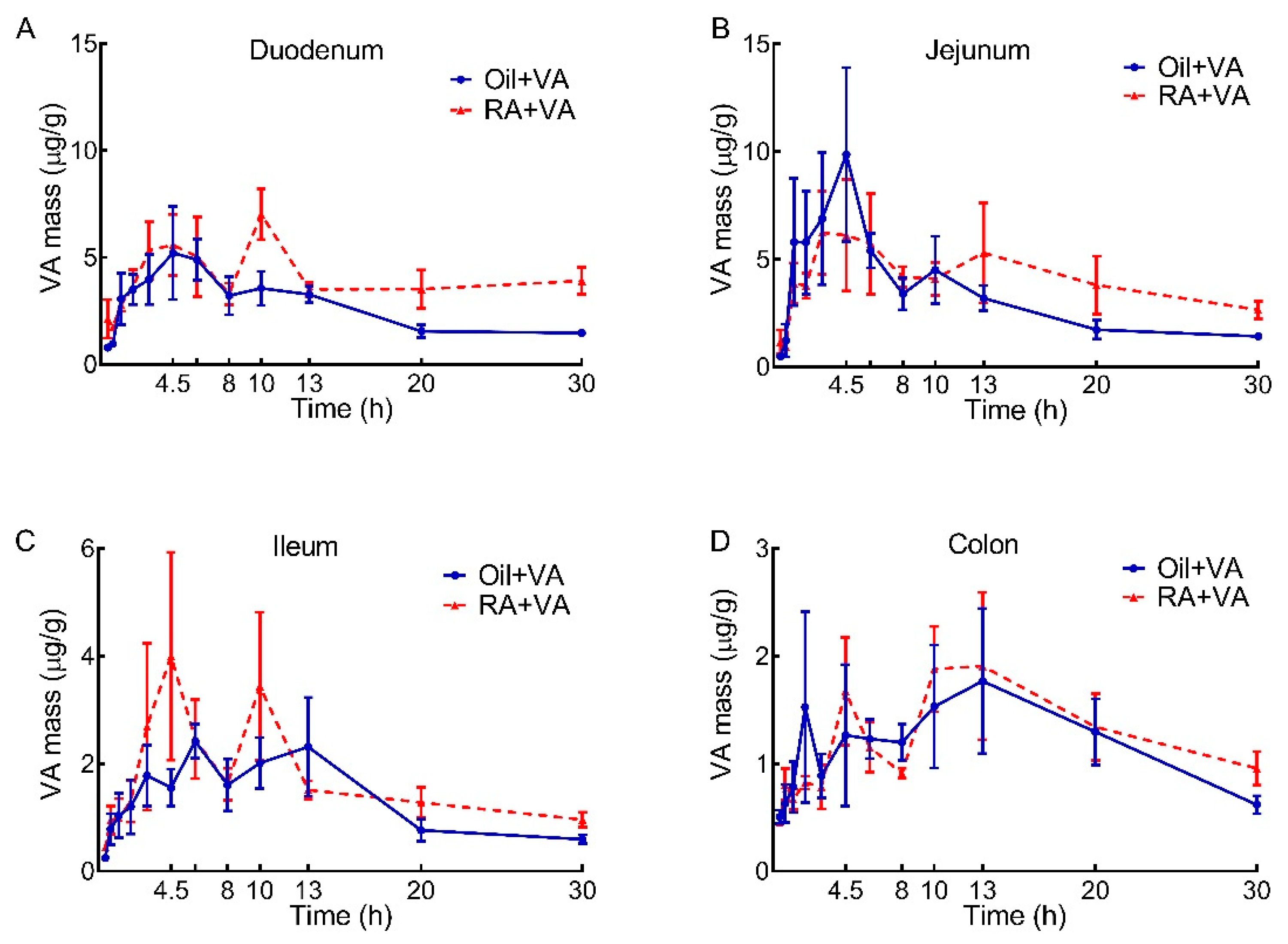
3.3. VA Distribution in Duodenum, Jejunum, Ileum and Colon
3.4. RA Pretreatment Stimulated the Uptake of 3H-Labeled VA in Duodenum, Jejunum, Ileum and Colon
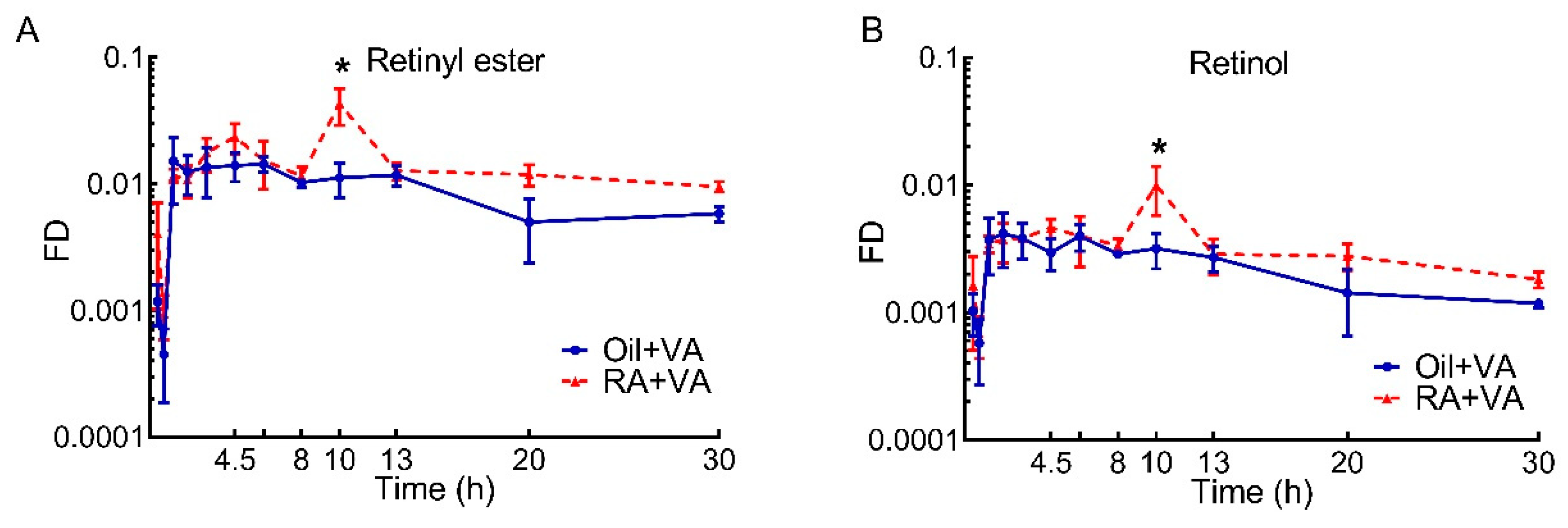
3.5. A Higher Proportion of Newly Ingested 3H Labeled VA Was Present in RE Than Retinol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am. J. Hum. Biol. 2007, 19, 1–19. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Horton, T.H. Fetal origins of developmental plasticity: Animal models of induced life history variation. Am. J. Hum. Biol. 2005, 17, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A. Programming by early nutrition: An experimental approach. J. Nutr. 1998, 128, 401S–406S. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, L.; De Magalhaes Filho, C.; Leneuve, P.; Xu, J.; Brunel, N.; Chatziantoniou, C.; Le Bouc, Y.; Holzenberger, M. Early postnatal nutrition determines somatotropic function in mice. Endocrinology 2009, 150, 314–323. [Google Scholar] [CrossRef]
- Gicquel, C.; El-Osta, A.; Le Bouc, Y. Epigenetic regulation and fetal programming. Best Pr. Res. Clin. Endocrinol. Metab. 2008, 22, 1–16. [Google Scholar] [CrossRef]
- Morelli, L. Postnatal development of intestinal microflora as influenced by infant nutrition. J. Nutr. 2008, 138, 1791S–1795S. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 10 November 2021).
- Ross, A.C.; Gardner, E.M. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. Adv. Exp. Med. Biol. 1994, 352, 187–200. [Google Scholar] [CrossRef]
- Chytil, F. The lungs and vitamin A. Am. J. Physiol. 1992, 262, L517–L527. [Google Scholar] [CrossRef]
- Arana, A.; Mendizabal, J.A.; Alzon, M.; Soret, B.; Purroy, A. The effect of vitamin A supplementation on postnatal adipose tissue development of lambs. J. Anim. Sci. 2008, 86, 3393–3400. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, Q.; Gong, M.; Chen, L.; Wen, E.Y.; Bi, Y.; Zhang, Y.; Shi, Y.; Qu, P.; Liu, Y.X.; et al. Vitamin A deficiency impairs postnatal cognitive function via inhibition of neuronal calcium excitability in hippocampus. J. Neurochem. 2012, 121, 932–943. [Google Scholar] [CrossRef]
- Ruhl, R.; Hanel, A.; Garcia, A.L.; Dahten, A.; Herz, U.; Schweigert, F.J.; Worm, M. Role of vitamin A elimination or supplementation diets during postnatal development on the allergic sensitisation in mice. Mol. Nutr. Food Res. 2007, 51, 1173–1181. [Google Scholar] [CrossRef]
- Semba, R.D. The role of vitamin A and related retinoids in immune function. Nutr. Rev. 1998, 56, S38–S48. [Google Scholar] [CrossRef]
- Sommer, A.; Katz, J.; Tarwotjo, I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am. J. Clin. Nutr. 1984, 40, 1090–1095. [Google Scholar] [CrossRef]
- Thornton, K.A.; Mora-Plazas, M.; Marin, C.; Villamor, E. Vitamin A deficiency is associated with gastrointestinal and respiratory morbidity in school-age children. J. Nutr. 2014, 144, 496–503. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, Y.; Yang, T.; Zhan, X.; Li, Z.; Hu, H.; Li, T.; Chen, J. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J. Clin. Biochem. Nutr. 2016, 59, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- WHO. Guideline: Vitamin A Supplementation in Infants and Children 6–59 Months of Age; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Mayo-Wilson, E.; Imdad, A.; Herzer, K.; Yakoob, M.Y.; Bhutta, Z.A. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: Systematic review and meta-analysis. BMJ 2011, 343, d5094. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Fawzi, W.W. Vitamin A supplementation: Implications for morbidity and mortality in children. J. Infect. Dis. 2000, 182 (Suppl. 1), S122–S133. [Google Scholar] [CrossRef]
- Rahmathullah, L.; Tielsch, J.M.; Thulasiraj, R.D.; Katz, J.; Coles, C.; Devi, S.; John, R.; Prakash, K.; Sadanand, A.V.; Edwin, N.; et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: Community based randomised trial in southern India. BMJ 2003, 327, 254. [Google Scholar] [CrossRef] [PubMed]
- Klemm, R.D.; Labrique, A.B.; Christian, P.; Rashid, M.; Shamim, A.A.; Katz, J.; Sommer, A.; West, K.P., Jr. Newborn vitamin A supplementation reduced infant mortality in rural Bangladesh. Pediatrics 2008, 122, e242–e250. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Taneja, S.; Bhatia, K.; Yoshida, S.; Kaur, J.; Dube, B.; Toteja, G.S.; Bahl, R.; Fontaine, O.; Martines, J.; et al. Efficacy of early neonatal supplementation with vitamin A to reduce mortality in infancy in Haryana, India (Neovita): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 1333–1342. [Google Scholar] [CrossRef]
- Awasthi, S.; Peto, R.; Read, S.; Clark, S.; Pande, V.; Bundy, D.; Team, D. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 2013, 381, 1469–1477. [Google Scholar] [CrossRef]
- Soofi, S.; Ariff, S.; Sadiq, K.; Habib, A.; Bhatti, Z.; Ahmad, I.; Hussain, M.; Ali, N.; Cousens, S.; Bhutta, Z.A. Evaluation of the uptake and impact of neonatal vitamin A supplementation delivered through the Lady Health Worker programme on neonatal and infant morbidity and mortality in rural Pakistan: An effectiveness trial. Arch. Dis. Child 2017, 102, 216–223. [Google Scholar] [CrossRef] [PubMed]
- West, K.P., Jr.; Christian, P.; Labrique, A.B.; Rashid, M.; Shamim, A.A.; Klemm, R.D.; Massie, A.B.; Mehra, S.; Schulze, K.J.; Ali, H.; et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: A cluster randomized trial. JAMA 2011, 305, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wray, A.E.; Green, M.H.; Ross, A.C. Compartmental modeling of whole-body vitamin A kinetics in unsupplemented and vitamin A-retinoic acid-supplemented neonatal rats. J. Lipid Res. 2014, 55, 1738–1749. [Google Scholar] [CrossRef]
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; von Lintig, J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef]
- Bachmann, H.; Desbarats, A.; Pattison, P.; Sedgewick, M.; Riss, G.; Wyss, A.; Cardinault, N.; Duszka, C.; Goralczyk, R.; Grolier, P. Feedback regulation of beta,beta-carotene 15,15′-monooxygenase by retinoic acid in rats and chickens. J. Nutr. 2002, 132, 3616–3622. [Google Scholar] [CrossRef]
- Ross, A.C.; Li, N.Q.; Wu, L. The components of VARA, a nutrient-metabolite combination of vitamin A and retinoic acid, act efficiently together and separately to increase retinyl esters in the lungs of neonatal rats. J. Nutr. 2006, 136, 2803–2807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Kayne, L.H.; D’Argenio, D.Z.; Meyer, J.H.; Hu, M.S.; Jamgotchian, N.; Lee, D.B. Analysis of segmental phosphate absorption in intact rats. A compartmental analysis approach. J. Clin. Investig. 1993, 91, 915–922. [Google Scholar] [CrossRef]
- Li, Y.; Wei, C.H.; Xiao, X.; Green, M.H.; Ross, A.C. Perturbed vitamin A status induced by iron deficiency is corrected by iron repletion in rats with pre-existing iron deficiency. J. Nutr. 2020, 150, 1989–1995. [Google Scholar] [CrossRef]
- Li, Y.; Wei, C.H.; Green, M.H.; Ross, A.C. Dietary iron repletion stimulates hepatic mobilization of vitamin A in previously iron-deficient rats as determined by model-based compartmental analysis. J. Nutr. 2020, 150, 1982–1988. [Google Scholar] [CrossRef]
- Ross, A.C. Retinol esterification by mammary gland microsomes from the lactating rat. J. Lipid Res. 1982, 23, 133–144. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fat-soluble vitamin intestinal absorption: Absorption sites in the intestine and interactions for absorption. Food Chem. 2015, 172, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.A.; Ong, D.E. Cell-specific immunohistochemical localization of a cellular retinol-binding protein (type two) in the small intestine of rat. Proc. Natl. Acad. Sci. USA 1985, 82, 4707–4711. [Google Scholar] [CrossRef]
- Levin, M.S.; Davis, A.E. Retinoic acid increases cellular retinol binding protein II mRNA and retinol uptake in the human intestinal Caco-2 cell line. J. Nutr. 1997, 127, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, C.J.; Green, J.B.; Green, M.H. Dietary retinoic acid alters vitamin A kinetics in both the whole body and in specific organs of rats with low vitamin A status. J. Nutr. 2005, 135, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Senoo, H.; Mezaki, Y.; Morii, M.; Hebiguchi, T.; Miura, M.; Imai, K. Uptake and storage of vitamin A as lipid droplets in the cytoplasm of cells in the lamina propria mucosae of the rat intestine. Cell Biol. Int. 2013, 37, 1171–1180. [Google Scholar] [CrossRef]
- Chen, B.; Liu, S.; Feng, D.; Xiao, L.; Yang, T.; Li, T.; Sun, W.; Chen, J. Vitamin A deficiency in the early-life periods alters a diversity of the colonic mucosal microbiota in rats. Front. Nutr. 2020, 7, 580780. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Cui, T.; Yang, T.; Liu, L.; Li, T.; Chen, J. Retinoic acid facilitates toll-like receptor 4 expression to improve intestinal barrier function through retinoic acid receptor beta. Cell Physiol. Biochem. 2017, 42, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Cui, T.; Liu, S.; Chen, B.; Wang, Y.; Yang, T.; Li, T.; Chen, J. Vitamin A supplementation improves the intestinal mucosal barrier and facilitates the expression of tight junction proteins in rats with diarrhea. Nutrition 2019, 57, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Schottstedt, T.; Muri, C.; Morel, C.; Philipona, C.; Hammon, H.M.; Blum, J.W. Effects of feeding vitamin A and lactoferrin on epithelium of lymphoid tissues of intestine of neonatal calves. J. Dairy Sci. 2005, 88, 1050–1061. [Google Scholar] [CrossRef]
- Tan, L.; Green, M.H.; Ross, A.C. Vitamin A kinetics in neonatal rats vs. adult rats: Comparisons from model-based compartmental analysis. J. Nutr. 2015, 145, 403–410. [Google Scholar] [CrossRef]
- Hodges, J.K.; Tan, L.; Green, M.H.; Ross, A.C. Vitamin A supplementation redirects the flow of retinyl esters from peripheral to central organs of neonatal rats raised under vitamin A-marginal conditions. Am. J. Clin. Nutr. 2017, 105, 1110–1121. [Google Scholar] [CrossRef][Green Version]

| Area under the Curve, μg/g × h | |||||
|---|---|---|---|---|---|
| Selected Time Interval, h | 0–8 | 8–13 | 13–20 | 20–30 | Total (0–30) |
| Oil + VA | 95.1 | 54.2 | 55.5 | 46.5 | 251 |
| RA + VA | 95.8 | 69.3 | 74.9 | 92.0 | 332 |
| Area under the Curve | |||||
|---|---|---|---|---|---|
| Selected Time Interval, h | 0–8 | 8–13 | 13–20 | 20–30 | Total (0–30) |
| Oil + VA | 0.132 | 0.080 | 0.090 | 0.088 | 0.39 |
| RA + VA | 0.156 | 0.282 | 0.117 | 0.147 | 0.702 |
| Area under the Curve, μg/g × h | ||||
|---|---|---|---|---|
| Intestinal Segment | Duodenum | Jejunum | Ileum | Colon |
| Oil + VA | 78.9 | 97.9 | 40.0 | 36.5 |
| RA + VA | 121 | 124 | 50.9 | 39.3 |
| Area under the Curve | ||||
|---|---|---|---|---|
| Intestinal Segment | Duodenum | Jejunum | Ileum | Colon |
| Oil + VA | 0.244 | 0.0792 | 0.0330 | 0.0339 |
| RA + VA | 0.406 | 0.108 | 0.0569 | 0.0379 |
| Area under the Curve | ||||||
|---|---|---|---|---|---|---|
| Selected Time Interval, h | 0–8 | 8–13 | 13–20 | 20–30 | Total (0–30) | |
| Retinyl ester | Oil + VA | 0.094 | 0.056 | 0.059 | 0.054 | 0.263 |
| RA + VA | 0.113 | 0.138 | 0.086 | 0.107 | 0.444 | |
| Retinol | Oil + VA | 0.025 | 0.015 | 0.014 | 0.013 | 0.067 |
| RA + VA | 0.028 | 0.032 | 0.020 | 0.023 | 0.103 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wei, C.-H.; Hodges, J.K.; Green, M.H.; Ross, A.C. Priming with Retinoic Acid, an Active Metabolite of Vitamin A, Increases Vitamin A Uptake in the Small Intestine of Neonatal Rats. Nutrients 2021, 13, 4275. https://doi.org/10.3390/nu13124275
Li Y, Wei C-H, Hodges JK, Green MH, Ross AC. Priming with Retinoic Acid, an Active Metabolite of Vitamin A, Increases Vitamin A Uptake in the Small Intestine of Neonatal Rats. Nutrients. 2021; 13(12):4275. https://doi.org/10.3390/nu13124275
Chicago/Turabian StyleLi, Yaqi, Cheng-Hsin Wei, J. Kalina Hodges, Michael H. Green, and A. Catharine Ross. 2021. "Priming with Retinoic Acid, an Active Metabolite of Vitamin A, Increases Vitamin A Uptake in the Small Intestine of Neonatal Rats" Nutrients 13, no. 12: 4275. https://doi.org/10.3390/nu13124275
APA StyleLi, Y., Wei, C.-H., Hodges, J. K., Green, M. H., & Ross, A. C. (2021). Priming with Retinoic Acid, an Active Metabolite of Vitamin A, Increases Vitamin A Uptake in the Small Intestine of Neonatal Rats. Nutrients, 13(12), 4275. https://doi.org/10.3390/nu13124275






