Abstract
Elucidating the mechanisms of bacterial translocation is crucial for the prevention and treatment of neonatal sepsis. In the present study, we aimed to evaluate the potential of lactoferrin to inhibit the development of late-onset blood infection in neonates. Our investigation evaluates the role of key stress factors leading to the translocation of intestinal bacteria into the bloodstream and, consequently, the development of life-threatening sepsis. Three stress factors, namely weaning, intraperitoneal administration of Gram-positive cocci and oral intake of Gram-negative rods, were found to act synergistically. We developed a novel model of rat pups sepsis induced by bacterial translocation and observed the inhibition of this process by supplementation of various forms of lactoferrin: iron-depleted (apolactoferrin), iron-saturated (hololactoferrin) and manganese-saturated lactoferrin. Additionally, lactoferrin saturated with manganese significantly increases the Lactobacillus bacterial population, which contributes to the fortification of the intestinal barrier and inhibits the translocation phenomenon. The acquired knowledge can be used to limit the development of sepsis in newborns in hospital neonatal intensive care units.
1. Introduction
Neonatal sepsis still represents an important cause of mortality and morbidity among infants [1]. Rapid deaths of newborns primarily result from the extremely short time that may elapse from the onset of an acute infection to the development of bloodstream infection (BSI) and sepsis. According to ECDC data (2011–2012, based on the European Centre for Disease Prevention and Control data), bloodstream infections in newborns are responsible for over 44.6% of all healthcare-associated infections [2]. Neonatal sepsis may be categorized as early onset sepsis (EOS) or late-onset sepsis (LOS). Early onset sepsis appears up to 72 h after delivery and is usually associated with colonization by high-virulence etiological factors, from both the maternal vaginal (Streptococcus agalactiae and Streptococcus pyogenes) and gastrointestinal (GI) microbiota (Escherichia coli and other Gram-negatives rods [3]. In contrast, late-onset sepsis is due to immature immunity including the skin, respiratory and gastrointestinal (GI) systems, need for invasive procedures, environmental exposures and prolonged hospital stay. In this case, positive blood cultures may be associated with both catheter-associated infection, nosocomial transmission and colonization factors [4].
Our attention has been focused on bloodstream infections originating from the gastrointestinal tract in very low birth weight infants. Bacterial translocation (BT), defined as invasion of indigenous intestinal bacteria through the mucosa into tissues, might therefore contribute to the development of sepsis [5]. Bacterial translocation from the gastrointestinal tract (GI) is an important pathway initiating late neonatal sepsis. The newborn’s own microbiota, depending on its qualitative and quantitative composition, external stress factors, immaturity of intestinal epithelium and mucosal immunity, as well as suboptimal nutrition, in particular lack of breast milk feeding, play major roles in facilitating bacterial translocation [2]. On the other side, there are reports claiming that supplementation with lactoferrin, probiotics or prebiotics could serve to prevent bacterial translocation in neonates and inhibit the development of sepsis [5].
Understanding the pathomechanism and methods of counteracting LOS in newborns is still very difficult due to the lack of an appropriate animal model. There have been several established neonatal rodent models, e.g., with stress induced by weaning, intestinal bacterial translocation and induced sepsis [6]. In rat pups, mother separation is a stress factor that stimulates immune response, increases levels of inflammatory triggers and often leads to gut microbiota distribution [7]. Neonate rats at the 12th postnatal day can be used as a model for late sepsis and preterm human newborns because of morphologically immature intestine with high permeability [8].
The aim of our work was to investigate the factors that the phenomenon of bacterial translocation depends on, as well as the mechanisms regulating intestinal leakage and ways to reduce the risk of late sepsis by feeding newborns. We strived to establish an experimental model of bacterial translocation induced by systemic inflammation triggered by intraperitoneal staphylococcal infection following the oral administration of Escherichia coli. Our study has shown that oral administration of various form of lactoferrin in the established model can prevent detrimental bacterial translocation. All tested forms, i.e., iron-depleted, iron-saturated and manganese-saturated lactoferrin, mitigated the translocation of E. coli from intestinal lumen to blood. Additionally, manganese-saturated protein had a positive impact on the population numbers on lactobacilli.
2. Materials and Methods
2.1. Animals
For a better understanding of the pathomechanism and for evaluating methods of counteracting LOS in newborns, we developed a new rat model and used it in the present research. Animal protocols conformed with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and approved by the Jagiellonian University Ethical Committee on Animal Experiments (no. 190/2012). Male albino Wistar rat pups [WistarKrf (Wi) Wu)] with a 20–30 g body weight were used in the experiment. Animals were housed in standard cages in temperature-controlled rooms (18–22 °C, 50–60% humidity) under a 12 h light cycle (06:00–18:00) and appropriate environmental enrichment was introduced in the cages. Standard laboratory pelleted formula (Labofeed B, Pasze Kcynia, Poland, containing 25% protein, 8% fat and 67% carbohydrates) and tap water were provided ad libitum. The entire process of developing our model required many preliminary experiments to determine the fastest weaning time, body weight of pups or the choice of a day of the experiment in which the phenomenon of intestinal translocation typical of LOS would take place.
Based on the pilot study results, day 12 was selected as appropriate for weaning and starting the experiment. From that day on, the animals can live independently. The possibility of taking water and food was additionally facilitated for the animals: bottles with an elongated cap were used, and the food was kept in the cage in a crumbled form, adapted to the size of the animal. The reduction in the amount of food and traces of its bites were observed daily. All animals, despite significant weight loss and a drop in body temperature, survived the withdrawal period. The experiment carried out provides not only scientific justification, but also practical possibilities of using its results.
2.2. Grouping
The first stage involved the experiment using 48 pups, which were randomly assigned to the experimental groups: non-weaned control group (NW, n = 6), weaned group (W, n = 6), weaned pups with intraperitoneal administration of Staphylococcus haemolyticus 186 (W+Sh, n = 6), weaned pups with oral administration of Escherichia coli 3A/1 (W+Ec, n = 6) and target model weaned group with administration of both intraperitoneal S. haemolyticus 186 and oral E. coli 3A/1 (W+Sh+Ec, n = 24).
The second stage of the study included 42 male pups that served as the model group (W+Sh+Ec), and were used to study the effect of oral administration of various lactoferrin forms for the following groups: metal-depleted apoLf (W+Sh+Ec+apoLf, n = 12), iron-saturated holoLf (W+Sh+Ec+holoLf, n = 12), manganese-saturated MnLf (W+Sh+Ec+MnLf, n = 12).
2.3. Procedures Performed on Animals
Weaning. For 11 days after birth, pups remained with the mother and were fed mother’s milk. On day 12 of the experiment, pups were weaned and transferred to separate cages (max. 6 pups per cage).
Temperature and weight measurement. The temperature from anus and weight of the animals were monitored on days 12, 15, 16 and 17 at 9:00 a.m.
Staphylococcus administration. Selected strain: coagulase-negative Staphylococcus haemolyticus 186 was isolated from blood of a very low birth weight neonate with clinical symptoms of sepsis. Utilization of this strain was approved by the Bioethics Committee of Jagiellonian University Medical College (opinion no. KBET/221/B/2011). Bacterial strain was inoculated into 10 mL liquid TSB broth (Becton Dickinson, Franklin Lakes, NJ, USA). The cultures were carried out overnight at 37 °C. After a double wash (10,000 rpm, 3 min, RT), the obtained pellets were suspended in PBS, the OD was adjusted to correspond to 1 × 109 cfu/mL and a volume of 200 µL (dose of 2 × 108 cfu) was administered intraperitoneally on day 15 to pups in groups W+Sh and W+ Sh+Ec (Table 1.)

Table 1.
Procedures performed on animals in individual study groups during the experiments.
Escherichia coli administration. Selected strain: Escherichia coli 3A/1 was isolated from an infant after approval by the Bioethics Committee of Jagiellonian University Medical College (no. KBET/75/B from 15 November 2007). The strain was selected based on the frequency analyses of the genes responsible for synthesis of proteins for acquisition of iron ions [9,10]. Bacterial cultures were inoculated into 10 mL liquid TSB broth (Becton Dickinson, USA). The culture was carried out overnight at 37 °C. After a double wash (10,000 rpm, 3 min, RT), the obtained pellets were suspended in PBS, the OD adjusted to correspond to 1 × 109 cfu/mL and a volume of 100 µL (dose of 1 × 108 cfu) was administered orally, twice on day 16 to pups in groups W+Ec and W+Ec+Sh (Table 1).
Blood collection. To collect blood for microbial culture, a drop of blood was taken from the incised tip of the tail. This procedure was performed on days 15 and 16, 4 h after administration of the bacterial suspensions to the animals.
Lactoferrin administration. On days 14–16, lactoferrin forms differing in metal saturation were administered twice a day orally at a dose of 300 mg/kg/day (every day between 8 and 9 a.m. and 14 and 15 p.m. in the treatment room) in appropriate groups. The modified lactoferrin forms were used: iron-depleted (apolactoferrin, apoLf, 1.2 ± 0.2% Fe saturation), iron-saturated (hololactoferrin, holoLf, 71.8 ± 6.5% Fe saturation) and manganese-saturated lactoferrin (MnLf, 1.2 ± 0.2% Fe saturation, 47.1 ± 2.0% Mn3+ saturation) [11].
Intestinal permeability was assessed following oral administration of FITC-Dextran (FD4, Sigma-Aldrich, St. Louis, MO, USA). Pups were given FITC-Dextran orally (50 mg/100 g body weight), 4 h before sacrifice. Whole blood was obtained by cardiac puncture at the time of necropsy. FITC-Dextran measurements were performed in a plate reader at a fluorescence excitation of 480 nm and an emission wavelength of 520 nm [12].
Euthanasia and necropsy. On day 17, all animals were euthanized by anesthesia overdose (Pentobarbital, Morbital, Pulawy, route of administration i.p. 100 mg/kg body weight). During necropsy, blood samples, spleen and feces from large intestine were collected. Changes such as colonic congestion, intestinal bloating and colon fragility were assessed.
2.4. Clinical Score (CS) as Symptoms of Sepsis
The symptoms of systemic inflammation were evaluated by inspecting changes in motor activity, lethargy, shivering and piloerection. Each one of these conditions was scored as 0 (no observable symptom), 1 (a noticeable symptom) or 2 (a severe symptom). The total score was calculated by adding the individual scores of each parameter. Ranging from 0 (normal) to 8 (serious condition), these were monitored on days 12, 15, 16 and 17.
2.5. Bacterial Load as Translocation Evidence
Spleen and feces were aseptically collected and homogenized in sterile PBS. Homogenized tissues were subjected to serial log fold dilutions in sterile saline. The bacterial load in blood, spleens and feces was determined by plating 10-fold serial dilutions on agar plates: Columbia agar medium (SIGMA) with 5% sheep blood for isolation of Staphylococcus spp. and on MacConkey agar (BioMaxima, Lublin, Poland) for isolation of Escherichia coli. The plates were incubated for 24 h at 37 °C and the colonies were isolated. Lactobacillus bacteria were isolated from feces and cultured on MRS plates (Biomaxima) in anaerobic conditions at 37 °C. Translocation was confirmed by positive culture of any enteric E. coli strains (either commensal or 3A/1) in the blood or spleen (shown as total E. coli). Differentiation of E. coli 3A/1 strain vs. commensal bacteria was performed using antibiotic resistance pattern (diffusion-disk method with the used of AMP10 = 14 mm, CXM30 = 19 mm, AMC30 = 19 mm, MXF5 = 22 mm, CIP30 = 25 mm).
2.6. Statistical Analysis
For statistical analyses and graphic presentations of the obtained results, the R program [13] with the datarium, ggpubr and rstatix libraries was used. A comparison of mean values across more variables based on repeated observations was conducted by the repeated measures ANOVA. Comparison of means between groups was based on ANOVA with the Tukey test.
3. Results
3.1. Physiological Effect of the Development of the Disorders in Pups
Body mass differences between day 17 (necropsy) and day 12 (weaning) were analyzed between the experimental groups. All weaned groups (W, W+Sh, W+Ec, W+Sh+Ec) demonstrated significant decrease in the body weight between days 12 and 17, as compared to the non-weaned control group (NW) (Figure 1A). Temperature changes were compared likewise and revealed a significant decrease in temperature between days 12 and 17 for the W and W+Sh+Ec groups in comparison to the non-weaned group (NW) (Figure 1B). Clinical scores on days 15, 16 and 17 were the highest for the W+Sh+Ec group, significantly different from all the other groups. At the same time, the non-weaned group (NW) differed significantly from W, W+Sh and W+Ec groups (Figure 1C).
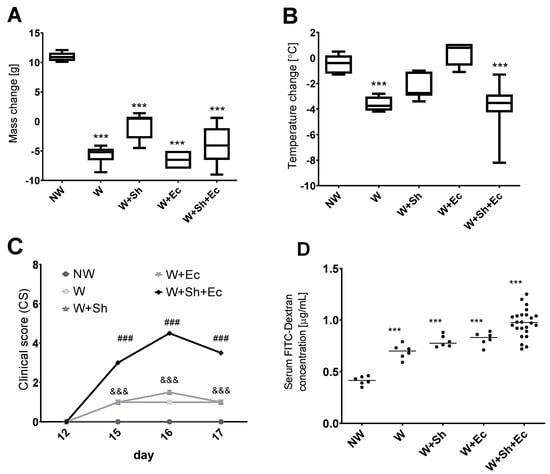
Figure 1.
Clinical parameters of animals in tested groups: change in weight (A) and temperature (B) between the day of necropsy (day 17) and weaning (day 12). (C) Clinical scores (reduced motor activity, lethargy, shivering, piloerection) were assessed on days 12, 15, 16 and 17. FITC-Dextran concentration was calculated using standard curve. (D) *** p < 0.001 vs. NW, ### p < 0.001 vs. NW, W, W+Ec and W+Sh, &&& p < 0.001 vs. NW and W+Sh+Ec. NW-non-weaned control group, W-weaned group, W+Sh-weaned pups with intraperitoneal administration of Staphylococcus haemolyticus 186, W+Ec-weaned pups with oral administration of Escherichia coli 3A/1, W+Sh+Ec-target model group weaned with administration of both intraperitoneal S. haemolyticus 186 and oral E. coli 3A/1.
3.2. Bacterial Load as Translocation Evidence
Weaning itself did not have a significant impact on the incidence of bacterial translocation from the intestinal lumen to the bloodstream (Figure 2A–C). However, the addition of another stress factor—intraperitoneal or orally delivered bacterial suspension—notably increased population numbers of E. coli, both in blood (Figure 2A,B) and spleen (Figure 2C), for respective groups (W+Sh, W+Ec and W+Sh+Ec vs. NW group). This increase was statistically significant only for the group with three stress factors (W+Sh+Ec). All tested animals in the model group (W+Sh+Ec) had E. coli present in their spleen compared to none in the NW group and 33% in intermediate groups (W, W+Sh and W+Ec). At the same time, fecal level of total E. coli was not altered (Figure 2D). Microbiological data were confirmed by analysis of serum FITC-Dextran concentrations. Unsurprisingly, the non-weaned control group (NW) showed the lowest levels of serum FITC-Dextran (a marker of intestinal translocation), significantly lower than all other groups (Figure 1D). On the other hand, the model group (W+Sh+Ec) had the highest levels of FITC-Dextran in serum, significantly higher than all other groups.
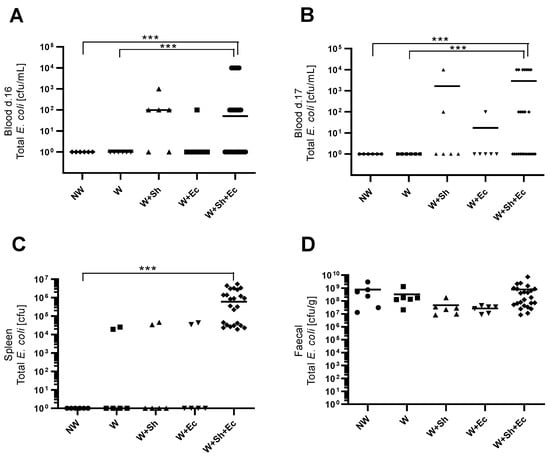
Figure 2.
Population numbers of total E. coli in blood d. 16 (A), blood d. 17 (B), spleen (C) and feces (D). *** p < 0.01. NW-non-weaned control group, W-weaned group, W+Sh-weaned pups with intraperitoneal administration of Staphylococcus haemolyticus 186, W+Ec-weaned pups with oral administration of Escherichia coli 3A/1, W+Sh+Ec-target model group weaned with administration of both intraperitoneal S. haemolyticus 186 and oral E. coli 3A/1.
3.3. Lactoferrin Supplementation
Oral administration of lactoferrin (no matter which form) seemed to be beneficial for the overall physiology of the tested animals. Negative changes in both mass (Figure 3A) and temperature (Figure 3B) in the course of the experiment were ameliorated in the groups in which lactoferrin was administered. There was also a slight decrease in the clinical scores of the lactoferrin-supplemented groups compared to the model (W+Sh+Ec) group, but no statistically significant changes were observed (Figure 3C). Orally supplemented lactoferrin mitigated the bacterial translocation of E.coli from intestinal lumen to blood (Figure 4A,B) and spleen (Figure 5C). We observed a significant decrease in total E. coli numbers in blood and spleen in the case of all tested forms of lactoferrin (regardless of metal saturation). At the same time, fecal levels of E. coli remained the same (Figure 4D) while population numbers of lactobacilli were significantly increased in the group supplemented with manganese-saturated lactoferrin (Figure 5). This phenomenon could be attributed to the release of manganese from this form of lactoferrin and stimulation of growth of these probiotic bacteria. FITC-Dextran levels confirm the previous results—translocation of this marker was significantly reduced in lactoferrin-supplemented groups compared to the model group (W+Sh+Ec, Figure 3D).
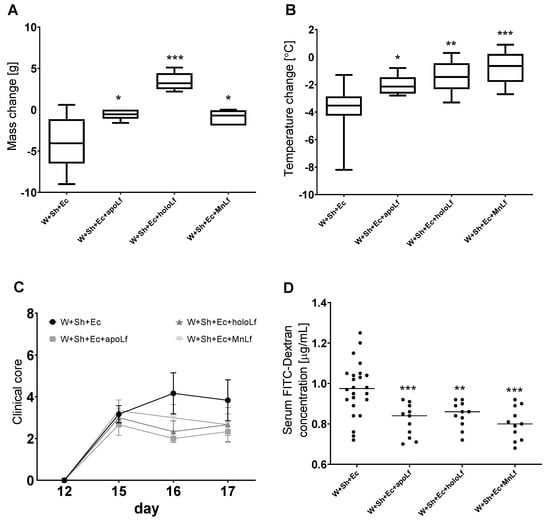
Figure 3.
Clinical parameters of animals in tested groups with lactoferrin supplementation: change in weight (A) and temperature (B) between the day of necropsy (day 17) and weaning (day 12). (C) Clinical scores (reduced motor activity, lethargy, shivering, piloerection) were assessed on days 12, 15, 16 and 17. Dextran concentration was calculated using standard curve for FITC-Dextran (D). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. W+Sh+Ec. W+Sh+Ec-target model group weaned with administration of both intraperitoneal S. haemolyticus 186 and oral E. coli 3A/1. W+Sh+Ec+apoLf-p.o. metal-depleted apoLf, W+Sh+Ec+holoLf-p.o. iron-saturated holoLf, W+Sh+Ec+MnLf-p.o. manganese-saturated MnLf.
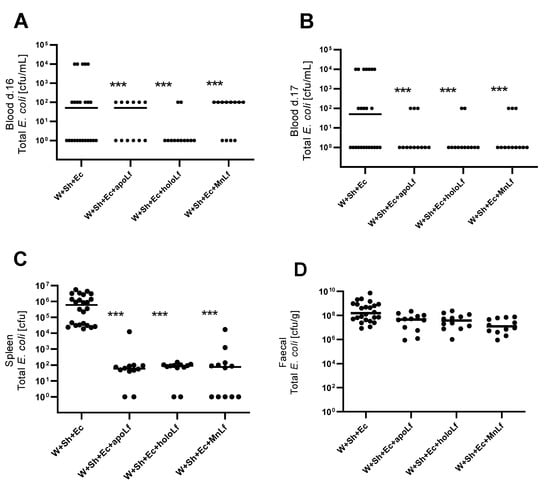
Figure 4.
Population numbers of total E. coli for groups with lactoferrin supplementation in blood d. 16 (A), blood d. 17 (B), spleen (C) and feces (D). *** p < 0.01 vs. W+Sh+Ec group. W+Sh+Ec-target model group weaned with administration of both intraperitoneal S. haemolyticus 186 and oral E. coli 3A/1. W+Sh+Ec+apoLf-p.o. metal-depleted apoLf, W+Sh+Ec+holoLf-p.o. iron-saturated holoLf, W+Sh+Ec+MnLf-p.o. manganese-saturated MnLf.
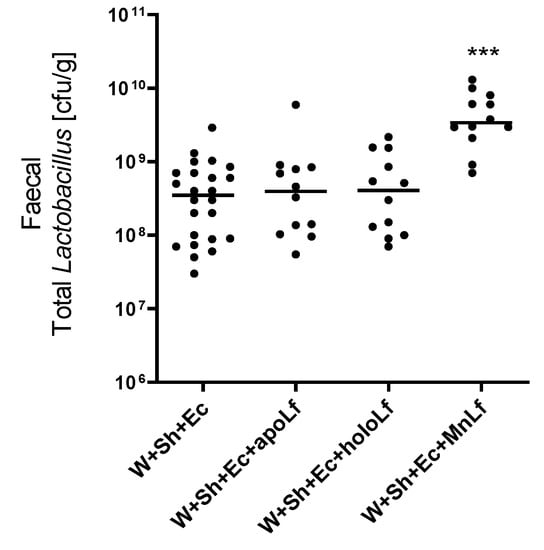
Figure 5.
Fecal population numbers of total lactobacilli for groups with lactoferrin supplementation. *** p < 0.01 vs. W+Sh+Ec and W+Sh+Ec+apoLf and W+Sh+Ec+holoLf. W+Sh+Ec-target model group weaned with administration of both intraperitoneal S. haemolyticus 186 and oral E. coli 3A/1. W+Sh+Ec+apoLf-p.o. metal-depleted apoLf, W+Sh+Ec+holoLf-p.o. iron-saturated holoLf, W+Sh+Ec+MnLf-p.o. manganese-saturated MnLf.
4. Discussion
Herein, we present the development of a novel animal model of neonatal sepsis induced by bacterial translocation and the application of such a model to elucidate the effect of oral supplementation with various forms of lactoferrin. We identified that lactoferrin saturated with manganese significantly increases the Lactobacillus bacterial population, which contributes to the fortification of the intestinal barrier and inhibits the translocation phenomenon. The acquired knowledge can be used to limit the development of sepsis in newborns in hospital neonatal intensive care units.
Neonatal sepsis remains a significant cause of mortality and morbidity for infants worldwide. Rapid deaths of newborns primarily result from the extremely short time that can elapse from the onset of an acute infection to the development of sepsis [2]. Isolation of the preterm newborns from the mother is a colossal stress factor affecting temporary intestinal translocation. This physiological phenomenon is considered desirable from an immunological perspective, leading to the development of immune tolerance to specific components of gut microflora colonizing a newborn’s digestive tract [2,14]. However, the colonization of a newborn’s GI tract with some maternal etiological factors (e.g., Streptococcus agalactiae) might facilitate the development of early onset sepsis occurring up to few days after delivery. This form of infection appears to be strongly associated with maternal microflora and perinatal disorders. Therefore, proper perinatal antibiotic therapy significantly reduces this type of condition. Late-onset sepsis, i.e., an acute infection leading to death up to several days after delivery, is mainly associated with lengthy hospitalization and complex diagnostic procedures [3,4]. Hospital-derived coagulase-negative staphylococci with various resistance mechanisms are the most frequent etiological factors causing LOS [15,16]. Recently, however, epidemiologists have observed an alarming occurrence: LOS is often caused by aerobic Gram-negative rods, particularly Escherichia coli, which are more often responsible for the death of newborns in comparison to staphylococcal sepsis [4,17].
Developing a suitable animal model corresponding to the physiological changes observed in a newborn’s organism during LOS has allowed research to verify the possibility of regulating this phenomenon through targeted nutrition. Both the advancement and LOS course result from many factors affecting a newborn’s organism, such as infections caused by highly virulent microorganisms and certain organ malfunctions occurring after an extended hospitalization. There were several unique features required for the establishment of the LOS animal model. In our study, we adapted a model of rat pups that were weaned on day 12 post-birth (group: W), when they were able to eat on their own. It has been revealed that weaning-related stress contributes to intestinal leakages and, subsequently, to gut–spleen translocation of E. coli, evidenced by positive cultures of E. coli in crushed fragments of the spleen (2 cases per 6). Interestingly, despite translocation, no other deteriorative parameters affecting the animals’ health were observed.
The E. coli strains isolated from the organs had the same drug resistance pattern as well as DNA profile as an E. coli originating from pups’ feces collected during necropsy on day 17 (data not shown) [18]. This was in line with the observation by Moussaoui team that Wistar puppies exposed to limited nesting stress from 2 to 10 days after birth exhibit increased total intestinal permeability [8]. Other studies also investigated a change in the intestinal barrier at weaning in the maternal separation model [19]. However, the presence of gut bacteria in the blood is only temporary and is followed by a quick clearance [5]. The literature has shown that bacterial translocation in rats is a common early life phenomenon. It is most probably related to the activity of submucosal macrophages, which, without initiating a pro-inflammatory immune response, effectively kill bacteria that have managed to overcome the intestinal epithelial barrier [20]. Based on our further research, it has been observed that the condition of animals was altered in the remaining two groups: W+Sh and W+Ec, in which the animals were subject to two stress factors acting simultaneously. In the W+Sh group, weaning served as the first stress factor, and one dose of a pure culture of Staphylococcus haemolyticus was administered intraperitoneally as a second stress factor. In the W+Ec group, weaning served as the first stress factor; however, a pure culture of E. coli was administered orally as a different second stress factor.
Even though we observed a slightly higher number of positive blood cultures of intestinal E. coli in the W+Sh group on day 16, we did not notice a significant deterioration in the animals’ health. Similar results were obtained in the W+Ec group, indicating that newborn rats can deal with two simultaneous stress factors quite effectively with only a minor deterioration in clinical parameters.
A completely different situation was observed in the last study group, where rats were subject to synchronized three-stressor action (W+Sh +Ec). When three stress factors acted synergistically, the intestinal barrier broke down, and Gram-negative rods (a dominant component of the intestinal microbiota) entered the bloodstream, causing bacteremia and endotoxemia. It subsequently led to a significant deterioration in all live, clinical and microbiological parameters. Based on these studies, it seems that the population of coagulase-negative staphylococci, which cause systemic infection symptoms that weaken the immune system, assists the phenomenon of translocation only when the newborn’s intestine presents an increased population of Gram-negative bacteria. Thanks to this model, bacterial translocation and its consequences for a newborn can be analyzed in detail.
Our further endeavors focused on elucidating which factors may determine the growth of the E. coli population in the neonatal gut. According to the current literature, ferric ions constitute a significant factor promoting the development of most Gram-negative rods. These ferric ions may appear in the intestine as a result of a small blood vessel rupture occurring throughout a digestive tract of a newborn—this phenomenon frequently occurs in necrotizing enterocolitis (NEC) [21,22]. Furthermore, stimulating the epithelial cells to produce tight junction proteins and enhancing the intestinal barrier might counteract bacterial translocation [23].
Theoretically, lactoferrin (Lf)—a protein found in colostrum and breast milk—can actively chelate Fe ions and has antimicrobial, anti-inflammatory and immunoregulatory properties [5,24]. Lf is natural compound that has been shown to play a key role in counteracting inflammatory homeostasis disorders associated with both aseptic and septic pathological conditions [25].
Feeding of Lf to neonatal rats before inducing an intestinal infection with E. coli was shown to enhance survival of the rat pups, reduce infection of the jejunum and ileum [26] and limit E. coli-related translocation to the liver and blood [27]. Lactoferrin (especially apolactoferrin) can sequester free ferric ions in the intestine and is therefore bacteriostatic for Gram-negative rods [11]. Additionally, lactoferrin can bind LPS, and thus inhibit the immunostimulatory effect of endotoxin on immune cells [23]. Research concluded that lactoferrin supplementation reduced the risk of late-onset infection by 40% and necrotizing enterocolitis by 60% [28]. Other studies showed that enteral lactoferrin supplementation significantly reduced late-onset sepsis incidence in very low birth weight newborns (VLBW) [29]. Another study demonstrated that stress factors such as a maternal separation (for 3 h per day from days 4 to 19 of life) of neonatal rats disrupted normal bacterial colonization of the gut, impaired colonic physiology and reduced host defenses. Adherent bacteria were dramatically increased in the colon of separated rats; in contrast, the number of lactobacilli was significantly reduced. Treatment with Lactobacillus strains restored normal gut function [19].
Therefore, it seems that the newborn’s organism, when exposed to at least three stress factors concurrently, cannot react appropriately, which leads to a rapid loss of intestinal barrier integrity and the development of LOS. Finding natural substances that will seal the newborn’s intestine should be a priority because any extended stay in the hospital will always lead to the phenomenon of translocation. It seems that lactoferrin containing Mn ions may be such a substance.
It is crucial to understand the limitations of the established model and performed experiments. The first stress factor—weaning—actually has two important components: weaned pups are deprived of milk (cease of breastfeeding) as well as the physical inter-action with their mother (cease of bonding). Further studies should be conducted to elucidate the impact of these two ‘subfactors’ by, e.g., oral supplementation of the weaned pups with animal milk/formula. What is more, due to the constraints of our study, we have focused on differentiating between the most ‘extreme’ species of lactoferrin—one devoid of metal ions (apolactoferrin), one loaded with iron (hololactoferrin) and finally the manganese-saturated one (MnLf). Naturally, it would be interesting to verify what effect the natural (10–20% saturated) lactoferrin would exert on the established model. Lastly, in this study, we focused on two groups of bacteria relevant for neonatal sepsis (Enterobacteriaceae and lactobacilli), and we were interested in their colony-forming ability. Our future studies will also include molecular analysis of fecal DNA using next-generation sequencing methods for more complex results.
The developed model allows us to analyze the translocation process taking place in the organism of newborns under the influence of several factors at the same time, all of which are relevant for the physiology and pathology of the neonatal gut. Separation from the mother, possibility of nosocomial infection and colonization of the intestine with potentially pathogenic bacterial species are all risk factors for the neonatal sepsis originating from the gut. Furthermore, our model allows the search for active substances that, when administered orally, have potent properties to counteract bacterial translocation by restoring the integrity of the neonatal intestinal barrier.
Author Contributions
M.P.-Z., G.M. contributed to conception and design of the study, realized laboratory experiments, wrote sections of the manuscript; A.B., B.S. contributed to conception of the animal model; M.P. organized the database and performed the statistical analysis; M.S. contributed to conception and design of the study. All authors contributed to manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by National Science Centre (Poland, project numbers: 2013/09/B/NZ6/00849, 2018/31/B/NZ6/02472 and DEC-2020/04/X/NZ5/00546).
Institutional Review Board Statement
Animal protocols conformed with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and approved by the Jagiellonian University Ethical Committee on Animal Experiments (no. 190/2012).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cortese, F.; Scicchitano, P.; Gesualdo, M.; Filaninno, A.; De Giorgi, E.; Schettini, F.; Laforgia, N.; Ciccone, M.M. Early and late infections in newborns: Where do we stand? A review. Pediatr. Neonatol. 2016, 57, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Wójkowska-Mach, J.; Chmielarczyk, A.; Strus, M.; Lauterbach, R.; Heczko, P. Neonate bloodstream infections in organization for economic cooperation and development countries: An update on epidemiology and prevention. J. Clin. Med. 2019, 8, 1750. [Google Scholar] [CrossRef] [Green Version]
- Wójkowska-Mach, J.; Borszewska-Kornacka, M.; Domanska, J.; Gadzinowski, J.; Gulczynska, E.; Helwich, E.; Agieszka, K.; Dorota, P.; Jerzy, S.; Jerzy, K.; et al. Early-onset infections of very-low-birth-weight infants in Polish neonatal intensive care units. Pediatr. Infect. Dis. J. 2012, 31, 691–695. [Google Scholar] [CrossRef]
- Wójkowska-Mach, J.; Gulczyńska, E.; Nowiczewski, M.; Borszewska-Kornacka, M.; Domańska, J.; Merritt, T.A.; Helwich, E.; Kordek, A.; Pawlik, D.; Gadzinowski, J.; et al. Late-onset bloodstream infections of Very-Low-Birth-Weight infants: Data from the Polish Neonatology Surveillance Network in 2009–2011. BMC Infect. Dis. 2014, 14, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, M.P. New concepts of microbial translocation in the neonatal intestine: Mechanisms and prevention. Clin. Perinatol. 2010, 37, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Moussaoui, N.; Braniste, V.; Ait-Belgnaoui, A.; Gabanou, M.; Sekkal, S.; Olier, M.; Théodorou, V.; Martin, P.; Houdeau, E. Changes in intestinal glucocorticoid sensitivity in early life shape the risk of epithelial barrier defect in maternal-deprived rats. PLoS ONE 2014, 9, e88382. [Google Scholar]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.; Cryan, J.; Dinan, T. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Moussaoui, N.; Jacobs, J.P.; Larauche, M.; Biraud, M.; Million, M.; Mayer, E.; Taché, Y. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: Influence of sex. J. Neurogastroenterol. Motil. 2017, 23, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strus, M.; Okoń, K.; Nowak, B.; Pilarczyk-Zurek, M.; Heczko, P.; Gawda, A.; Ciszek-Lenda, M.; Skowron, B.; Baranowska, A.; Marcinkiewicz, J. Distinct effects of Lactobacillus plantarum KL30B and Escherichia coli 3A1 on the induction and development of acute and chronic inflammation. Cent.-Eur. J. Immunol. 2015, 40, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilarczyk-Zurek, M.; Strus, M.; Adamski, P.; Heczko, P.B. The dual role of Escherichia coli in the course of ulcerative colitis. BMC Gastroenterol. 2016, 16, 128. [Google Scholar] [CrossRef] [Green Version]
- Majka, G.; Pilarczyk-Zurek, M.; Baranowska, A.; Skowron, B.; Strus, M. Lactoferrin Metal Saturation—Which Form Is the Best for Neonatal Nutrition? Nutrients 2020, 12, 3340. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Blaut, M. Small intestinal permeability and gut-transit time determined with low and high molecular weight fluorescein isothiocyanate-dextrans in C3H mice. Nutrients 2018, 10, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing; Team RC: Wroclaw, Poland, 2013. [Google Scholar]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Marchant, E.A.; Boyce, G.K.; Sadarangani, M.; Lavoie, P.M. Neonatal sepsis due to coagulase-negative staphylococci. Clin. Dev. Immunol. 2013, 2013, 586076. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.E. Gram negative organisms and viral infections in neonatal sepsis. BMJ 2020, 371, m4248. [Google Scholar] [CrossRef]
- Pilarczyk-Zurek, M.; Chmielarczyk, A.; Gosiewski, T.; Tomusiak, A.; Adamski, P.; Zwolinska-Wcislo, M.; Mach, T.; Heczko, P.B.; Strus, M. Possible role of Escherichia coli in propagation and perpetuation of chronic inflammation in ulcerative colitis. BMC Gastroenterol. 2013, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Weber, B.; Saurer, L.; Mueller, C. Intestinal macrophages: Differentiation and involvement in intestinal immunopathologies. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 171–184. [Google Scholar]
- Macdonald, A.; Green, J. Necrotising enterocolitis and neonatal sepsis: A literature review. J. Neonatal Nurs. 2018, 24, 80–85. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Majka, G.; Więcek, G.; Śróttek, M.; Śpiewak, K.; Brindell, M.; Koziel, J.; Marcinkiewicz, J.; Strus, M. The impact of lactoferrin with different levels of metal saturation on the intestinal epithelial barrier function and mucosal inflammation. Biometals 2016, 29, 1019–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majka, G.; Śpiewak, K.; Kurpiewska, K.; Heczko, P.; Stochel, G.; Strus, M.; Brindell, M. A high-throughput method for the quantification of iron saturation in lactoferrin preparations. Anal. Bioanal. Chem. 2013, 405, 5191–5200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in aseptic and septic inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, M.P.; Bennett, S.H.; Hwang, F.F.; Yu, C. Neonatal small bowel epithelia: Enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals 2004, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Edde, L.; Hipolito, R.B.; Hwang, F.F.; Headon, D.R.; Shalwitz, R.A.; Sherman, M.P. Lactoferrin protects neonatal rats from gut-related systemic infection. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 281, G1140–G1150. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Lu, C.; Wang, Q.; Pan, B.; Wang, Q.; Tian, J.; Ge, L. Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta-Analysis With Trial Sequential Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1186. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).