Abstract
Background: The effects of the fat-to-muscle ratio (FMR) on hyperuricemia and a reduction in the estimated glomerular filtration rate (eGFR) are still unclear. Methods: Data from the China National Health Survey were used to explore the associations of the FMR with hyperuricemia and reduced eGFR. The fat mass and muscle mass were measured through bioelectrical impedance analysis. Mediation analysis was used to estimate the mediated effect of hyperuricemia on the association between the FMR and reduced eGFR. Results: A total of 31171 participants were included. For hyperuricemia, compared with the Q1 of the FMR, the ORs (95% CI) of Q2, Q3 and Q4 were 1.60 (1.32–1.95), 2.31 (1.91–2.80) and 2.71 (2.15–3.43) in men and 1.91 (1.56–2.34), 2.67 (2.12–3.36) and 4.47 (3.40–5.89) in women. For the reduced eGFR, the ORs (95% CI) of Q2, Q3 and Q4 of the FMR were 1.48 (1.18–1.87), 1.38 (1.05–1.82) and 1.45 (1.04–2.04) in men aged 40–59, but no positive association was found in younger men or in women. Hyperuricemia mediated the association between the FMR and reduced eGFR in men. The OR (95% CI) of the indirect effect was 1.08 (1.05–1.10), accounting for 35.11% of the total effect. Conclusions: The FMR was associated with hyperuricemia and reduced eGFR, and the associations varied based on sex and age. The effect of the FMR on the reduced eGFR was significantly mediated by hyperuricemia in men.
1. Introduction
Hyperuricemia, which is defined as elevated serum uric acid (SUA) when the SUA production exceeds its excretion, has been found to be associated with various health outcomes [1,2]. Data from the China National Health Survey (CNHS) revealed an almost 20% prevalence of hyperuricemia among adults in mainland China [3]. Hyperuricemia was also suggested to be one of the most important risk factors for kidney dysfunction [4,5,6], which has received increasing attention as a global public health issue.
People with kidney dysfunction are more likely to develop end-stage renal disease and cardiovascular disease and have a higher risk of mortality [5,7,8]. Elevated SUA was reported to be associated with kidney dysfunction, and previous epidemiological studies and basic science suggest that hyperuricemia is an important risk factor for the development of chronic kidney disease (CKD) [1].
As one of the shared risk factors for hyperuricemia and kidney dysfunction, excess adiposity was demonstrated to have a significant role in elevating the SUA in the general Chinese population in our previous studies [3,9,10]. Although plenty of adiposity measurements, such as the body mass index (BMI), body fat percentage, waist circumference, waist-to-height ratio, fat mass index, etc., have been applied in clinical practice as indicators of chronic disease, the common limitation of these indicators is that they only consider the effect of fat, while ignoring the skeletal muscle, which is another important tissue for maintaining metabolism health. The fat-to-muscle ratio (FMR), therefore, has been proposed as an alternative approach for assessing excess adiposity [11]. In recent years, researchers have begun to explore the value of the FMR for the identification of members of populations at a high risk of developing cardiometabolic disorders, such as type-2 diabetes and metabolic syndrome, and they found that the FMR is positively associated with an increased risk of such outcomes [11,12,13]. However, the associations of the FMR with hyperuricemia and reduced kidney function, both of which are important risk factors for cardiovascular disease and even death [1,8], are still unclear. Furthermore, as excess adiposity is considered as a common risk factor for hyperuricemia and kidney dysfunction, and hyperuricemia is strongly associated with kidney dysfunction, we hypothesized that there could be a potential mediating role of hyperuricemia in the pathway from FMR to reduced kidney function (which is termed as an indirect effect).
The glomerular filtration rate (GFR) is the ideal overall index of kidney function, but the measurement is cumbersome and impractical for the general detection and management of kidney disease [14]. In this case, the estimated GFR (eGFR) acquired by equations is widely used as a surrogate of GFR, especially in large-scale epidemiological studies. Here, using data from the CNHS, a nationwide cross-sectional study conducted in mainland China, we aimed to explore the role of FMR in, and its associations with, hyperuricemia and reduced eGFR in Chinese adults, and we further explored the possible mediating role of hyperuricemia in the association between the FMR and reduced eGFR.
2. Materials and Methods
2.1. Study Population
The CNHS was conducted from 2012 to 2017 by the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The study protocol has been published previously [15]. Briefly, using a multi-stage, stratified cluster sampling method, the CNHS recruited representative adults from eleven provinces in mainland China. In the sampling procedure, the geographic regions, degree of urbanization and economic development level, assessed according to the local GDP, were considered.
The inclusive criteria for the study population were an age of 20–80 years and the status of having lived in the local area for at least one year. The exclusive criteria meant that people with severe mental or physical disease, pregnant women or military personnel in active service were discounted. In this study, we used data restricted to Han Chinese individuals so as to reduce potential heterogeneity in terms of genetic or socio-cultural backgrounds.
The study was carried out in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Bioethical Committee of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (No.029-2013). All participants provided written informed consent before the survey.
2.2. Data Collection and Measurements
A standardized questionnaire was designed to collect demographic and health-related information through face-to-face interview. All staff underwent a training program to guarantee their capability of conducting precise data collection and measurements. Information on demographic characteristics (sex, date of birth, permanent address, educational level), health-related lifestyle (alcohol drinking, cigarette smoking) and personal disease history (hypertension, diabetes, cardiovascular disease) were collected during the survey.
The standing height was measured to the nearest 0.1 cm using a fixed stadiometer. The weight, fat mass and muscle mass were measured through bioelectrical impedance analysis (BIA) (TANITA BC-420, Tanita, Tokyo, Japan). The average value of three blood pressure (BP) readings was recorded using a digital BP measurement device (Omron HEM-907, Omron, Osaka, Japan) [15].
A blood sample was collected from each participant by venepuncture after an overnight fast of at least 8 h. The SUA, creatinine, serum lipids and fasting plasma glucose were tested in the laboratory of the General Hospital of the Chinese People’s Liberation Army. The SUA and creatine were measured by oxidization with the specific enzyme uricase.
The eGFR was calculated according to the equation developed by the adaptation of the modification of diet in renal disease (MDRD) equation, based on data from Chinese CKD patients [16], which is written as:
For male, eGFR = 175 × Scr−1.234 × age–0.179
For female, eGFR = 175 × Scr−1.234 × age–0.179 × 0.79
Above, Scr is the serum creatinine in mg/dl and age is in years.
2.3. Definitions
Hyperuricemia was defined as an SUA level higher than 360 μmol/L (~6 mg/dl) in women and 420 μmol/L (~7 mg/dl) in men [17]. There is a debate regarding the most appropriate cutoff for the eGFR in identifying reduced kidney function. The two cutoffs most often used to exclude individuals from reference interval studies are <60 mL/min/1.73 m2 and <90 mL/min/1.73 m2 [18]. Some studies supported the notion that the use of 90 mL/min/1.73 m2 can be more beneficial in regard to health outcomes [19]. As the early identification of kidney dysfunction using a higher cutoff value may have greater public health significance, in this study, we used 90 mL/min/1.73 m2 to define a reduced eGFR.
Hypertension was defined as a systolic BP of 140 mmHg or higher, a diastolic BP of 90 mmHg or higher, the use of any antihypertensive medication or any self-reported history of diagnosed hypertension [20]. Dyslipidemia was defined according to the Chinese guidelines for the management of dyslipidemia in adults [21]. Diabetes was defined as self-reported or ever-diagnosed disease or as a fasting plasma glucose (FPG) over 7.0 mmol/L. The definitions of cigarette smoking and alcohol drinking were consistent with our previous publications [3,15]. BMI was categorized into three groups: under/normal weight (BMI < 24 kg/m2), overweight (BMI ≥ 24 kg/m2 but <28 kg/m2) and obesity (BMI ≥ 28 kg/m2) [22]. FMR was calculated as the fat mass in kilograms divided by the muscle mass in kilograms.
2.4. Statistical Analyses
After excluding individuals with missing values for the FMR, SUA and eGFR, the final analytic sample included 31171 participants. Continuous data were presented as means with standard deviations (SDs), except for the eGFR, which was presented as the median (interquartile range, IQR) because of its high skew. Categorical variables were shown as frequencies and proportions. For descriptive purposes, the basic characteristics of the study population were presented according to presence or absence of HUA and reduced eGFR. The LMS (lambda, mu, sigma) method was used to construct the sex-specific growth curves of the FMR with advanced age [23].
The FMR was classified into four groups according to its centile distribution in each sex, i.e., the Q1 (less than P25), Q2 (P25–P49), Q3 (P50−P74) and Q4 (P75 and above). We analyzed the associations of the FMR with HUA and reduced eGFR using logistic regression models, in which sampling clusters and strata were considered using the Surveylogistic procedure in the statistical software SAS. Multivariable-adjusted odds ratios (ORs) were reported with their 95% confidence intervals (CIs). To avoid reverse causality resulting from the declined kidney function accompanied by significant muscle waste in senior participants, the association analyses of the FMR and reduced eGFR were restricted to people aged 20–59 years.
The covariates included in the multivariable logistic regression models varied based on different study aims. Several models were developed. Model 1 was adjusted for the demographic, health-related lifestyle and personal disease history information. Model 2 was additionally adjusted for BMI to explore the effect of the FMR independently from BMI. In the analyses of the association between the FMR and reduced eGFR, another model, Model 3, was designed to adjust for the HUA status in order to explore whether there was a modified effect of HUA on the association. We also performed age-stratified analyses to investigate whether the associations varied based on age.
Given the associations between the FMR, HUA and reduced eGFR, we performed a mediation analysis to further explore whether there was an indirect effect of HUA on the association between the FMR and reduced eGFR. The methodology and hypothesis were described in detail in our previous study [24]. In the mediation analysis, the FMR was categorized into two groups based on its sex-specific cutoff values by calculating the Youden Index (the maximum value of sensitivity + specificity − 1), yielded by the covariates’ adjusted receiver operator characteristic (ROC) curves. We further investigated the association between HUA and reduced eGFR using multivariable logistic regression models, and the results are presented in the Supplementary Materials.
All p values were two-sided, and a p value of less than 0.05 was considered to be statistically significant. Analyses were performed with SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). The GAMLSS package in R (version 4.0, R Core Team, Vienna, Austria) was used to perform the LMS method. As there were substantial sex-based differences in the FMR, HUA and eGFR, we performed all the analyses accounting for variations by sex.
3. Results
3.1. Basic Characteristics
The general characteristics of the study population are summarized in Table 1. Among the overall 31171 participants, 40.2% were male, with an average age of 48.71 ± 13.36 years. HUA cases were predominant among males (24.65% vs. 11.56% in women), people living in the urban areas (18.83% vs. 13.27% in rural areas), individuals with a higher educational level (18.59% in the college groups vs. 15.46% in the elementary/lower group), ever-smokers (23.81% vs. 13.93% in never-smokers), ever-drinkers (23.17% vs. 12.51% in never-drinkers), overweight or obese people (24.93% vs. 9.47% in the under/normal weight group) and participants in higher FMR categories (29.39% in the Q4 group vs. 6.48% in the Q1 group). Similar effects of the age trend and urban–rural and educational disparities on the prevalence of reduced eGFR were observed. Men, ever-smokers, alcohol drinkers, overweight/obese people and individuals with a higher FMR also had a higher prevalence of reduced eGFR. The age- and sex-specific prevalence of HUA and reduced eGFR are summarized in Supplementary Table S1.

Table 1.
Basic characteristics of the study population (n = 31171).
3.2. Fat-to-Muscle Ratio and Its Association with Hyperuricemia
The sex-specific growth curves of the FMR are presented in Figure 1. The FMR slightly increased with advanced age in women, but in men, the FMR increased from early adulthood to middle age and was then preserved until senior age. The overall and age-stratified associations between the FMR and HUA are presented in Figure 2 and Supplementary Table S2. Model 2 shows that the effect of the increased centile groups of FMR on the risk of HUA was independent from the BMI. The forest plots revealed that people in higher FMR categories were more likely to have HUA. Compared with the Q1 of FMR, the ORs (95% CI) of the Q2, Q3 and Q4 groups in men were 1.60 (1.32–1.95), 2.31 (1.91–2.80) and 2.71 (2.15–3.43), respectively. In women, the magnitudes were larger, and the ORs of the Q2, Q3 and Q4 FMR groups were 1.91 (1.56–2.34), 2.67 (2.12–3.36) and 4.47 (3.40–5.89), respectively.
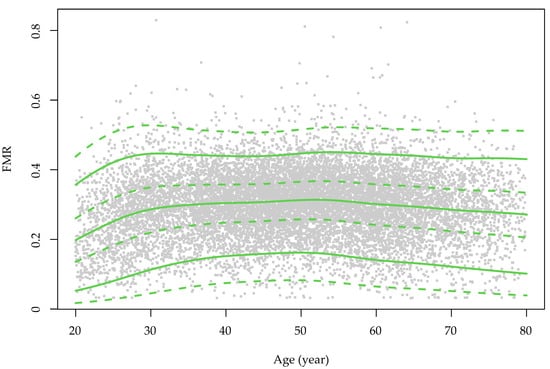
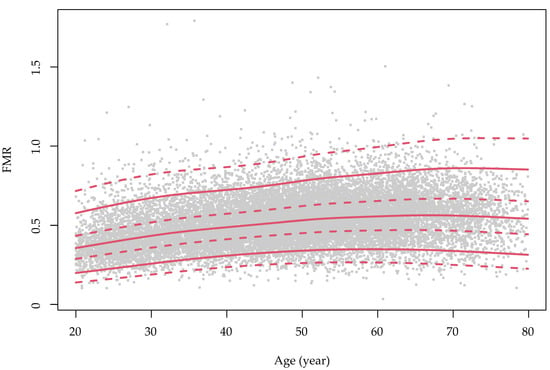
Figure 1.
The sex-specific growth curve of the fat-to-muscle ratio over the course of ageing in the study population. FMR: fat-to-muscle ratio. The green color is for males and red is for females. The grey dots represent the distribution of the FMR values for each sex.
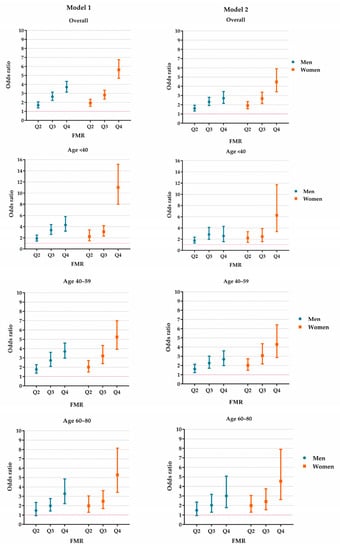
Figure 2.
The effect of FMR on HUA in the study population. Model 1 was adjusted for age, rural/urban location, educational level, study sites, alcohol drinking status (male only), smoking status (male only), hypertension, diabetes, dyslipidemia and serum creatine. Model 2 was additionally adjusted for body mass index based on Model 1. FMR: fat-to-muscle ratio. HUA: hyperuricemia. Q2–Q4: the values fall in the 25–49th, 50–74th and 75–100th centiles of FMR.
The age-stratified analyses revealed that, in men, the ORs of Q2–Q4 of FMR were similar between different age groups, but in women, the Q4 FMR group seemed to have a greater effect among younger females. Compared with the Q1 FMR group, Q4 group had an OR of 6.27 (3.35–11.73) among the group of participants aged <40, and we observed ORs of 4.29 (2.86–6.41) and 4.55 (2.62–7.89) in the groups aged 40–59 and 60 or above, respectively. In both the overall and the age-stratified analyses, the highest FMR group, Q4, had a greater effect among females than their male counterparts. Further details are available in Figure 2 and Supplementary Table S2.
3.3. The Association between FMR and Reduced eGFR
The associations between the FMR and reduced eGFR are presented in Figure 3 and Supplementary Tables S3 and S4. In men, the magnitude of the ORs became smaller when HUA was adjusted in the regression models. The ORs (95% CI) of Q2, Q3 and Q4 of FMR were 1.26 (1.03–1.54), 1.19 (0.93–1.52) and 1.22 (0.92–1.63), respectively. However, in females, the FMR was not found to be associated with reduced eGFR in any FMR category. The positive associations in Model 1 and Model 2 disappeared when we adjusted for BMI and HUA in Model 3.
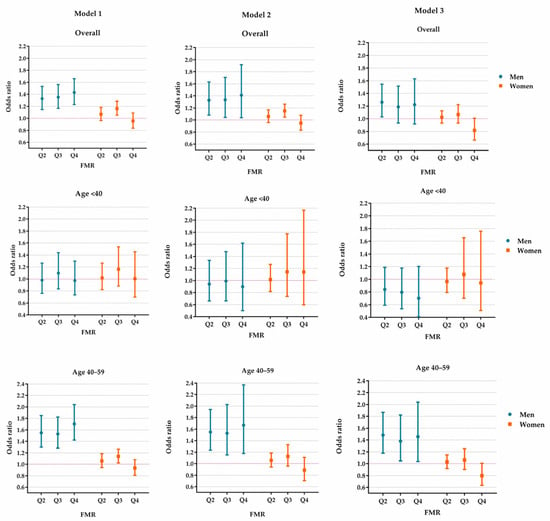
Figure 3.
The effect of FMR on reduced eGFR in the study population aged 20–59. Model 1 was adjusted for age, rural/urban location, educational level, alcohol drinking status, smoking status, hypertension, diabetes and dyslipidemia. Model 2 was additionally adjusted for body mass index based on Model 1. Model 3 was additionally adjusted for hyperuricemia based on Model 2. FMR: fat-to-muscle ratio. Q2–Q4: the values fall in the 25–49th, 50–74th and 75–100th centiles of FMR.
The age-stratified analyses revealed a possible modification effect of age on the associations in men. The FMR was positively associated with reduced eGFR in the older men (aged 40–59 years) but not in men younger than 40. Notably, in women, after adjusting for BMI and HUA, there was no positive association between the FMR and reduced eGFR in different age groups (Figure 3 and Supplementary Tables S3 and S4).
3.4. The Mediation Analysis of the Association between FMR and Reduced eGFR
HUA had strong effect on the reduced eGFR, and the associations between HUA and reduced eGFR are shown in Supplementary Table S5. As no positive association between the FMR and reduced eGFR was observed in females, we only applied the mediation analysis to male participants. The result showed that HUA mediated the association between the FMR and reduced eGFR remarkably, and the indirect effect accounted for 35.11% of the total effect. The OR (95% CI) of the indirect effect was 1.08 (1.05–1.10) (Table 2).

Table 2.
The mediation analysis of hyperuricemia’s effect on the association between the FMR and reduced eGFR among men aged 20–59.
4. Discussion
In this representative sample of Chinese adults, the FMR was found to be independently associated with hyperuricemia and reduced eGFR, and the associations varied based on both sex and age. Furthermore, the effect of the FMR on reduced eGFR was significantly mediated by hyperuricemia in men.
Some studies have documented the FMR as an indicator for identifying members of populations at a high risk of developing cardiovascular diseases and its risk factors [11,12,13,25,26]. Nevertheless, to the best of our knowledge, this is the first study that explored the associations of the FMR with HUA and reduced eGFR in Chinese adults. The primary findings of this study suggest that the FMR can be used as an indicator of HUA independently from the traditional excess adiposity indexes, such as BMI, and a higher FMR is also associated with a reduced eGFR. In addition, by deconstructing the total effect of the FMR on reduced eGFR, our study revealed indirect associations between the FMR, HUA and reduced eGFR.
In recent years, the function of the skeletal muscle and the balance of MM and FM have raised great concern. Termed as “sarcopenia” and “sarcopenic obesity”, the loss of muscle mass and severe imbalance between the distribution of muscle and fat tissue with ageing are believed to be associated with various health hazards [27]. The associations of the FMR with HUA and reduced eGFR highlight the importance of exploring alternative indicators to BMI and focusing our attention on the balance of the skeletal muscle and fat mass in the early identification of the individuals at a high-risk of HUA and reduced eGFR in community-dwelling populations.
In contrast with traditional adiposity indicators, such as the BMI, body fat percentage or waist circumference, the FMR can reflect the relative relationship and imbalance between the skeletal muscle and fat mass. The decline in skeletal MM accompanied by an increase in FM may lead to a dual metabolic burden, which leads to a high risk of developing cardiometabolic disorders [25]. Although the precise mechanism of the relationship between the FMR and HUA is not clear, previous studies have shown that an increased FMR is closely related to degradation of insulin and chronic inflammation and can result in impaired cardiometabolic functions [28,29]. These shared factors may contribute to research demonstrating the relationship between FMR and HUA.
Sex differences in the trajectory of the FMR with ageing were observed. The decrease in MM due to enhanced catabolism has been suggested to be combined with obesity, thereby resulting in changes in the FMR [30]. Compared with men, women had an increased FMR with ageing, and this may partially explain the stronger effect of the FMR on HUA in females in the current study. Another possible reason is that there is a sex-based disparity in the risk of the onset of HUA during ageing, with the risk declined in men but increased in women [3,9].
Overweight and obesity have been suggested to increase the risk of kidney dysfunction in previous studies. Madero et al. reported that adiposity indicators, such as the BMI and waist circumference, were associated with kidney function decline [28]. Wang et al.’s study supported the notion that the combined effects of dyslipidemia and high adiposity were significantly associated with the decline in the eGFR in a middle-aged Chinese population, especially in men [31]. Impaired insulin resistance, chronic inflammatory reactions, lipid toxicity and the chronic and excessive activation of the sympathetic nervous system may contribute to the development of kidney dysfunction and, eventually, CKD [32]. The analyses of body composition indicators other than those that only represent the fat mass have brought new insights on our understanding of the prevention of kidney dysfunction. Kim et al. investigated the effects of the fat mass and muscle mass index (MMI, muscle divided by height) on CKD and revealed that a higher FM and lower MMI, but not BMI, were associated with a higher risk of CKD among Korean older adults [33]. The findings of our study also support the notion that men with a higher FMR are more likely to have a reduced eGFR, even after the adjustment for BMI. However, this association was not observed in females. The effects of sex-based differences and disparities on the prevalence and progression of kidney dysfunction may be related to the direct effects of sex steroids on the kidneys, sex differences in NO metabolism and oxidative stress and the gender-differential impacts of comorbidities and lifestyle risk factors [34]. The reason underlying the sex disparity observed in this study is still not clear but may be related to the competing risk of more predominant effects caused by increased fat mass over the course of aging in women. The age-variable associations between the FMR and reduced eGFR in men highlight the importance of focusing our attention on the senior members of society, among whom sarcopenic obesity or sarcopenia occurs more frequently.
Although hyperuricemia is believed to be a major contributor to the progression of kidney dysfunction, the role of HUA in the development of CKD was firstly viewed as one that can be solely attributed to the retention of SUA caused by the GFR fall [35]. Although some Mendelian randomization studies have found no evidence of the casual role of serum urate in CKD [36], a great number of epidemiological, experimental and clinical studies have suggested that uric acid has an important pathogenic role in kidney disease [4]. The potential mechanism underlying this procedure may be related to inflammation, endothelial dysfunction and the activation of the renin-angiotensin system [1]. Clinical practice has also suggested that uric-acid-lowering therapy can benefit people by slowing the progression of CKD [37]. In addition to the strong associations between HUA and kidney function, our findings also revealed that HUA may play a mediating role in the effect of the FMR on reduced eGFR. This finding suggests that the management of HUA may bring additional health benefits aiding in the prevention of reduced eGFR. Although the cross-sectional design of our study cannot provide information on the time sequence of HUA and reduced eGFR in the study population, the analysis still has value for our understanding of the effects of muscle health and body composition imbalance on reduced kidney function, as well as its prevention and management.
A major strength of our study is that the data were derived from a representative national survey, with a high degree of heterogeneity. Hence, it provides a unique dataset that enabled us to explore the effect of the fat-to-muscle ratio, which is a novel indicator that represents skeletal muscle health and the balance between the FM and MM, as well as their effects on various health outcomes. In addition, the training programs and the on-site quality control strategies used here ensured the credibility of the results. The limitations of our study should also be acknowledged. First, it should be recognized that the use of eGFR as an estimation of kidney function should be approached with caution. As previous studies have demonstrated, dietary factors, such as meat consumption, can influence the creatine levels [14]. Moreover, as blood creatine is predominantly derived from muscle, the estimation of the GFR based on creatine may lead to underestimated kidney function measurements among senior people and, in contrast, overestimation among younger people, especially in the case of muscular young men [14]. Nevertheless, as we limited the analysis of the eGFR to people aged 20–59, the direction of the positive association between the FMR and eGFR in the senior group tends toward the null hypothesis. Thus, it does not lead to a reversed conclusion. Secondly, it is true that the varied estimation formulas used to calculate the GFR, and the “one-off” testing of the serum creatine level, with no 3-month observation, which was used to calculate the eGFR, may have caused a misclassification in this study. Additionally, diet and other environmental factors may have affected the measurements of the level of serum creatine. Nevertheless, as the purpose of this study was the association exploration, rather than the prevalence or the disease burden estimation, these limitations are acceptable and would not reverse the study conclusions. Thirdly, as mentioned above, the nature of the study’s cross-sectional design limited the causal inference of the exposure and health outcomes, and future longitudinal data are still needed in order to perform a validation analysis.
5. Conclusions
In summary, our study, for the first time, investigated the possible role of the fat-to-muscle ratio as a novel indicator for identifying individuals at a high risk of HUA and reduced eGFR in a representative Chinese adult population. The FMR was found to be positively associated with HUA and reduced eGFR, with the additional effects of sex and age differences. People with a higher FMR were more likely to have HUA in both sexes and were more likely to have a reduced eGFR in the case of middle-aged men. HUA may act as a mediator in the association between the FMR and reduced eGFR in men. The FMR should be used in community health screening for, and the co-management of, elevated serum uric acid, which should be considered in kidney dysfunction prevention strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14194193/s1, Table S1: The cross-over prevalence of hyperuricemia and reduced eGFR in FMR groups, stratified by sex; Table S2: The independent effect of FMR on hyperuricemia in the study population; Table S3: The independent effect of FMR on reduced eGFR in the male study population aged 20–59; Table S4: The independent effect of FMR on reduced eGFR in the female study population aged 20–59; Table S5: The association between hyperuricemia and reduced eGFR in the study population.
Author Contributions
Conceptualization, H.H. and G.S.; data curation, H.H. and G.S.; formal analysis, H.H.; funding acquisition, G.S.; investigation, H.H., L.P. (Li Pan), D.W., F.L., J.D., L.P. (Lize Pa), X.W., Z.C., X.R., H.W., X.P., J.Z. and G.S.; methodology, H.H.; project administration, H.H., L.P. (Li Pan), D.W., F.L., J.D., L.P. (Lize Pa), X.W., Z.C., X.R., H.W., X.P., J.Z. and G.S.; resources, D.W., F.L., J.D., L.P. (Lize Pa), X.W., Z.C., X.R., H.W., X.P., J.Z. and G.S.; software, H.H.; supervision, L.P. (Li Pan) and G.S.; validation, H.H., L.P. (Li Pan) and G.S.; visualization, H.H.; writing—original draft, H.H.; writing—review and editing, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Basic Research Program of the Ministry of Science and Technology of China (grant No. 2013FY114100), CAMS Innovation Fund for Medical Sciences (CIFMS) (grant No. 2021-I2M-1-023) and the National Key R&D Program of China (grant No. 2016YFC0900600/2016YFC0900601).
Institutional Review Board Statement
The study was carried out in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Bioethical Committee of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (No. 029-2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated or analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We appreciate all the participants of the CNHS and gratefully thank all staff members of the CNHS who offered their considerable time and effort in this survey. We also thank Guangjin Zhu for her professional advice and effort in the field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis. Care. Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Pan, L.; Ren, X.; Wang, D.; Du, J.; Cui, Z.; Zhao, J.; Wang, H.; Wang, X.; Liu, F.; et al. The Effect of Body Weight and Alcohol Consumption on Hyperuricemia and Their Population Attributable Fractions: A National Health Survey in China. Obes. Facts. 2022, 15, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Nakagawa, T.; Kanbay, M.; Kuwabara, M.; Kumar, A.; Arroyo, F.E.G.; Jimenez, C.; Sasai, F.; Kang, D.; Jensen, T.; et al. Hyperuricemia in Kidney Disease: A Major Risk Factor for Cardiovascular Events, Vascular Calcification, and Renal Damage. Semin. Nephrol. 2020, 40, 574–585. [Google Scholar] [CrossRef]
- Park, J.H.; Jo, Y.; Lee, J. Renal effects of uric acid: Hyperuricemia and hypouricemia. Korean. J. Intern. Med. 2020, 35, 1291–1304. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Han, L.; Xu, G.; Ran, J.M. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: A meta-analysis of 15 cohort studies. PLoS ONE 2014, 9, e100801. [Google Scholar] [CrossRef]
- Levey, A.S.; Atkins, R.; Coresh, J.; Cohen, E.P.; Collins, A.J.; Eckardt, K.U.; Nahas, M.E.; Jaber, B.L.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives–a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef]
- Rahimlu, M.; Shab-Bidar, S.; Djafarian, K. Body Mass Index and All-cause Mortality in Chronic Kidney Disease: A Dose–response Meta-analysis of Observational Studies. J. Renal Nutr. 2017, 27, 225–232. [Google Scholar] [CrossRef]
- He, H.; Guo, P.; He, J.; Zhang, J.; Niu, Y.; Niu, Y.; Chen, S.; Guo, F.; Liu, F.; Zhang, R.; et al. Prevalence of hyperuricemia and the population attributable fraction of modifiable risk factors: Evidence from a general population cohort in China. Front. Public Health 2022, 10, 936717. [Google Scholar] [CrossRef]
- He, H.; Pan, L.; Ren, X.; Wang, D.; Du, J.; Cui, Z.; Zhao, J.; Wang, H.; Wang, X.; Liu, F.; et al. Joint Effect of Beer, Spirits Intake, and Excess Adiposity on Hyperuricemia Among Chinese Male Adults: Evidence From the China National Health Survey. Front. Nutr. 2022, 9, 806751. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Carrillo, H.; Correa-Bautista, J.; Schmidt-RioValle, J.; González-Jiménez, E.; Correa-Rodríguez, M.; González-Ruíz, K.; García-Hermoso, A. Fat-to-Muscle Ratio: A New Anthropometric Indicator as a Screening Tool for Metabolic Syndrome in Young Colombian People. Nutrients 2018, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.G.; Song, H.J.; Song, Y.R. Fat-to-muscle ratio as a predictor of insulin resistance and metabolic syndrome in Korean adults. J. Cachexia. Sarcopenia. Muscle 2020, 11, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Lee, J.; Kwon, Y. Fat-to-Muscle Ratios and the Non-Achievement of LDL Cholesterol Targets: Analysis of the Korean Genome and Epidemiology Study. J. Cardiovasc. Dev. Dis. 2021, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, P.; Stevens, P.E.; Lamb, E.J. Estimated glomerular filtration rate. BMJ 2014, 348, g264. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Pan, L.; Pa, L.; Cui, Z.; Ren, X.; Wang, D.; Liu, F.; Wang, X.; Du, J.; Wang, H.; et al. Data Resource Profile: The China National Health Survey (CNHS). Int. J. Epidemiol. 2018, 47, 1734–1735f. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zuo, L.; Chen, J.; Luo, Q.; Yu, X.; Li, Y.; Xu, J.; Huang, S.; Wang, L.; Huang, W.; et al. Modified Glomerular Filtration Rate Estimating Equation for Chinese Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2006, 17, 2937–2944. [Google Scholar] [CrossRef]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez Lozada, L.G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Nouri, K. Low-risk cutoff of 90 ml/min/1.73 m2 for the estimated glomerular filtration rate and the importance of the equation for patients with acute coronary syndrome. Clin. Chim. Acta 2021, 523, 532–533. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Holdenrieder, S.; Neumann, F.J.; Lahu, S.; Cassese, S.; Joner, M.; Xhepa, E.; Kufner, S.; Wiebe, J.; Laugwitz, K.L.; et al. Prognostic value of glomerular function estimated by Cockcroft-Gault creatinine clearance, MDRD-4, CKD-EPI and European Kidney Function Consortium equations in patients with acute coronary syndromes. Clin. Chim. Acta 2021, 523, 106–113. [Google Scholar] [CrossRef]
- Yu, C.; Ren, X.; Cui, Z.; Pan, L.; Zhao, H.; Sun, J.; Wang, Y.; Chang, L.; Cao, Y.; He, H.; et al. A diagnostic prediction model for hypertension in Han and Yugur population from the China National Health Survey (CNHS). Chinese Med. J.-Peking 2022. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Joint Committee Issued Chinese Guideline for the Management of Dyslipidemia in Adults. Chinese guideline for the management of dyslipidemia in adults. Chin. J. Cardiol. 2007, 35, 390–419. [Google Scholar]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar]
- Tomkinson, G.R.; Carver, K.D.; Atkinson, F.; Daniell, N.D.; Lewis, L.K.; Fitzgerald, J.S.; Lang, J.J.; Ortega, F.B. European normative values for physical fitness in children and adolescents aged 9–17 years: Results from 2 779 165 Eurofit performances representing 30 countries. Brit. J. Sport. Med. 2018, 52, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Pan, L.; Liu, F.; Ren, X.; Cui, Z.; Pa, L.; Zhao, J.; Wang, D.; Du, J.; Wang, H.; et al. The Mediation Effect of Body Composition on the Association Between Menopause and Hyperuricemia: Evidence From China National Health Survey. Front. Endocrinol. 2022, 13, 879384. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, J.; Ruan, Y.; Zhang, Z.; Sun, J.; Chen, H. The association between fat-to-muscle ratio and metabolic disorders in type 2 diabetes. Diabetol. Metab. Syndr. 2021, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Y.; Zhang, H.; Chen, C.; Wang, Y.; Zhang, J.; Xia, F.; Benedict, C.; Tan, X.; Lu, Y. Total and regional fat-to-muscle mass ratio measured by bioelectrical impedance and risk of incident type 2 diabetes. J. Cachexia. Sarcopenia. Muscle 2021, 12, 2154–2162. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia. Sarcopenia. Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Madero, M.; Katz, R.; Murphy, R.; Newman, A.; Patel, K.; Ix, J.; Peralta, C.; Satterfield, S.; Fried, L.; Shlipak, M.; et al. Comparison between Different Measures of Body Fat with Kidney Function Decline and Incident CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 893–903. [Google Scholar] [CrossRef]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.; et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef]
- Volpato, S.; Bianchi, L.; Lauretani, F.; Lauretani, F.; Bandinelli, S.; Guralnik, J.M.; Zuliani, G.; Ferrucci, L. Role of Muscle Mass and Muscle Quality in the Association Between Diabetes and Gait Speed. Diabetes. Care 2012, 35, 1672–1679. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Li, J.; Gao, X.; Han, Y.; Teng, W.; Shan, Z.; Lai, Y. Combined Effects of Dyslipidemia and High Adiposity on the Estimated Glomerular Filtration Rate in a Middle-Aged Chinese Population. Diabetes. Metab. Syndr. Obes. 2021, 14, 4513–4522. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, J.I.; Weir, M.R. Obesity and Kidney Disease. Prog. Cardiovasc. Dis. 2018, 61, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, H.; Kim, G.; Isobe, T.; Sakae, T.; Oh, S. Relationships of Fat and Muscle Mass with Chronic Kidney Disease in Older Adults: A Cross-Sectional Pilot Study. Int. J. Env. Res. Pub. He 2020, 17, 9124. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sanchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transpl. 2013, 28, 2221–2228. [Google Scholar] [CrossRef]
- Jordan, D.M.; Choi, H.K.; Verbanck, M.; Topless, R.; Won, H.; Nadkarni, G.; Merriman, T.R.; Do, R. No causal effects of serum urate levels on the risk of chronic kidney disease: A Mendelian randomization study. PLoS Med. 2019, 16, e1002725. [Google Scholar] [CrossRef]
- Sato, Y.; Feig, D.I.; Stack, A.G.; Kang, D.; Lanaspa, M.A.; Ejaz, A.A.; Sánchez-Lozada, L.G.; Kuwabara, M.; Borghi, C.; Johnson, R.J. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat. Rev. Nephrol. 2019, 15, 767–775. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).