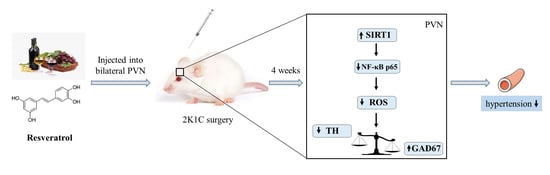
Resveratrol in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension by Regulation of ROS and Neurotransmitters
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PVN Cannula Implantation
2.3. Preparation and Grouping of Animal Models
2.4. Measurement of Blood Pressure
2.5. Collection of Blood and Tissue Samples
2.6. Immunofluorescence Staining
2.7. Western Blotting
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Result
3.1. Blood Pressure
3.2. Plasma NE
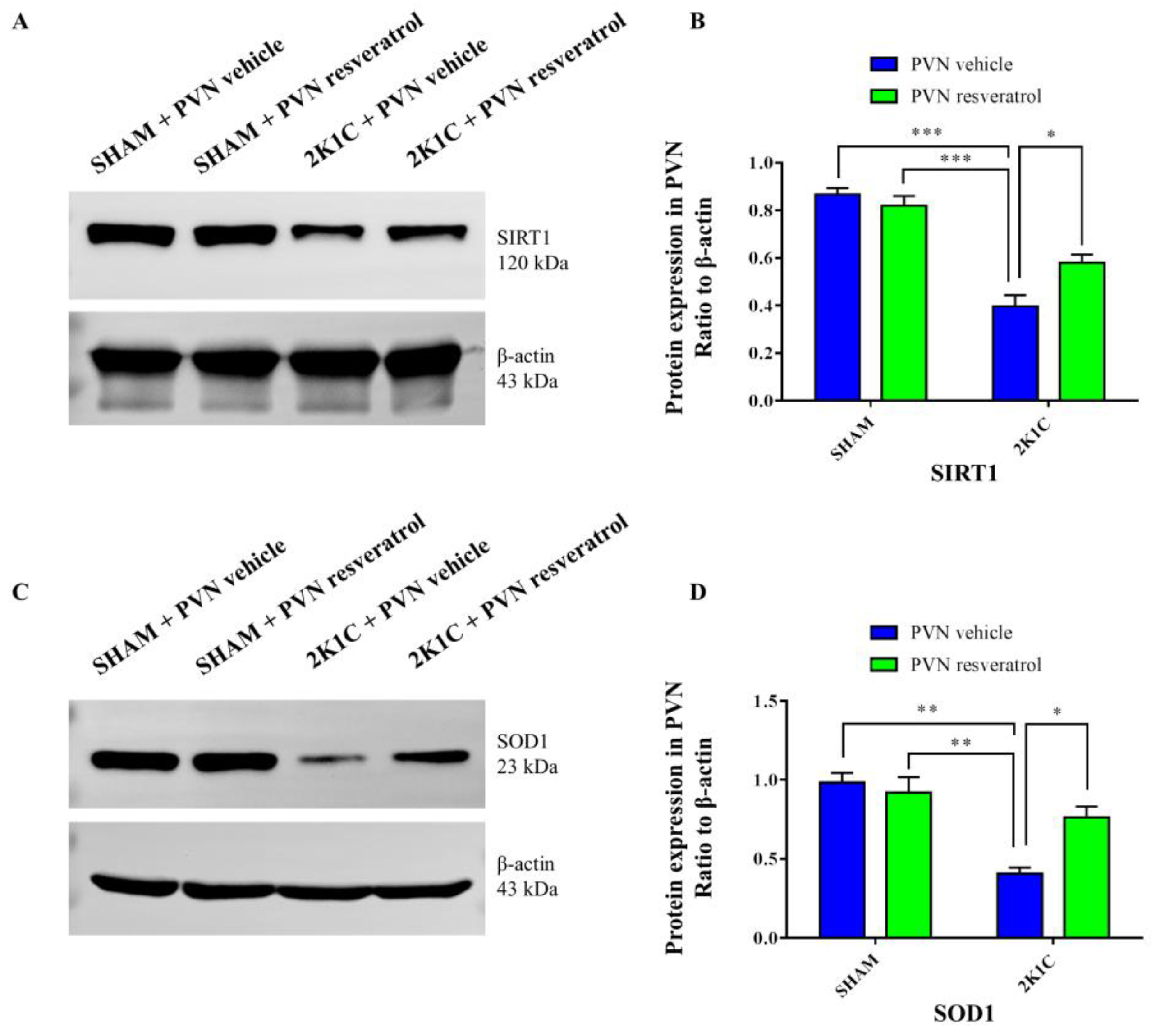
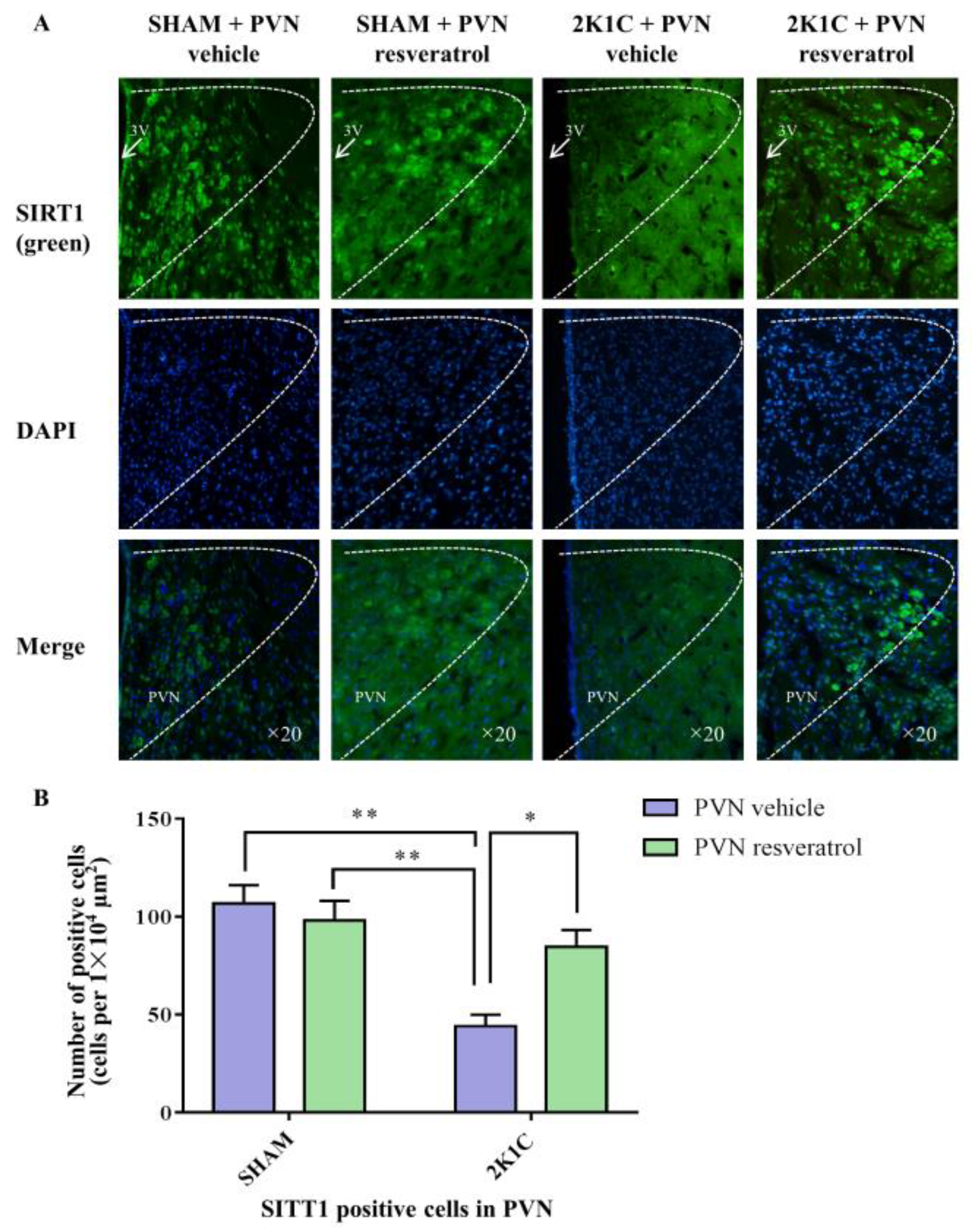
3.3. SIRT1 Expression in PVN
3.4. NF-κB Activity in the PVN
3.5. NAD(P)H Oxidase Activity in the PVN
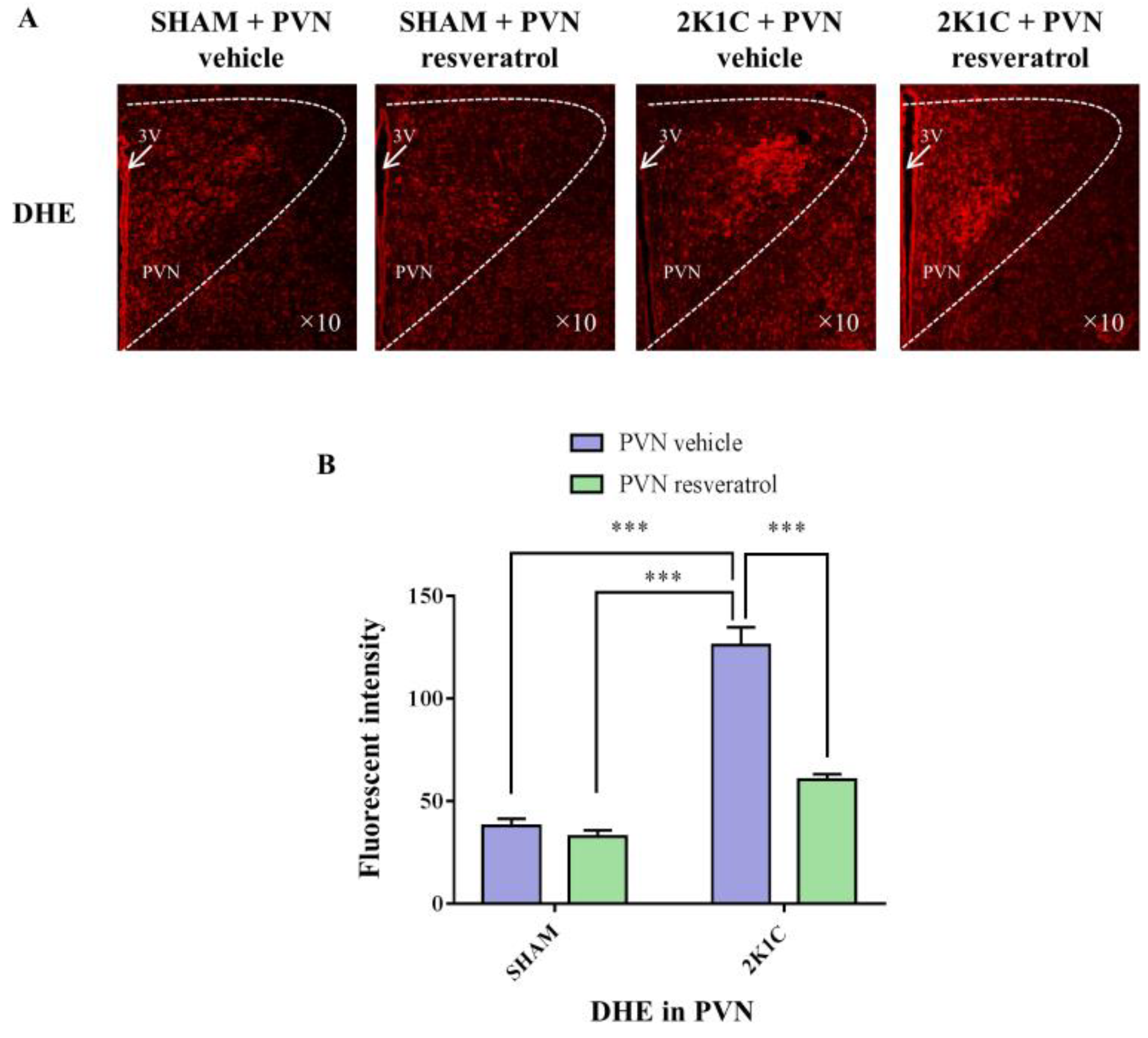
3.6. ROS Production in PVN
3.7. SOD1 Protein Expression in the PVN
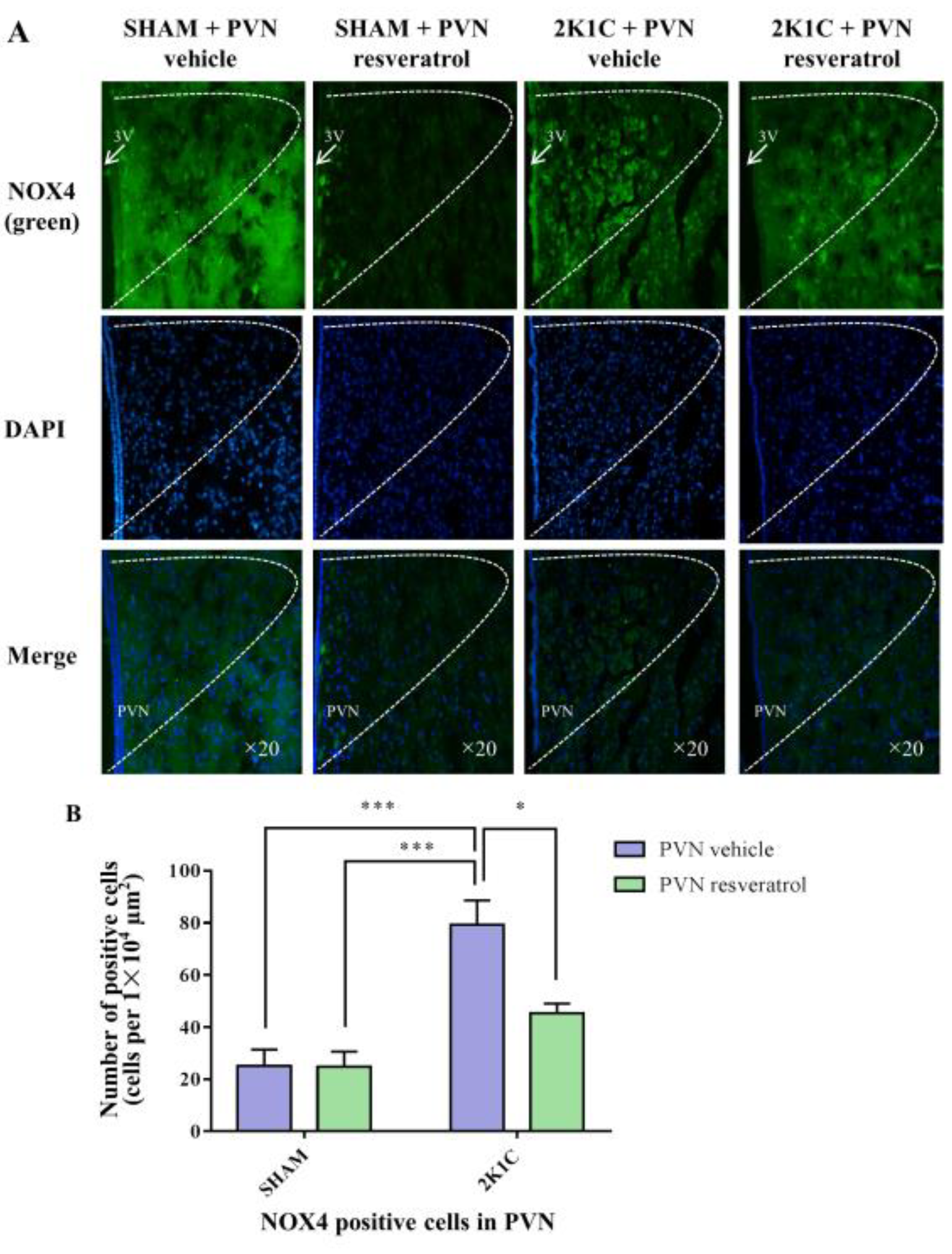
3.8. NOX4 Expression in PVN
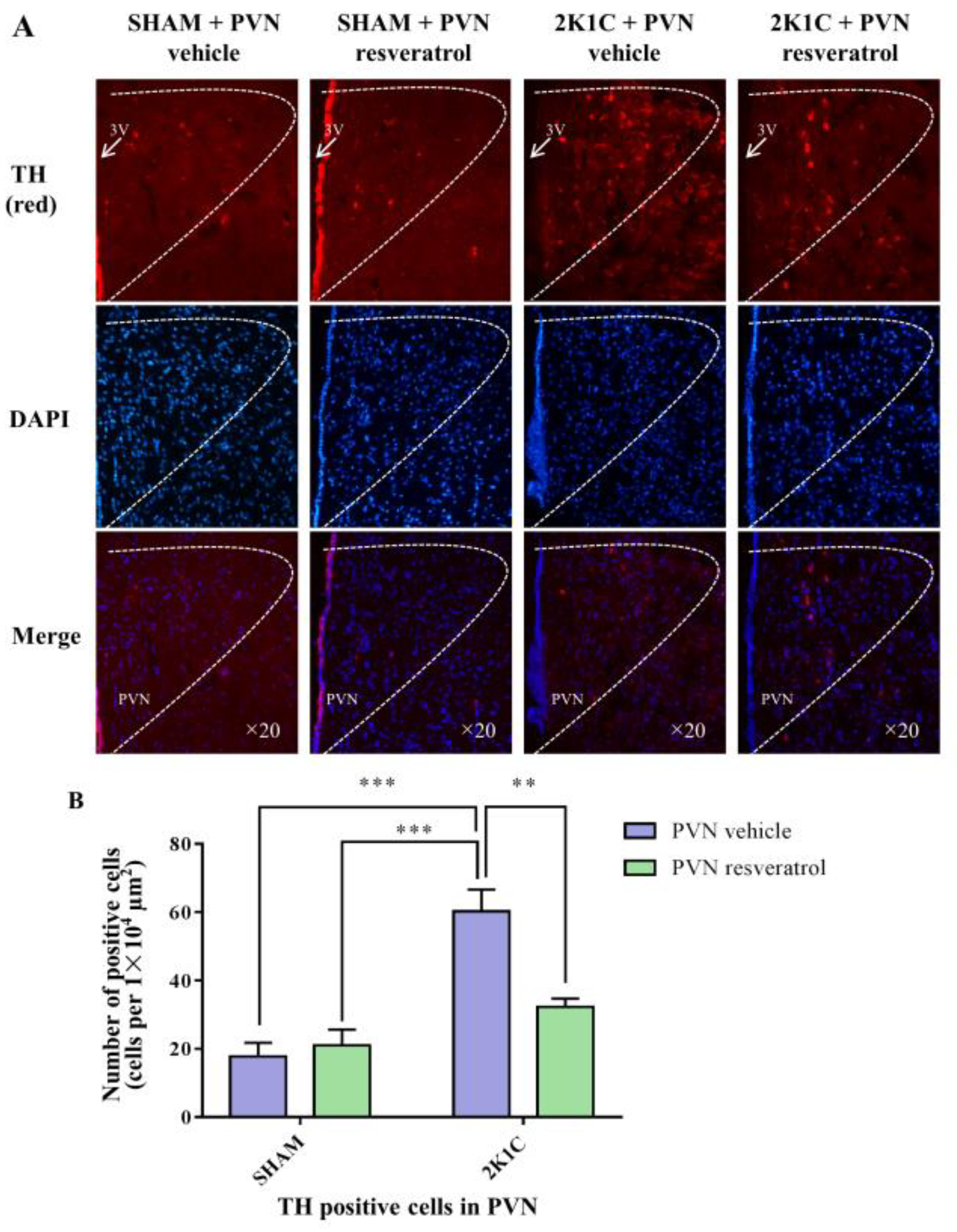
3.9. TH Expression in the PVN
3.10. GAD67 Expression in the PVN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVN | Hypothalamic Paraventricular Nucleus |
| SNA | sympathetic nerve activity |
| SIRT1 | Silent Mating type information regulation 2 homolog-1 |
| ROS | reactive oxygen species |
| NOX | NAD(P)H oxidase |
| RSNA | renal sympathetic nerve activity |
| NF-κB | nuclear factor-kappa B |
| TH | Tyrosine hydroxylase |
| GAD67 | Glutamate decarboxylase 67 |
| NE | norepinephrine |
| Glu | glutamate |
| GABA | γ-aminobutyric acid |
| 2K1C | two-kidney one-clamp |
| SHAM | sham operation |
| aCSF | artificial cerebrospinal fluid |
| SBP | systolic blood pressure |
| DHE | Dihydroethidium |
| RVLM | rostral ventrolateral medulla |
| ARC | arcuate nucleus of the hypothalamus |
References
- Richards, E.M.; Li, J.; Stevens, B.R.; Pepine, C.J.; Raizada, M.K. Gut Microbiome and Neuroinflammation in Hypertension. Circ. Res. 2022, 130, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Bo, J.H.; Zheng, F.; Zhang, F.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Salusin-beta in Intermediate Dorsal Motor Nucleus of the Vagus Regulates Sympathetic-Parasympathetic Balance and Blood Pressure. Biomedicines 2021, 9, 1118. [Google Scholar] [CrossRef] [PubMed]
- Cayupe, B.; Morgan, C.; Puentes, G.; Valladares, L.; Burgos, H.; Castillo, A.; Hernandez, A.; Constandil, L.; Rios, M.; Saez-Briones, P.; et al. Hypertension in Prenatally Undernourished Young-Adult Rats Is Maintained by Tonic Reciprocal Paraventricular-Coerulear Excitatory Interactions. Molecules 2021, 26, 3568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Pachuau, J.; Li, D.P.; Chen, S.R.; Pan, H.L. Group III metabotropic glutamate receptors regulate hypothalamic presympathetic neurons through opposing presynaptic and postsynaptic actions in hypertension. Neuropharmacology 2020, 174, 108159. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Ju, Y.; Wang, X.; Sun, X.; Fang, Y.; Chen, K. Transcriptomic and Metabolomic Basis of Short- and Long-Term Post-Harvest UV-C Application in Regulating Grape Berry Quality Development. Foods 2021, 10, 625. [Google Scholar] [CrossRef]
- Gutierrez Aguilar, G.F.; Alquisiras-Burgos, I.; Franco-Perez, J.; Pineda-Ramirez, N.; Ortiz-Plata, A.; Torres, I.; Pedraza-Chaverri, J.; Aguilera, P. Resveratrol Prevents GLUT3 Up-Regulation Induced by Middle Cerebral Artery Occlusion. Brain Sci. 2020, 10, 651. [Google Scholar] [CrossRef]
- Astley, C.; Houacine, C.; Zaabalawi, A.; Wilkinson, F.; Lightfoot, A.P.; Alexander, Y.; Whitehead, D.; Singh, K.K.; Azzawi, M. Nanostructured Lipid Carriers Deliver Resveratrol, Restoring Attenuated Dilation in Small Coronary Arteries, via the AMPK Pathway. Biomedicines 2021, 9, 1852. [Google Scholar] [CrossRef]
- Grujic-Milanovic, J.; Jacevic, V.; Miloradovic, Z.; Jovovic, D.; Milosavljevic, I.; Milanovic, S.D.; Mihailovic-Stanojevic, N. Resveratrol Protects Cardiac Tissue in Experimental Malignant Hypertension Due to Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Properties. Int. J. Mol. Sci. 2021, 22, 5006. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Perinatal Resveratrol Therapy Prevents Hypertension Programmed by Maternal Chronic Kidney Disease in Adult Male Offspring: Implications of the Gut Microbiome and Their Metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Kung, H.C.; Lin, K.J.; Kung, C.T.; Lin, T.K. Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease. Biomedicines 2021, 9, 918. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Deng, J.; Liu, X.; Li, Q.; Zhang, J.; Bai, H.; Zhang, J. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol. J. Cell. Mol. Med. 2021, 25, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, L.; Wu, W.; Wu, J.; Sun, Z.; Ren, J. USP22 Protects against Myocardial Ischemia-Reperfusion Injury via the SIRT1-p53/SLC7A11-Dependent Inhibition of Ferroptosis-Induced Cardiomyocyte Death. Front. Physiol. 2020, 11, 551318. [Google Scholar] [CrossRef] [PubMed]
- Nishi, E.E.; Lopes, N.R.; Gomes, G.N.; Perry, J.C.; Sato, A.Y.S.; Naffah-Mazzacoratti, M.G.; Bergamaschi, C.T.; Campos, R.R. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens. Res. 2019, 42, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Hartlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Xue, B.; Beltz, T.G.; Johnson, R.F.; Guo, F.; Hay, M.; Johnson, A.K. PVN adenovirus-siRNA injections silencing either NOX2 or NOX4 attenuate aldosterone/NaCl-induced hypertension in mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H733–H741. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhou, H.; Feng, X.M.; Gao, Q.; Ding, L.; Tang, C.S.; Zhu, G.Q.; Zhou, Y.B. Superoxide anions in the paraventricular nucleus mediate cardiac sympathetic afferent reflex in insulin resistance rats. Acta Physiol. 2014, 212, 267–282. [Google Scholar] [CrossRef]
- Bai, J.; Yu, X.J.; Liu, K.L.; Wang, F.F.; Jing, G.X.; Li, H.B.; Zhang, Y.; Huo, C.J.; Li, X.; Gao, H.L.; et al. Central administration of tert-butylhydroquinone attenuates hypertension via regulating Nrf2 signaling in the hypothalamic paraventricular nucleus of hypertensive rats. Toxicol. Appl. Pharmacol. 2017, 333, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Schraivogel, D.; Kuhn, T.M.; Rauscher, B.; Rodriguez-Martinez, M.; Paulsen, M.; Owsley, K.; Middlebrook, A.; Tischer, C.; Ramasz, B.; Ordonez-Rueda, D.; et al. High-speed fluorescence image-enabled cell sorting. Science 2022, 375, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Javed Shaikh, M.A.; Thangavelu, L.; Singh, S.K.; Rama Raju Allam, V.S.; Jha, N.K.; et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Fan, Y.; He, Q.; Li, Y.; Wu, D.; Jiang, E. Exogenous H2S Ameliorates High Salt-Induced Hypertension by Alleviating Oxidative Stress and Inflammation in the Paraventricular Nucleus in Dahl S Rats. Cardiovasc. Toxicol. 2022, 22, 477–491. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Yin, J.; Shi, Y.; Tan, J.; Zheng, L.; Wang, C.; Li, X.; Xue, M.; Liu, J.; et al. TLR4 participates in sympathetic hyperactivity Post-MI in the PVN by regulating NF-kappaB pathway and ROS production. Redox Biol. 2019, 24, 101186. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, J.; Yin, J.; Hu, H.; Shi, Y.; Wang, Y.; Xue, M.; Li, X.; Liu, J.; Li, Y.; et al. Targeting blockade of nuclear factor-kappaB in the hypothalamus paraventricular nucleus to prevent cardiac sympathetic hyperinnervation post myocardial infarction. Neurosci. Lett. 2019, 707, 134319. [Google Scholar] [CrossRef]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef]
- Deng, H.J.; Zhou, C.H.; Huang, L.T.; Wen, L.B.; Zhou, M.L.; Wang, C.X. Activation of silent information regulator 1 exerts a neuroprotective effect after intracerebral hemorrhage by deacetylating NF-kappaB/p65. J. Neurochem. 2021, 157, 574–585. [Google Scholar] [CrossRef]
- Ramlan, H.; Damanhuri, H.A. Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging. Biomolecules 2022, 12, 449. [Google Scholar] [CrossRef]
- Frerker, B.; Rohde, M.; Muller, S.; Bien, C.G.; Kohling, R.; Kirschstein, T. Distinct Effects of Stereotactically Injected Human Cerebrospinal Fluid Containing Glutamic Acid Decarboxylase Antibodies into the Hippocampus of Rats on the Development of Spontaneous Epileptic Activity. Brain Sci. 2020, 10, 123. [Google Scholar] [CrossRef]
- Idalencio, R.; Lopes, T.M.; Soares, S.M.; Pompermaier, A.; de Alcantara Barcellos, H.H.; Kalichak, F.; Fagundes, M.; de Oliveira, C.M.; Barcellos, L.J.G. Effect of levodopa/carbidopa on stress response in zebrafish. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2021, 207, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Yu, X.J.; Wang, X.M.; Li, H.B.; Li, Y.; Bai, J.; Qi, J.; Zhang, N.; Liu, K.L.; Zhang, Y.; et al. Bilateral Paraventricular Nucleus Upregulation of Extracellular Superoxide Dismutase Decreases Blood Pressure by Regulation of the NLRP3 and Neurotransmitters in Salt-Induced Hypertensive Rats. Front. Pharmacol. 2021, 12, 756671. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, B.; He, R. Effect of the changes of NMDA receptor in hypothalamic paraventricular nucleus on cardiac function and sympathetic nervous activity in rats with heart failure. Biochem. Biophys. Res. Commun. 2017, 493, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Yu, X.J.; Yang, Q.; Wang, X.M.; Xia, W.J.; Li, H.B.; Liu, K.L.; Yi, Q.Y.; Kang, Y.M. Inhibition of Maternal c-Src Ameliorates the Male Offspring Hypertension by Suppressing Inflammation and Neurotransmitters in the Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Gao, F.; Li, H.H.; Cardinale, J.P.; Elks, C.; Zang, W.J.; Yu, X.J.; Xu, Y.Y.; Qi, J.; Yang, Q.; et al. NF-kappaB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic Res. Cardiol. 2011, 106, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Cao, Y.K.; Liu, Y.X.; Wang, R.; Wu, Y.M. Microinjection of resveratrol into rostral ventrolateral medulla decreases sympathetic vasomotor tone through nitric oxide and intracellular Ca2+ in anesthetized male rats. Acta Pharmacol. Sin. 2008, 29, 906–912. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.J.; Xiao, T.; Chi, H.L.; Zhu, G.Q.; Kang, Y.M. Nrf1 Knock-Down in the Hypothalamic Paraventricular Nucleus Alleviates Hypertension Through Intervention of Superoxide Production-Removal Balance and Mitochondrial Function. Cardiovasc. Toxicol. 2021, 21, 472–489. [Google Scholar] [CrossRef]
- Su, Q.; Yu, X.J.; Wang, X.M.; Peng, B.; Bai, J.; Li, H.B.; Li, Y.; Xia, W.J.; Fu, L.Y.; Liu, K.L.; et al. Na(+)/K(+)-ATPase Alpha 2 Isoform Elicits Rac1-Dependent Oxidative Stress and TLR4-Induced Inflammation in the Hypothalamic Paraventricular Nucleus in High Salt-Induced Hypertension. Antioxidants 2022, 11, 288. [Google Scholar] [CrossRef]
- Gao, H.L.; Yu, X.J.; Zhang, Y.; Wang, C.L.; Lei, Y.M.; Yu, J.Y.; Zong, D.M.; Liu, K.L.; Zhang, D.D.; Li, Y.; et al. Astaxanthin Ameliorates Blood Pressure in Salt-Induced Prehypertensive Rats Through ROS/MAPK/NF-kappaB Pathways in the Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 1045–1057. [Google Scholar] [CrossRef]
- Ding, L.; Kang, Y.; Dai, H.B.; Wang, F.Z.; Zhou, H.; Gao, Q.; Xiong, X.Q.; Zhang, F.; Song, T.R.; Yuan, Y.; et al. Adipose afferent reflex is enhanced by TNFalpha in paraventricular nucleus through NADPH oxidase-dependent ROS generation in obesity-related hypertensive rats. J. Transl. Med. 2019, 17, 256. [Google Scholar] [CrossRef]
- Kang, Y.; Ding, L.; Dai, H.; Wang, F.; Zhou, H.; Gao, Q.; Xiong, X.; Zhang, F.; Song, T.; Yuan, Y.; et al. Intermedin in Paraventricular Nucleus Attenuates Ang II-Induced Sympathoexcitation through the Inhibition of NADPH Oxidase-Dependent ROS Generation in Obese Rats with Hypertension. Int. J. Mol. Sci. 2019, 20, 4217. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, S.; Bolor-Erdene, E.; Wang, L.; Tian, D.; Mei, Y. NAMPT/SIRT1 Attenuate Ang II-Induced Vascular Remodeling and Vulnerability to Hypertension by Inhibiting the ROS/MAPK Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1974265. [Google Scholar] [CrossRef] [PubMed]
- Castrejon-Tellez, V.; Villegas-Romero, M.; Rubio-Ruiz, M.E.; Perez-Torres, I.; Carreon-Torres, E.; Diaz-Diaz, E.; Guarner-Lans, V. Effect of a Resveratrol/Quercetin Mixture on the Reversion of Hypertension Induced by a Short-Term Exposure to High Sucrose Levels Near Weaning and a Long-Term Exposure That Leads to Metabolic Syndrome in Rats. Int. J. Mol. Sci. 2020, 21, 2231. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.Z.; Wu, Z.T.; Wang, W.; Tan, X.; Yang, Y.H.; Wang, Y.K.; Li, M.L.; Wang, W.Z. SIRT1 exerts anti-hypertensive effect via FOXO1 activation in the rostral ventrolateral medulla. Free Radic. Biol. Med. 2022, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.F.; Zhang, H.; Zheng, P.; Chen, S.; Gu, Z.; Zhou, J.J.; Phaup, J.G.; Chang, H.M.; Yeh, E.T.H.; Pan, H.L.; et al. Impaired Kv7 channel activity in the central amygdala contributes to elevated sympathetic outflow in hypertension. Cardiovasc. Res. 2022, 118, 585–596. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, H. Modulation of Sirt1 and FoxO1 on Hypothalamic Leptin-Mediated Sympathetic Activation and Inflammation in Diet-Induced Obese Rats. J. Am. Heart Assoc. 2021, 10, e020667. [Google Scholar] [CrossRef]
- Qi, J.; Yu, X.J.; Fu, L.Y.; Liu, K.L.; Gao, T.T.; Tu, J.W.; Kang, K.B.; Shi, X.L.; Li, H.B.; Li, Y.; et al. Exercise Training Attenuates Hypertension Through TLR4/MyD88/NF-kappaB Signaling in the Hypothalamic Paraventricular Nucleus. Front. Neurosci. 2019, 13, 1138. [Google Scholar] [CrossRef]
- Xu, M.L.; Yu, X.J.; Zhao, J.Q.; Du, Y.; Xia, W.J.; Su, Q.; Du, M.M.; Yang, Q.; Qi, J.; Li, Y.; et al. Calcitriol ameliorated autonomic dysfunction and hypertension by down-regulating inflammation and oxidative stress in the paraventricular nucleus of SHR. Toxicol. Appl. Pharmacol. 2020, 394, 114950. [Google Scholar] [CrossRef]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef]
- Meng, F.; Yang, M.; Chen, Y.; Chen, W.; Wang, W. miR-34a induces immunosuppression in colorectal carcinoma through modulating a SIRT1/NF-kappaB/B7-H3/TNF-alpha axis. Cancer Immunol. Immunother. 2021, 70, 2247–2259. [Google Scholar] [CrossRef]
- Dasgupta, A.; Shukla, S.K.; Vernucci, E.; King, R.J.; Abrego, J.; Mulder, S.E.; Mullen, N.J.; Graves, G.; Buettner, K.; Thakur, R.; et al. SIRT1-NOX4 signaling axis regulates cancer cachexia. J. Exp. Med. 2020, 217, e20190745. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Zhang, A.Q.; Zhao, X.F.; Cardinale, J.P.; Elks, C.; Cao, X.M.; Zhang, Z.W.; Francis, J. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res. Cardiol. 2011, 106, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Hristova, K.; Fedacko, J.; El-Kilany, G.; Cornelissen, G. Chronic heart failure: A disease of the brain. Heart Fail. Rev. 2019, 24, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, S.; Krukoff, T.L. Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension 2006, 48, 1130–1136. [Google Scholar] [CrossRef]
- Bardgett, M.E.; Holbein, W.W.; Herrera-Rosales, M.; Toney, G.M. Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-alpha. Hypertension 2014, 63, 527–534. [Google Scholar] [CrossRef][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Fu, L.-Y.; Liu, K.-L.; Li, R.-J.; Qiao, J.-A.; Yu, X.-J.; Yu, J.-Y.; Li, Y.; Feng, Z.-P.; Yi, Q.-Y.; et al. Resveratrol in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension by Regulation of ROS and Neurotransmitters. Nutrients 2022, 14, 4177. https://doi.org/10.3390/nu14194177
Qi J, Fu L-Y, Liu K-L, Li R-J, Qiao J-A, Yu X-J, Yu J-Y, Li Y, Feng Z-P, Yi Q-Y, et al. Resveratrol in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension by Regulation of ROS and Neurotransmitters. Nutrients. 2022; 14(19):4177. https://doi.org/10.3390/nu14194177
Chicago/Turabian StyleQi, Jie, Li-Yan Fu, Kai-Li Liu, Rui-Juan Li, Jin-An Qiao, Xiao-Jing Yu, Jia-Yue Yu, Ying Li, Zhi-Peng Feng, Qiu-Yue Yi, and et al. 2022. "Resveratrol in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension by Regulation of ROS and Neurotransmitters" Nutrients 14, no. 19: 4177. https://doi.org/10.3390/nu14194177
APA StyleQi, J., Fu, L.-Y., Liu, K.-L., Li, R.-J., Qiao, J.-A., Yu, X.-J., Yu, J.-Y., Li, Y., Feng, Z.-P., Yi, Q.-Y., Jia, H., Gao, H.-L., Tan, H., & Kang, Y.-M. (2022). Resveratrol in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension by Regulation of ROS and Neurotransmitters. Nutrients, 14(19), 4177. https://doi.org/10.3390/nu14194177