Parabacteroides distasonis Properties Linked to the Selection of New Biotherapeutics
Abstract
1. Introduction
2. Materials and Method
2.1. Bacterial Strains and Culture Conditions
2.2. Cell Lines and Culture Conditions
2.3. Tolerance to Gastric Conditions
2.4. Bacterial Adhesion to Caco-2 Cell Line
2.5. Transepithelial Electrical Resistance
2.6. Immunomodulation Assay on HT-29 and PBMC Cells
2.7. Statistical Analysis
3. Results
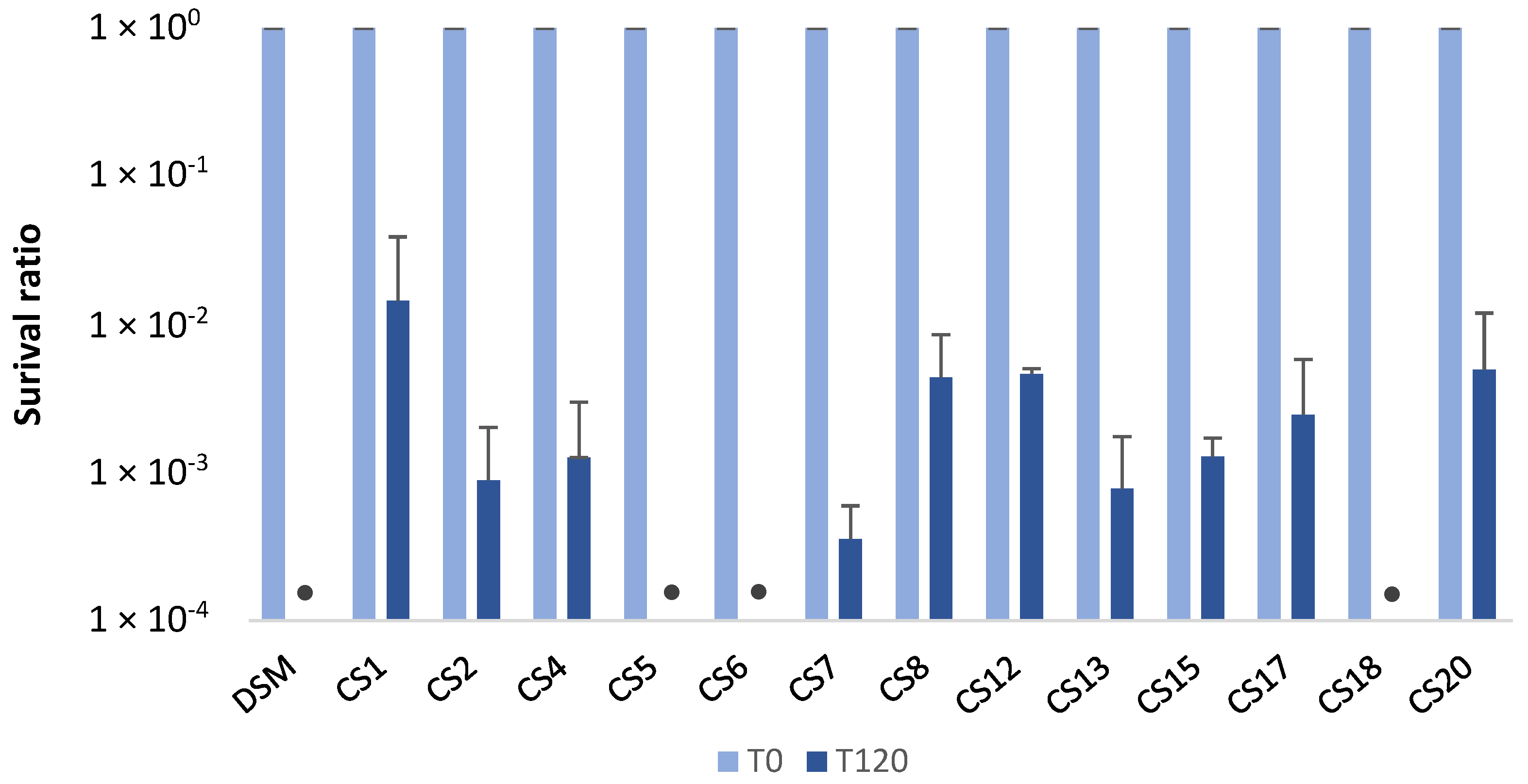
3.1. Survival Capacity of P. Distasonis to Gastric Conditions
3.2. Adhesion Capacities of P. Distasonis Strains to Caco-2 Cells
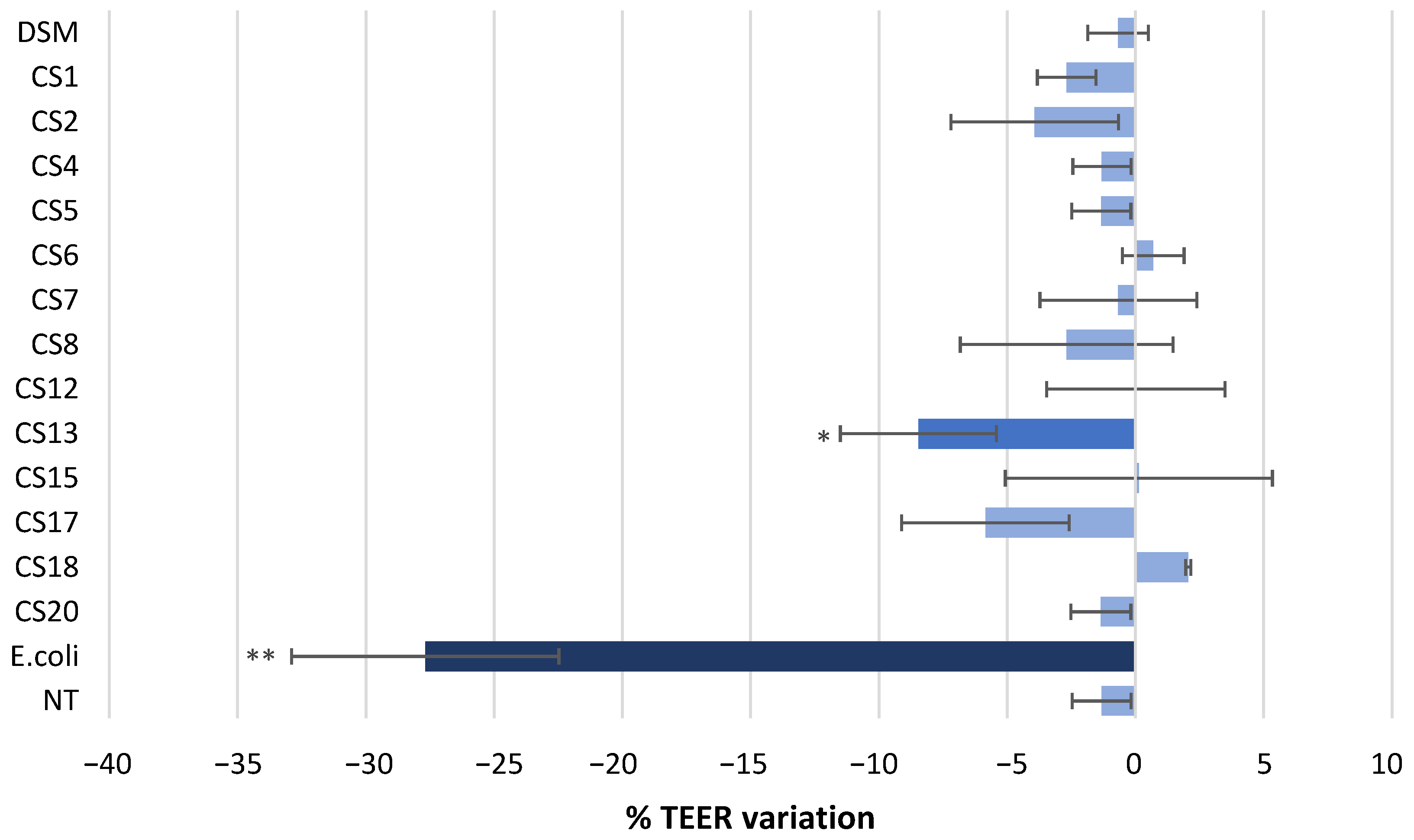
3.3. Effect of P. Distasonis on Caco-2 Monolayer Integrity
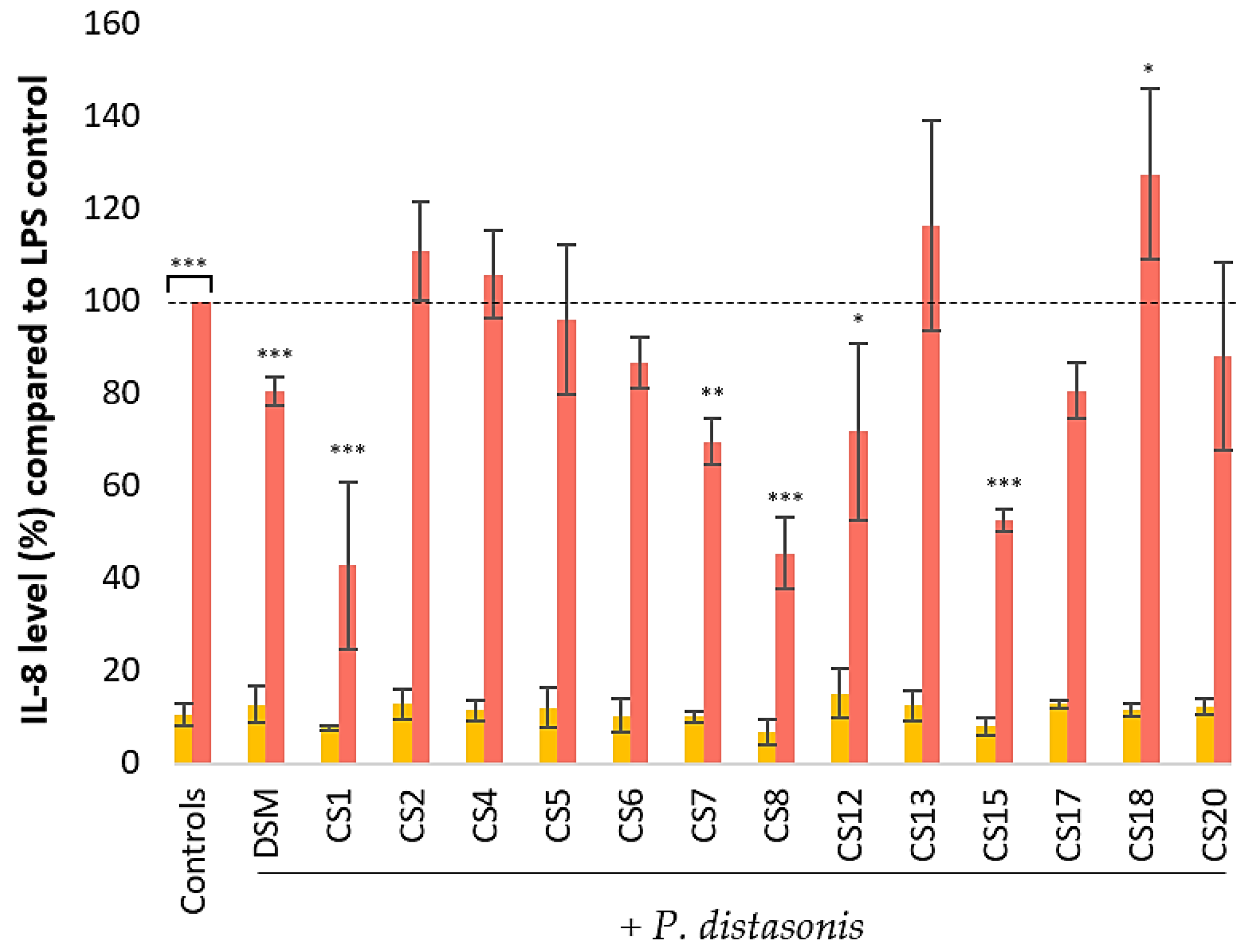
3.4. Effect of P. Distasonis on IL-8 Production by Untreated and LPS-Stimulated HT-29 Cells
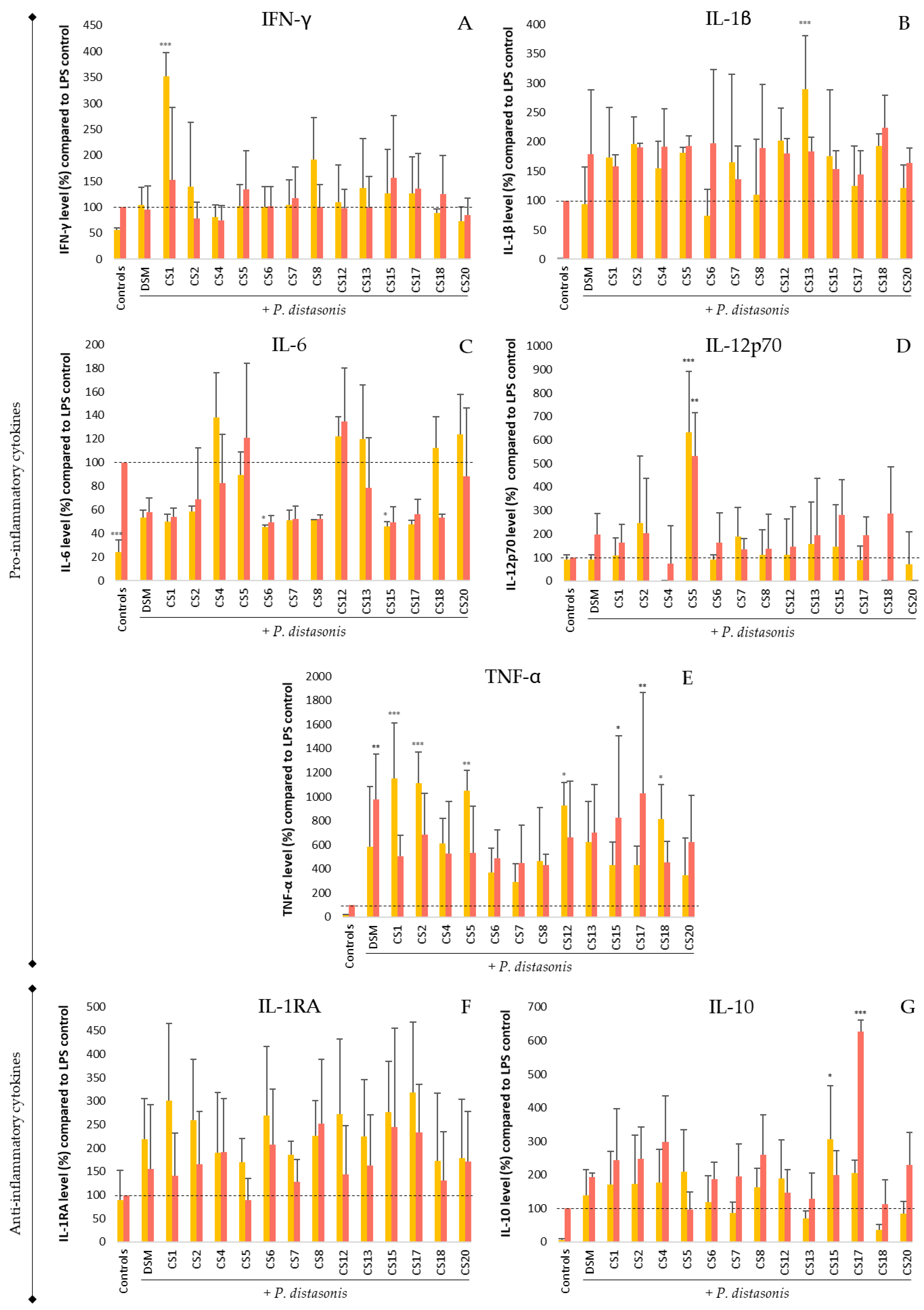
3.5. Immunomodulation of P. Distasonis on Untreated and LPS-Stimulated PBMC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doré, J.; Multon, M.-C.; Béhier, J.-M.; Affagard, H.; Andremont, A.; Barthélémy, P.; Batitsa, R.; Bonneville, M.; Bonny, C.; Boyaval, G.; et al. The Human Gut Microbiome as Source of Innovation for Health: Which Physiological and Therapeutic Outcomes Could We Expect? Therapies 2017, 72, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E. An Integrated Catalog of Reference Genes in the Human Gut Microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.B.; Hsiao, E.Y. Microbiomes as Sources of Emergent Host Phenotypes. Science 2019, 365, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Talham, G.L.; Jiang, H.-Q.; Bos, N.A.; Cebra, J.J. Segmented Filamentous Bacteria Are Potent Stimuli of a Physiologically Normal State of the Murine Gut Mucosal Immune System. Infect. Immun. 1999, 67, 1992–2000. [Google Scholar] [CrossRef]
- Jandhyala, S.M. Role of the Normal Gut Microbiota. WJG 2015, 21, 8787. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 46. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26. [Google Scholar] [CrossRef]
- Youssef, M.; Ahmed, H.Y.; Zongo, A.; Korin, A.; Zhan, F.; Hady, E.; Umair, M.; Shahid Riaz Rajoka, M.; Xiong, Y.; Li, B. Probiotic Supplements: Their Strategies in the Therapeutic and Prophylactic of Human Life-Threatening Diseases. Int. J. Mol. Sci. 2021, 22, 11290. [Google Scholar] [CrossRef]
- McFarland, L.V. From Yaks to Yogurt: The History, Development, and Current Use of Probiotics. Clin. Infect. Dis. 2015, 60, S85–S90. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Fu, L.; Wang, J. Protocol for Fecal Microbiota Transplantation in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2018, 2018, 8941340. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Choo, J.M.; Wang, Y.; Leong, L.E.X.; Costello, S.P.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Bacterial Viability in Faecal Transplants: Which Bacteria Survive? EBioMedicine 2019, 41, 509–516. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, Y.-S.; Kim, Y.; Lee, S.-H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.-S.; Lim, H.S.; Kim, M.-S.; et al. Gut Commensal Bacteroides Acidifaciens Prevents Obesity and Improves Insulin Sensitivity in Mice. Mucosal. Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Kainulainen, V.; Suutarinen, M.; Heini, T.; Bowers, J.R.; Jasso-Selles, D.; Lemmer, D.; Valentine, M.; Barnes, R.; Engelthaler, D.M.; et al. Isolation of Anti-Inflammatory and Epithelium Reinforcing Bacteroides and Parabacteroides spp. from A Healthy Fecal Donor. Nutrients 2020, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, B.; Assohoun, A.L.W.; Boutillier, D.; Peucelle, V.; Desramaut, J.; Boudebbouze, S.; Croyal, M.; Waligora-Dupriet, A.-J.; Rhimi, M.; Grangette, C.; et al. Identification of New Potential Biotherapeutics from Human Gut Microbiota-Derived Bacteria. Microorganisms 2021, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, B.; Assohoun, A.L.W.; Boutillier, D.; Súkeníková, L.; Desramaut, J.; Boudebbouze, S.; Salomé-Desnoulez, S.; Hrdý, J.; Waligora-Dupriet, A.-J.; Maguin, E.; et al. In Vitro Characterization of Gut Microbiota-Derived Commensal Strains: Selection of Parabacteroides distasonis Strains Alleviating TNBS-Induced Colitis in Mice. Cells 2020, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral Administration of Parabacteroides distasonis Antigens Attenuates Experimental Murine Colitis through Modulation of Immunity and Microbiota Composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Kane, A.; Lee, K.; Xu, Q.; Wu, X.; Roper, J.; Mason, J.B.; Crott, J.W. Parabacteroides distasonis Attenuates Toll-like Receptor 4 Signaling and Akt Activation and Blocks Colon Tumor Formation in High-fat Diet-fed Azoxymethane-treated Mice. Int. J. Cancer 2018, 143, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Kane, A.V.; Wu, X.; Crott, J.W. Parabacteroides distasonis Attenuates Tumorigenesis, Modulates Inflammatory Markers and Promotes Intestinal Barrier Integrity in Azoxymethane-Treated A/J Mice. Carcinogenesis 2020, 41, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Conway, P.L.; Brown, I.L.; Evans, A.J. In Vitro Utilization of Amylopectin and High-Amylose Maize (Amylomaize) Starch Granules by Human Colonic Bacteria. Appl Env. Microbiol. 1999, 65, 4848–4854. [Google Scholar] [CrossRef]
- Xu, J.; Mahowald, M.A.; Ley, R.E.; Lozupone, C.A.; Hamady, M.; Martens, E.C.; Henrissat, B.; Coutinho, P.M.; Minx, P.; Latreille, P.; et al. Evolution of Symbiotic Bacteria in the Distal Human Intestine. PLoS Biol. 2007, 5, e156. [Google Scholar] [CrossRef] [PubMed]
- Chamarande, J.; Cunat, L.; Caillet, C.; Mathieu, L.; Duval, J.F.L.; Lozniewski, A.; Frippiat, J.-P.; Alauzet, C.; Cailliez-Grimal, C. Surface Properties of Parabacteroides distasonis and Impacts of Stress-Induced Molecules on Its Surface Adhesion and Biofilm Formation Capacities. Microorganisms 2021, 9, 1602. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Waśko, A.; Paduch, R.; Skrzypek, T.; Sroka-Bartnicka, A. The Effect of Cell Surface Components on Adhesion Ability of Lactobacillus Rhamnosus. Antonie Van Leeuwenhoek 2014, 106, 751–762. [Google Scholar] [CrossRef]
- Playford, R.J.; Choudhry, N.; Kelly, P.; Marchbank, T. Effects of Bovine Colostrum with or without Egg on In Vitro Bacterial-Induced Intestinal Damage with Relevance for SIBO and Infectious Diarrhea. Nutrients 2021, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef]
- Edebol Carlman, H.M.T.; Rode, J.; König, J.; Repsilber, D.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Kiselev, A.; Pruessner, J.C.; Brummer, R.J. Probiotic Mixture Containing Lactobacillus helveticus, Bifidobacterium longum and Lactiplantibacillus plantarum Affects Brain Responses to an Arithmetic Stress Task in Healthy Subjects: A Randomised Clinical Trial and Proof-of-Concept Study. Nutrients 2022, 14, 1329. [Google Scholar] [CrossRef]
- Chamarande, J.; Cunat, L.; Alauzet, C.; Cailliez-Grimal, C. In Silico Study of Cell Surface Structures of Parabacteroides distasonis Involved in Its Maintenance within the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 9411. [Google Scholar] [CrossRef]
- Alvarez, C.-S.; Badia, J.; Bosch, M.; Giménez, R.; Baldomà, L. Outer Membrane Vesicles and Soluble Factors Released by Probiotic Escherichia Coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight Junction Proteins in Intestinal Epithelial Cells. Front. Microbiol. 2016, 7, 1981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]

| P. distasonis | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSM 20701T | CS1 | CS2 | CS4 | CS5 | CS6 | CS7 | CS8 | CS12 | CS13 | CS15 | CS17 | CS18 | CS20 | ||||
| Abiotic | Homo-aggregation | ||||||||||||||||
| Adhesion | Strongest adhesion capacity | ||||||||||||||||
| Biofilm formation | Strongest biofilm formation capacity | ||||||||||||||||
| SGJ tolerance | Strongest SGJ tolerance | ||||||||||||||||
| Caco-2 | Adhesion | Strongest adhesion capacity | |||||||||||||||
| maintain TEER | Enhance or damage TEER | ||||||||||||||||
| HT-29 | IL-8 production (NT) | ||||||||||||||||
| Decrease IL-8 production (LPS) | Strongest anti-inflammation properties | ||||||||||||||||
| PBMC | Pro-inf. production (NT) | Never increase more than LPS control | |||||||||||||||
| Anti-inf. production (NT) | Increase IL-10 significantly more than LPS control | ||||||||||||||||
| Decrease pro-inf. production (LPS) | |||||||||||||||||
| Increase anti-inf. production (LPS) | Increase IL-10 significantly more than LPS control | ||||||||||||||||
| NT: non-treated; LPS: LPS-stimulated cells | Positive effect or capacity | Negative effect or capacity | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamarande, J.; Cunat, L.; Pavlov, N.; Alauzet, C.; Cailliez-Grimal, C. Parabacteroides distasonis Properties Linked to the Selection of New Biotherapeutics. Nutrients 2022, 14, 4176. https://doi.org/10.3390/nu14194176
Chamarande J, Cunat L, Pavlov N, Alauzet C, Cailliez-Grimal C. Parabacteroides distasonis Properties Linked to the Selection of New Biotherapeutics. Nutrients. 2022; 14(19):4176. https://doi.org/10.3390/nu14194176
Chicago/Turabian StyleChamarande, Jordan, Lisiane Cunat, Nadine Pavlov, Corentine Alauzet, and Catherine Cailliez-Grimal. 2022. "Parabacteroides distasonis Properties Linked to the Selection of New Biotherapeutics" Nutrients 14, no. 19: 4176. https://doi.org/10.3390/nu14194176
APA StyleChamarande, J., Cunat, L., Pavlov, N., Alauzet, C., & Cailliez-Grimal, C. (2022). Parabacteroides distasonis Properties Linked to the Selection of New Biotherapeutics. Nutrients, 14(19), 4176. https://doi.org/10.3390/nu14194176





