Abstract
Cross-sectional studies have demonstrated an association between abdominal obesity, determined by waist circumference (WC), and vitamin D (25(OH)D) deficiency in older adults. However, longitudinal evidence is based only on general obesity determined using body mass index (BMI). We investigated whether abdominal obesity is associated with the incidence of 25(OH)D insufficiency (>30 and ≤50 nmol/L) and deficiency (≤30 nmol/L), and whether vitamin D supplementation modifies these associations. We included 2459 participants aged ≥50 years from the English Longitudinal Study of Ageing (ELSA) with 25(OH)D sufficiency (>50 nmol/L) at baseline. Abdominal obesity was defined as >88 cm for women and >102 cm for men. After 4 years, 25(OH)D concentrations were reassessed. Multinomial logistic regression models controlled by covariates were performed. Abdominal obesity increased the risk of the incidence of 25(OH)D insufficiency (RRR = 1.36; 95% CI: 1.01–1.83) and deficiency (RRR = 1.64; 95% CI: 1.05–2.58). These risks were maintained when excluding individuals who took vitamin D supplementation (RRR = 1.38; 95% CI: 1.02–1.88) and (RRR = 1.62; 95% CI: 1.02–2.56). Abdominal obesity is associated with the risk of incidence of low 25(OH)D concentrations. WC seems to be an adequate tool for screening individuals with obesity and at potential risk of developing these conditions.
1. Introduction
Aging reduces the cutaneous synthesis of vitamin D, a prohormone that plays an essential role in endocrine-metabolic responses. Vitamin D deficiency affects about 75% of the worldwide older population [1]. Although a consensus to classify serum 25-hydroxyvitamin D (25(OH)D) concentrations has not yet been established, the Institute of Medicine (IOM) defines serum concentrations of 25(OH)D < 30 nmol/L as deficient; 30 to 50 nmol/L as insufficient; and >50 nmol/L as sufficient, since such values are capable of supplying the needs of 97.5% of the North American population [2].
In addition to aging, the black race, lower education, smoking, physical inactivity, periods of low sun incidence (autumn and winter), living alone, and a lower consumption of important sources of vitamin D (salmon, oily fish, cod liver oil, and mushrooms) [3,4,5,6] are associated with serum 25(OH)D deficiency. Moreover, 25(OH)D can become trapped in adipose tissue due to the strong expression of vitamin D receptors in this tissue, leading to a significant reduction in the bioavailability of the prohormone [7,8,9,10]. Thus, obesity, especially when restricted to the abdominal region, which is common during the aging process, is another determinant factor of inadequate serum 25(OH)D concentrations in older adults [3,4,5,11,12].
Cross-sectional studies report associations between higher body mass index (BMI), waist circumference (WC), skin folds, and lower serum 25(OH)D concentrations [7] in 453 individuals aged ≥65 years, as well as an association between abdominal obesity (WC ≥ 90 cm for men and ≥80 cm for women) and serum 25(OH)D deficiency (<50 nmol/L) in individuals aged between 20–70 years [13]. However, the few longitudinal studies on this topic do not consider abdominal obesity and present discrepant results. Ding and collaborators [8] found that an increase in BMI, the percentage of trunk fat, and the waist-to-hip ratio (WHR) were associated with the risk of serum 25(OH)D deficiency (<50 nmol/L) in older people in 2.6 years of follow-up. In contrast, González-Molero and collaborators [14] found no association between obesity (BMI ≥ 30 kg/m2) and the risk of serum 25(OH)D deficiency (<50 nmol/L) in individuals aged 18–77 years in a six-year follow-up period.
Given the possibility of coexistence of a normal BMI and visceral fat accumulation [15], WC stands out as a more adequate measure for investigating the association between abdominal obesity and the incidence of low concentrations of 25(OH)D in older adults. However, this analysis has not been explored longitudinally. Moreover, vitamin D supplementation could increase 25(OH)D concentrations in individuals with obesity, leading to an underestimation of such associations. Therefore, we have tested the following hypotheses in the present study: (i) abdominal obesity is associated with the risk of 25(OH)D insufficiency and deficiency later in life, and (ii) vitamin D supplementation modifies the effect of these associations.
2. Materials and Methods
2.1. Study Population
The English Longitudinal Study of Ageing (ELSA) is a panel study conducted in England with a representative sample of community-dwelling individuals aged ≥50 years [16]. The study commenced in 2022. The interviews were conducted biannually and the health examination, i.e., nurse visit, occurs every four years for the collection of blood samples and anthropometric data, as well as the application of performance tests.
The baseline for the present study was wave 6 of the ELSA Study (2012–2013), as it was from this period that serum 25(OH)D concentrations began to be collected. Wave 6 was composed of 9169 individuals, 5870 of whom had valid 25(OH)D data. A total of 3339 individuals were excluded due to serum 25(OH)D insufficiency or deficiency at baseline. Thirty-four others were excluded due to a lack of information on abdominal obesity, and 38 were excluded due to a lack of information on covariates. Therefore, the final analytical sample of the present investigation was 2459 individuals. The sample selection process is shown in Figure 1.
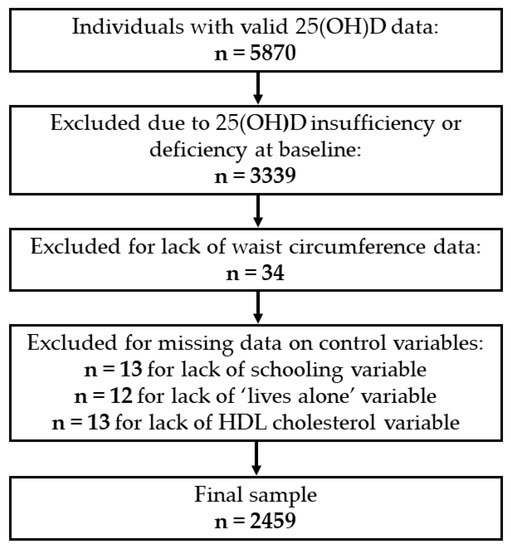
Figure 1.
Selection of individuals at baseline (2012–2013). 25(OH)D, 25-hydroxyvitamin D.
2.2. Vitamin D Assessment
Vitamin D was assessed based on serum 25(OH)D concentrations collected by a nurse during the four-year visits. The samples were analyzed at the Royal Victoria Infirmary (United Kingdom). The analyses were performed in duplicate using the DiaSorin Liaison immunoassay, which has an analytical sensitivity of 7.5 nmol/L with a variation coefficient of 8.7% to 9.4%. The lab that performed the analyses is part of the Vitamin D External Quality Assessment Schemes (DEQAS). Serum 25(OH)D concentrations were stratified according to IOM cutoff points: sufficiency (>50 nmol/L), insufficiency (>30 to ≤50 nmol/L), and deficiency (≤30 nmol/L) [2].
2.3. Abdominal Obesity
Abdominal obesity was evaluated based on WC measured at baseline using a non-elastic metric tape positioned at the midpoint between the last rib and upper margin of the iliac crest. The measurement was taken twice at the end of the expiration phase of the respiratory cycle. If the measurements differed by more than 3 cm, a third was taken. The mean of the two valid measurements or the two closest if three were taken was used for the analysis. Abdominal obesity was determined as WC > 88 cm for women and >102 cm for men [17].
2.4. Covariates
In line with previous studies involving the factors associated with serum 25(OH)D deficiency and obesity [3,4,8], the following covariates were collected at the baseline and incorporated into the study.
The sociodemographic characteristics were gender, age (50–59, 60–69, 70–79, and ≥80 years), race (white or non-white), total household wealth (including financial, housing and physical wealth, such as jewelry and artwork, which were categorized in quintiles), living alone (yes or no) and schooling years, based on the English three-way education system (>13 years; 12–13 years; ≤11 years).
The lifestyle habits considered were smoking status (non-smokers, ex-smokers, or smokers), frequency of alcohol intake (never/rarely, often, daily, or did not answer), and practice of physical activity. Physical activity was assessed using a validated instrument used in the Health Survey for England (HSE), which is based on frequency (“more than once a week”, “once a week”, “one to three times a month” or “almost never”) and intensity of the activity performed (vigorous, moderate, or light) [18]. The combination of this information allowed the classification of individuals in terms of lifestyle as active (light, moderate, or vigorous activity at least once a week) or sedentary (no weekly physical activity) [19].
Clinical conditions were recorded based on self-reports of a medical diagnosis of cancer, lung disease, heart disease, stroke, osteoporosis, osteoarthritis, systemic arterial hypertension, and diabetes. The presence of depressive symptoms was considered when the score on the shortened version of The Center for Epidemiologic Studies—Depression Scale was ≥ 4 (CES-D) [20].
BMI was calculated dividing weight in kilograms by height in meters squared (kg/m2) and classified as underweight (<18.5 kg/m2), normal weight (≥18.5 and <25 kg/m2), overweight (≥25 and <30 kg/m2) and obesity (≥30 kg/m2) [21].
The biochemical measures were triglycerides (high when ≥150 mg/dL) [22], total cholesterol (high when ≥200 mg/dL), LDL cholesterol (high when ≥100 mg/dL) and HDL cholesterol (low when <40 mg/dL for men and <50 mg/dL for women) [23]. As abdominal obesity is associated with an increase in systemic inflammation, C-reactive protein was considered high when ≥3 mg/L [22].
The season of the year in which the blood samples were collected was another control variable considered and was categorized as spring (March to May), summer (June to August), autumn (September to November), or winter (December to February). The use of vitamin D supplementation and carbamazepine was also considered, the latter of which is an antiseizure agent with the potential to reduce serum 25(OH)D levels [24].
2.5. Statistical Analyses
Descriptive statistics were used for the characteristics of the individuals at baseline according to the absence/presence of abdominal obesity. Continuous quantitative variables were expressed as mean and standard deviation values, and qualitative variables were expressed as proportions. Comparisons between individuals with and without abdominal obesity were performed using the chi-square test and Student’s t-test. A p-value < 0.05 was considered indicative of statistical significance.
To analyze whether abdominal obesity was associated with the risk of incidence of serum 25(OH)D insufficiency and deficiency, multinomial logistic regression models were performed and controlled by sociodemographic, behavioral, clinical, and biochemical characteristics. For such, univariate analyses were first performed with the control variables using the stepwise forward method, and variables with a p-value ≤ 0.20 were incorporated into the multivariate models. Those with a p-value < 0.05 after the adjustments remained in the final model and were considered significantly associated with the outcome [25].
As individuals who received vitamin D supplementation could be a source of confusion, a sensitivity model was performed excluding such individuals. In all analyses, “individuals without abdominal obesity” and “vitamin D sufficiency” were the reference categories for the comparisons. All analyses were conducted in Stata 16® program (StataCorp, College Station, TX, USA).
3. Results
Among 2459 individuals with serum 25(OH)D sufficiency at baseline, the mean age was 66 years (SD ± 8.5), and the majority were women (53.9%), white, had low schooling, were ex-smokers, had frequent alcohol intake, and were physically active. The most prevalent health conditions were osteoarthritis, hypertension, and heart disease. High frequencies of hypercholesterolemia and high serum levels of LDL cholesterol were also found. A total of 10.3% had a medical diagnosis of osteoporosis and only 4.5% received vitamin D supplementation at baseline (Table 1 and Table 2).

Table 1.
Sociodemographic characteristics of 2459 individuals at baseline, ELSA Study (2012).

Table 2.
Lifestyle habits, clinical conditions, and biochemical measures of 2459 individuals at baseline, ELSA Study (2012).
The individuals with abdominal obesity at baseline represented 43.6% of the sample and were older, predominantly women, had lower schooling, had lower income, and were more likely to report rarely consuming alcohol compared to those without abdominal obesity. Individuals with abdominal obesity had higher frequencies of hypertension, osteoarthritis, diabetes, high C-reactive protein, low HDL cholesterol, and hypertriglyceridemia, and lower frequencies of high total cholesterol and LDL cholesterol than those without abdominal obesity (p < 0.05). In addition, according to BMI, the individuals with abdominal obesity presented a higher prevalence of obesity (Table 1 and Table 2).
In the fully adjusted multinomial logistic regression model, abdominal obesity increased the risk of serum 25(OH)D insufficiency by 36% (RRR = 1.36; 95% CI: 1.01 to 1.83, p = 0.043) and the risk of serum 25(OH)D deficiency by 64% (RRR = 1.64; 95% CI: 1.05 to 2.58, p = 0.031) (Table 3).

Table 3.
Final adjusted model for incidence of serum 25(OH)D insufficiency and deficiency during 4-year follow-up according to abdominal obesity status, ELSA Study (2012–2016).
Even with the exclusion of individuals who received vitamin D supplementation in the sensitivity analyses, the results were confirmed, where abdominal obesity remained a risk factor for serum 25(OH)D insufficiency (RRR = 1.38; 95% CI: 1.02 to 1.88, p = 0.037) and deficiency (RRR = 1.62; 95% CI: 1.02 to 2.56, p = 0.040) (Table 4).

Table 4.
Final adjusted model for incidence of serum 25(OH)D insufficiency and deficiency during 4-year follow-up according to abdominal obesity status in individuals without use of vitamin D supplementation, ELSA Study (2012–2016).
4. Discussion
The findings from this large representative sample of English older adults showed that abdominal obesity was associated with the incidence of serum 25(OH)D insufficiency and deficiency after a four-year follow-up period. Moreover, considering those who did not use vitamin D supplementation, the risk of 25(OH)D insufficiency and deficiency among individuals with abdominal obesity was maintained.
According to previous cross-sectional studies [3,4,7,8,12,14,26,27], the higher adiposity demonstrated by both WC and BMI is associated with lower serum 25(OH)D concentrations in older adults. Analyzing 5356 older Irish adults, Laird and collaborators [3] found that obesity (BMI ≥ 30 kg/m2) was associated with serum 25(OH)D concentrations < 50 nmol/L. Snjider and collaborators [7] found inverse associations between WC and BMI with serum 25(OH)D concentrations in a sample composed of 453 older adults in The Netherlands. Analyzing a sample of Chinese individuals between 20–70 years of age, Zhang and collaborators [13] found that abdominal obesity (WC ≥ 90 cm for men and ≥80 cm for women) was associated with a greater likelihood of 25(OH)D deficiency (<50 nmol/L) in men and premenopausal women.
In contrast, longitudinal studies offer conflicting results. Ding and collaborators [8] found that increases in the BMI, percentage of trunk fat, and WHR were associated with a greater risk of serum 25(OH)D deficiency (<50 nmol/L) in 859 older adults in Tasmania over a 2.6-year follow-up period. In a study conducted in Spain, however, González-Molero and collaborators [14] found no association between obesity (BMI ≥ 30 kg/m2) and a greater risk of serum 25(OH)D deficiency (<50 nmol/L) in 1226 individuals between 18–77 years of age over a six-year follow-up period.
In addition to presenting conflicting results, the longitudinal studies measured obesity by the BMI and WHR, which do not reflect the distribution and accumulation of fat in older adults as well as WC. BMI is not sensitive to changes in the body fat redistribution pattern throughout the aging process. Although better than BMI, the WHR is not sensitive to differences in the distribution of adipose tissue, as the accumulation of fat in women older than 50 years of age is no longer restricted to the hips and thighs, occurring also in the abdominal region. Therefore, the WC seems to be the preferable measure, as it enables an evaluation of adiposity compatible with the obesity profile in both sexes and is a better predictor of visceral adipose tissue compared to BMI and WHR with the advance in age [28,29,30,31]. Therefore, to the best of our knowledge, the present study is the first to identify that abdominal obesity measured by WC is associated with the risk of the incidence of both serum 25(OH)D insufficiency and deficiency in a four-year follow-up period among individuals aged 50 or older.
The present results are possibly justified by the expression of vitamin D receptors in adipocytes, which makes adipose tissue a kind of vitamin D reservoir that sequesters 25(OH)D circulating in the organism, leading to a reduction in its bioavailability and contributing to a greater risk of the development of serum 25(OH)D insufficiency and deficiency in individuals with obesity [11,12,13,14]. Another possible mechanism is the lower conversion of cholecalciferol into 25(OH)D in the liver of individuals with obesity compared to those without obesity. As obesity is associated with non-alcoholic fatty liver disease, the liver in these individuals may have an impaired capacity to metabolize 25(OH)D, interfering in its distribution to the kidneys, where the conversion of 25(OH)D to the active form of vitamin D (1,25(OH)2D) takes place [14,32].
The present findings also demonstrate stability in the effect of abdominal obesity as a risk factor for the incidence of serum 25(OH)D insufficiency and deficiency following the exclusion of individuals who took vitamin D supplementation. Studies have shown that vitamin D supplementation does not seem to have a significant effect on the treatment of obesity and its complications [33,34], which may support the finding of our sensitivity analysis, where the exclusion of individuals who took vitamin D supplementation does not increase the strength of the association between obesity and the risk of incidence of 25(OH)D insufficiency and deficiency.
The present study has strengths and limitations that need to be acknowledged. The strengths were the use of a large representative sample of community-dwelling English individuals aged 50 years or older. Moreover, a wide range of information was included on sociodemographic, behavioral, and clinical characteristics, which enabled rigorous control in the multinomial logistic regression models. The reasonably long follow-up period is another strong point of this study.
Among the limitations, there is the impossibility of extrapolating the results to institutionalized individuals. The losses during follow-up constitute another limitation, but this is an unavoidable occurrence in longitudinal studies. Finally, despite WC not reflecting visceral adipose tissue as directly as more sophisticated methods, such as X-ray absorptiometry (DEXA), computed tomography (CT), and bioelectrical impedance analysis (BIA) [13,35], it is an easy, low-cost measure and an important predictor of metabolic risk [21,36,37,38], and did not impede us from finding the expected associations.
5. Conclusions
In conclusion, abdominal obesity was associated with the risk of incidence of serum 25(OH)D insufficiency and deficiency in English older adults, and the effect of this association appears to be confirmed by excluding individuals who were users of vitamin D supplementation. Our findings highlight the importance of early adoption of obesity screening strategies, to avoid its complications, as well as identify those at potential risk of developing 25(OH)D deficiency, enabling adequate treatment for both conditions. Further investigation is still needed to better elucidate the mechanisms involved in the association between abdominal obesity and vitamin D deficiency. It is also important to know how the trajectories of decline in serum concentrations of 25(OH)D occur in individuals with and without obesity.
Author Contributions
Conceptualization, T.B.P.d.S. and T.d.S.A.; methodology, T.B.P.d.S., M.M.L. and T.d.S.A.; validation, T.B.P.d.S., M.M.L. and T.d.S.A.; formal analysis, T.B.P.d.S. and T.d.S.A.; investigation, T.B.P.d.S., M.M.L. and T.d.S.A.; resources, A.S., C.d.O. and T.d.S.A.; data curation, T.B.P.d.S., M.M.L., M.L.B.D. and T.d.S.A.; writing—original draft preparation, T.B.P.d.S., M.M.L., M.L.B.D. and T.d.S.A.; writing—review and editing, T.B.P.d.S., M.M.L., M.L.B.D., A.S., C.d.O. and T.d.S.A.; visualization, T.B.P.d.S., M.M.L. and T.d.S.A.; supervision, T.d.S.A.; project administration, T.d.S.A.; funding acquisition, C.d.O. and T.d.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Economic and Social Research Council, grant number ES/T008822/11. ELSA is supported by the National Institute on Aging USA, grant number R01AG017644. This work was supported by the Sao Paulo Research Foundation—FAPESP, grant number 2020/06716-1 to T.B.P.d.S. and grant number 2018/13917-3 to T.d.S.A.; the National Council of Scientific and Technological Development—CNPq, grant numbers 303981/2017-2 and 303577/2020-7 to T.d.S.A.; and the Coordination for the Improvement of Higher Education Personnel—Institutional Internationalization Program—CAPES-PrInt, grant number 88887.570076/2020-00 to T.d.S.A. The funders had no involvement in the manuscript.
Institutional Review Board Statement
The ELSA Study was conducted in accordance with the Declaration of Helsinki and approved by The National Research Ethics Service (London Multicenter Research Ethics Committee [MREC 01/2/91]).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The English Longitudinal Study of Ageing data are available to the scientific community from the UK Data Service for researchers who meet the criteria for access to confidential data, under conditions of the End User License http://ukdataservice.ac.uk/media/455131/cd137-enduserlicence.pdf (accessed on 6 October 2022). The data can be accessed from: https://beta.ukdataservice.ac.uk/datacatalogue/series/series?id=200011#!/access-data (accessed on 6 October 2022). Contact with the UK Data Service regarding access to the English Longitudinal Study of Ageing can be made through the website https://www.ukdataservice.ac.uk/about-us/contact (accessed on 6 October 2022), by phone +44-(0)1206-872143, or by email at help@ukdataservice.ac.uk.
Acknowledgments
The authors are grateful to the participants of the ELSA Study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; O’Halloran, A.M.; Carey, D.; Healy, M.; O’Connor, D.; Moore, P.; Shannon, T.; Molloy, A.M.; Kenny, R.A. The Prevalence of Vitamin D Deficiency and the Determinants of 25(OH)D Concentration in Older Irish Adults: Data from The Irish Longitudinal Study on Ageing (TILDA). J. Gerontol. A Biol. Sci. Med. 2018, 73, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Aspell, N.; Laird, E.; Healy, M.; Shannon, T.; Lawlor, B.; O’Sullivan, M. The Prevalence and Determinants of Vitamin D Status in Community-Dwelling Older Adults: Results from the English Longitudinal Study of Ageing (ELSA). Nutrients 2019, 11, 1253. [Google Scholar] [CrossRef]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Deeg, D.J.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef]
- Ding, C.; Parameswaran, V.; Blizzard, L.; Burgess, J.; Jones, G. Not a simple fat-soluble vitamin: Changes in serum 25-(OH)D levels are predicted by adiposity and adipocytokines in older adults. J. Intern. Med. 2010, 268, 501–510. [Google Scholar] [CrossRef]
- Sousa-Santos, A.R.; Afonso, C.; Santos, A.; Borges, N.; Moreira, P.; Padrão, P.; Fonseca, I.; Amaral, T.F. The association between 25(OH)D levels, frailty status and obesity indices in older adults. PLoS ONE 2018, 13, e0198650. [Google Scholar] [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef]
- Brock, K.; Huang, W.Y.; Fraser, D.R.; Ke, L.; Tseng, M.; Stolzenberg-Solomon, R.; Peters, U.; Ahn, J.; Purdue, M.; Mason, R.S.; et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J. Steroid Biochem. Mol. Biol. 2010, 121, 462–466. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, K.; Beirne, A.; Casey, M.; McNulty, H.; Ward, M.; Hoey, L.; Molloy, A.; Laird, E.; Healy, M.; Strain, J.J.; et al. Determinants of 25-hydroxyvitamin D in older Irish adults. Age Ageing 2015, 44, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, P.; Zhu, Y.; Chang, H.; Wang, X.; Liu, W.; Zhang, Y.; Huang, G. Higher visceral fat area increases the risk of vitamin D insufficiency and deficiency in Chinese adults. Nutr. Metab. 2015, 12, 50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González-Molero, I.; Rojo-Martínez, G.; Morcillo, S.; Gutierrez, C.; Rubio, E.; Pérez-Valero, V.; Esteva, I.; Ruiz de Adana, M.S.; Almaraz, M.C.; Colomo, N.; et al. Hypovitaminosis D and incidence of obesity: A prospective study. Eur. J. Clin. Nutr. 2013, 67, 680–682. [Google Scholar] [CrossRef]
- Bosello, O.; Vanzo, A. Obesity paradox and aging. Eat. Weight Disord. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Mindell, J.; Biddulph, J.P.; Hirani, V.; Stamatakis, E.; Craig, R.; Nunn, S.; Shelton, N. Cohort profile: The health survey for England. Int. J. Epidemiol. 2012, 41, 1585–1593. [Google Scholar] [CrossRef]
- National Heart, Lung, Blood Institute; National Institute of Diabetes, & Kidney Diseases (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report; No. 98; National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1998. [Google Scholar] [PubMed]
- Joint Health Surveys Unit, National Centre Social Research and University College London Research Department of Epidemiology and Public Health. The Health Survey for England Physical Activity Validation Study: Substantive Report; NHS Information Centre for Health and Social Care: Leeds, UK, 2008. [Google Scholar]
- Luiz, M.M.; Máximo, R.; Oliveira, D.C.; Ramírez, P.C.; de Souza, A.F.; Delinocente, M.L.B.; Steptoe, A.; de Oliveira, C.; Alexandre, T. Association of Serum 25-Hydroxyvitamin D Deficiency with Risk of Incidence of Disability in Basic Activities of Daily Living in Adults > 50 Years of Age. J. Nutr. 2020, 150, 2977–2984. [Google Scholar] [CrossRef]
- Steffick, D.E. Documentation of Affective Functioning Measures in the Health and Retirement Study; HRS Documentation Report DR-005; Survey Research Center at the Institute for Social Research: Ann Arbor, MI, USA, 2000. [Google Scholar]
- World Health Organization (Ed.) Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-120894-9. [Google Scholar]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Fedder, D.O.; Koro, C.E.; L’Italien, G.J. New National Cholesterol Education Program III Guidelines for Primary Prevention Lipid-Lowering Drug Therapy. Circulation 2002, 105, 152–156. [Google Scholar] [CrossRef]
- LoPinto-Khoury, C.; Brennan, L.; Mintzer, S. Impact of carbamazepine on vitamin D levels: A meta-analysis. Epilepsy Res. 2021, 178, 106829. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, G.; Greenland, S. Simulation Study of Confounder-Selection Strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Bani-issa, W.; Eldeirawi, K.; Harfil, S.; Fakhry, R. Vitamin D Deficiency and Its Determinants in Adults: A Sample from Community-Based Settings in the United Arab Emirates. Int. J. Endocrinol. 2017, 2017, 3906306. [Google Scholar] [CrossRef] [PubMed]
- Stokić, E.; Kupusinac, A.; Tomić-Naglić, D.; Zavišić, B.K.; Mitrović, M.; Smiljenić, D.; Soskić, S.; Isenović, E. Obesity and vitamin D deficiency: Trends to promote a more proatherogenic cardiometabolic risk profile. Angiology 2015, 66, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Sainsbury, A.; Stock Conference 2008 Working Group. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 2009, 10, 154–167. [Google Scholar] [CrossRef]
- Price, G.M.; Uauy, R.; Breeze, E.; Bulpitt, C.J.; Fletcher, A.E. Weight, shape, and mortality risk in older persons: Elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am. J. Clin. Nutr. 2006, 84, 449–460. [Google Scholar] [CrossRef]
- de Carvalho, D.H.T.; Scholes, S.; Santos, J.L.F.; de Oliveira, C.; Alexandre, T.S. Does Abdominal Obesity Accelerate Muscle Strength Decline in Older Adults? Evidence from the English Longitudinal Study of Ageing. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1105–1111. [Google Scholar] [CrossRef]
- Swainson, M.G.; Batterham, A.M.; Tsakirides, C.; Rutherford, Z.H.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef]
- Wamberg, L.; Pedersen, S.B.; Rejnmark, L.; Richelsen, B. Causes of vitamin D deficiency and effect of vitamin D supplementation on metabolic complications in obesity: A review. Curr. Obes. Rep. 2015, 4, 429–440. [Google Scholar] [CrossRef]
- Jamka, M.; Woźniewicz, M.; Walkowiak, J.; Bogdański, P.; Jeszka, J.; Stelmach-Mardas, M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: A systematic review with meta-analysis. Eur. J. Nutr. 2016, 55, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Grundy, S.M.; Li, X.; Adams-Huet, B.; Vega, G.L. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: The Dallas Heart Study. Nutr. Diabetes 2016, 6, e221. [Google Scholar] [CrossRef]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ponnalagu, S.D.; Bi, X.; Henry, C.J. Is waist circumference more strongly associated with metabolic risk factors than waist-to-height ratio in Asians? Nutrition 2019, 60, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Osayande, O.E.; Azekhumen, G.N.; Obuzor, E.O. A comparative study of different body fat measuring instruments. Niger. J. Physiol. Sci. 2018, 33, 125–128. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).