1. Introduction
Osteoarthritis (OA) is the most prevalent chronic joint disease, increases in prevalence with age [
1], and affects most individuals over 65 [
2,
3]; it is a disease of the entire joint, involving the cartilage, joint lining, ligaments, and bone. OA is characterized by the breakdown of the cartilage, bony changes of the joints, deterioration of tendons and ligaments, and various degrees of inflammation of the joint lining [
4].
OA tends to affect commonly used joints, such as the hands and spine, and the weight-bearing joints, such as the hips and knees. OA symptoms include joint pain and stiffness [
5,
6].
OA multi-factorial etiology includes oxidative stress and the overproduction of reactive oxygen species (ROS), which regulate intracellular-signaling processes, chondrocyte senescence and apoptosis, extracellular matrix synthesis and degradation, and synovial inflammation and dysfunction of the subchondral bone [
7].
The term oxidative stress represents the inbalance between the cells’ ROS and the cells’ antioxidant capacity. High levels of oxidative stress may damage the cells by oxidizing lipids and altering the DNA and protein structure [
8].
ROS are free radicals containing oxygen molecules, including hydroxyl radical (OH
−), hydrogen peroxide (H
2O
2), superoxide anion (O
2−), nitric oxide (NO), and hypochlorite ion (OCl
−). The presence of unpaired electrons in the valence shell causes ROS to be short-lived, unstable, and highly reactive to achieve stability [
4]. The major sites of ROS generation include the mitochondria (through oxidative phosphorylation), non-mitochondrial membrane-bound nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and xanthine oxidase (XO). The body has multiple mechanisms for scavenging ROS; the antioxidant system includes enzymatic and non-enzymatic antioxidants, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione (GSH), NADPH ubiquinone oxidoreductase (NQO1), paraoxonases (PON), and natural antioxidants such as ascorbic acid (vitamin C), α-tocopherol (vitamin E), and carotenoids [
9,
10].
Obesity is linked with OA through (1) mechanical stress and (2) the adipokine system. Recent epidemiological data showed an increased risk of hand osteoarthritis in obese patients, underlying the role of the systemic inflammatory system [
11] and adipokines, released by adipose tissue [
12,
13].
Environmental factors influencing the prevalence of OA are modifiable risk factors and include occupation, diet, level of physical activity, and obesity [
14,
15].
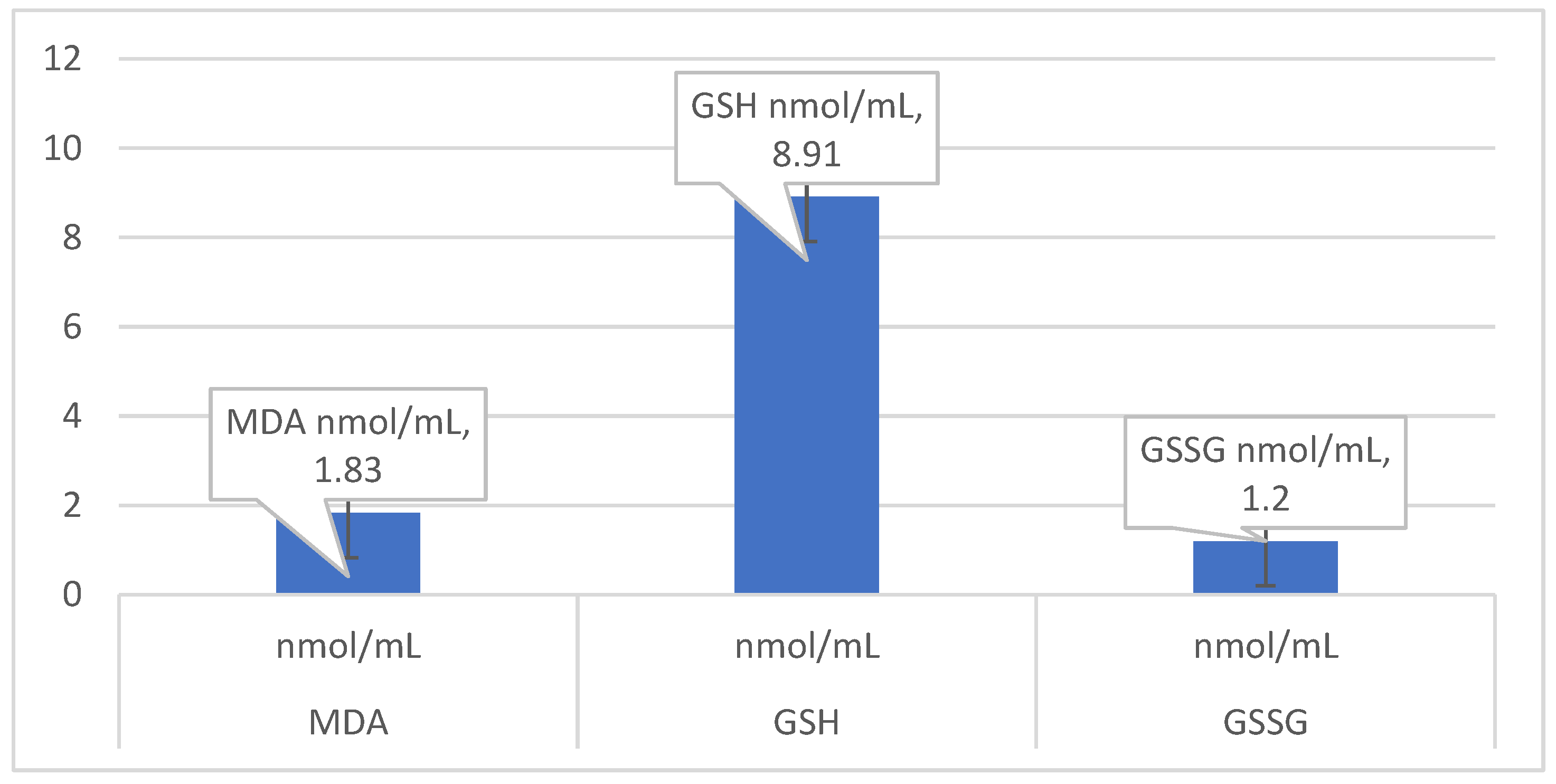
In our study, we chose to determine the following oxidative status markers: malonyl-dialdehyde (MDA); glutathione, and the ratio of GSH/GSSG. MDA is the primary marker of lipid peroxidation. The prime targets of ROS attack are the polyunsaturated fatty acids in the membrane lipids causing lipid peroxidation (LPO), which may lead to disorganization of the cell structure and function. Further decomposition of peroxidized lipids yields various end-products, including malondialdehyde (MDA) [
16]. The measurement of MDA is widely used as an indicator of LPO [
17].
Glutathione is an antioxidant produced in cells, with a structure of tripeptide (cysteine, glycine, and glutamic acid) existing either in a reduced (GSH) or oxidized (GSSG) form [
18]. It is produced by the liver and is involved in many processes. Experimental studies on rats showed that mechanical stress promotes the accumulation of reactive oxygen species (ROS) in chondrocytes in vivo, resulting in chondrocyte apoptosis and leading to osteoarthritis development in a rat model. Osteoarthritis development was inhibited by oral administration of N-acetyl cysteine (NAC), and it was demonstrated to have efficacy in reducing cartilage degradation and inflammation markers as well as significant improvements in pain and functional outcomes [
19,
20].
Few studies in the literature on human subjects have investigated oxidative markers from blood samples; to our knowledge, most of the studies focused on preclinical models.
Less is known about the relationship between the oxidative status markers, diet and lifestyle in osteoarthritis patients. Our study aimed to assess the oxidative status (lipid peroxidation and antioxidant status) in relation to the diet and physical activity of patients with OA. To our knowledge, this is the first study in Romania that established a relationship between the oxidative status of the body reflected in the biochemical parameters, physical activity, and diet. Because diet and lifestyle are related to body mass index, the second objective of our study was to establish a relationship between weight status and oxidative markers in patients with OA.
2. Materials and Methods
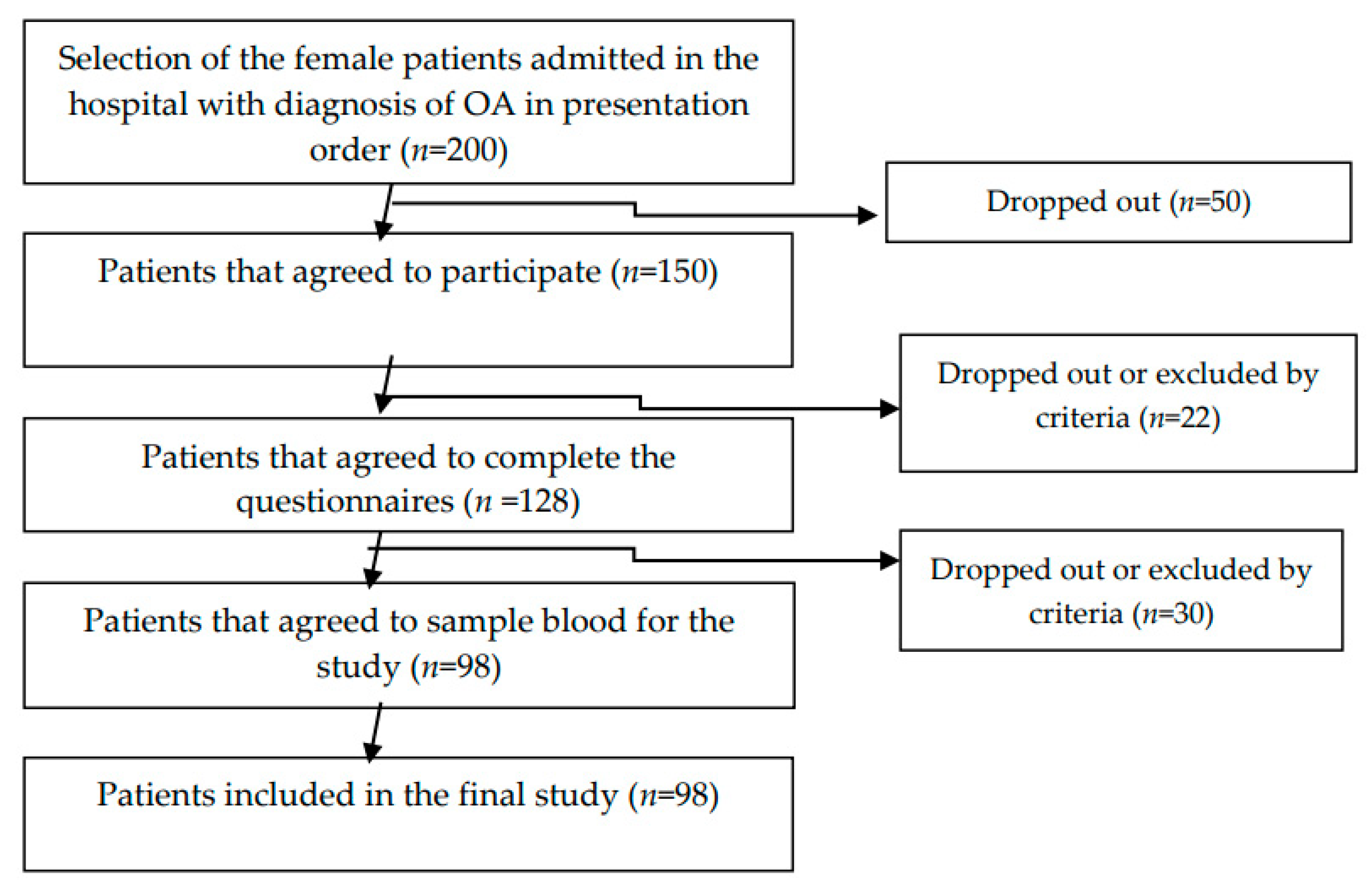
We used a cross-sectional study on 98 female osteoarthritis patients diagnosed according to the American College of Rheumatology (ACR) criteria for the classification of OA. The data were collected from July 2019 to December 2019. The exclusion criteria were: refusal of patients to participate in the study; patients with psychiatric disorders; VSH (sedimentation rate) > 30 mm/h, CRP > 1.5 (C-Reactive Protein); inflammatory arthritis; and crystal arthritis. The diagram flow of the sample selection is presented in
Figure 1.
The 5 mL peripheric blood samples were collected from patients after an overnight fast (10–12 h) in two ethylenediaminetetraacetic acid tubes. MDA is a stable product of the reaction between oxidative radicals and lipids [
3]. Free plasma MDA was determined using the spectrofluorimetric method described by Conti [
21]. This method is based on the reaction between MDA and thiobarbituric acid with fluorescent adduct synthesis proportionally to MDA concentration. A Perkin Elmer spectrofluorometer was used for emission intensity measurement at 534 nm in the synchronous fluorescence system at a 14 nm wavelength difference (Δλ) between excitation and emission. A calibration curve performed with known MDA concentrations was used. The concentration values were expressed in nmol/mL.
2.1. The GSH/GSSG Ratio Assessment
The GSH/GSSG ratio was calculated using the method proposed by Vats [
22]. A total of 50 mL of blood was treated with 450 mL of 10% solution of m-phosphoric acid and centrifuged for 10 min at 1000×
g. For the determination of GSH, 0.1 mL of the supernatant was diluted with 1.8 mL of 0.1 M phosphate buffer (pH 8) continuing 5 nmol/L EDTA after which 0.1 mL solution of o-phthalaldehyde in methanol (1 mg/mL) was added. After 15 min of incubation, the fluorescence emitted at 420 nm was measured with an excitation of 350 nm. The GSSG was estimated in 250 μL supernatant which was incubated for 30 min with 40 nmol/L N-ethylmaleimide. Then, 0.65 mL 0.1N NaOH was added, followed by adding 0.1 mL reaction mixture formed by 1.8 mL 0.1N NaOH and 0.1 mL o-phthalaldehyde solution.
The GSH and GSSG concentration calculation was based on calibration curves obtained with GSH and GSSG known concentrations and processed similarly. The GSH ratio was calculated.
2.2. Questionnaire and Variables
The research team applied a structured questionnaire to the study’s participants consisting of (1) a demographic section with a personal medical history and (2) a validated food frequency questionnaire. We used a food frequency questionnaire section to assess the dietary intake of vegetables and fruit. The food frequency questionnaire (FFQ) is part of a valid questionnaire adapted to Romanian habits (EPIC Norfolk). We tested and re-tested the questionnaire on a sample of 30 respondents and calculated Spearman’s correlation coefficient to assess the reliability (r = 0.766). The questionnaires were administered by interview. We calculated Kappa statistics to assess the inter-rater agreement; the kappa result was 0.8.
In the FFQ section, we assessed the food and beverages intake-habits as different frequencies: never or less than 1/month; 1–3 times/month; once/week; 2–4 times/week; 5–6 times/week; once/day; 2–3 times/day; 4–5 times/day; more than six times/day. A portion of fruit/vegetables was considered to be 80 g of a medium-size fruit, 1/2 cup of chopped/cooked vegetables, 30 g of dried fruit, or 150 mL of fruit or vegetable juice.
Anthropometric parameters weight and height were measured during the hospitalization. Body mass index (BMI) was calculated “as weight (kg) divided by the square of height (m2)”, and accordingly, obesity was defined from a BMI of >30 kg/m2. The nutritious status of the patients was divided as underweight BMI < 18.5 kg/m2; normoweight: BMI = 18.5–24.9 kg/m2; overweight: BMI = 25–29.9 kg/m2; obesity type 1 BMI = 30–34.9 kg/m2; obesity type 2 BMI = 35–39.9 kg/m2; and type 3 BMI > 40 kg/m2.
Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) [
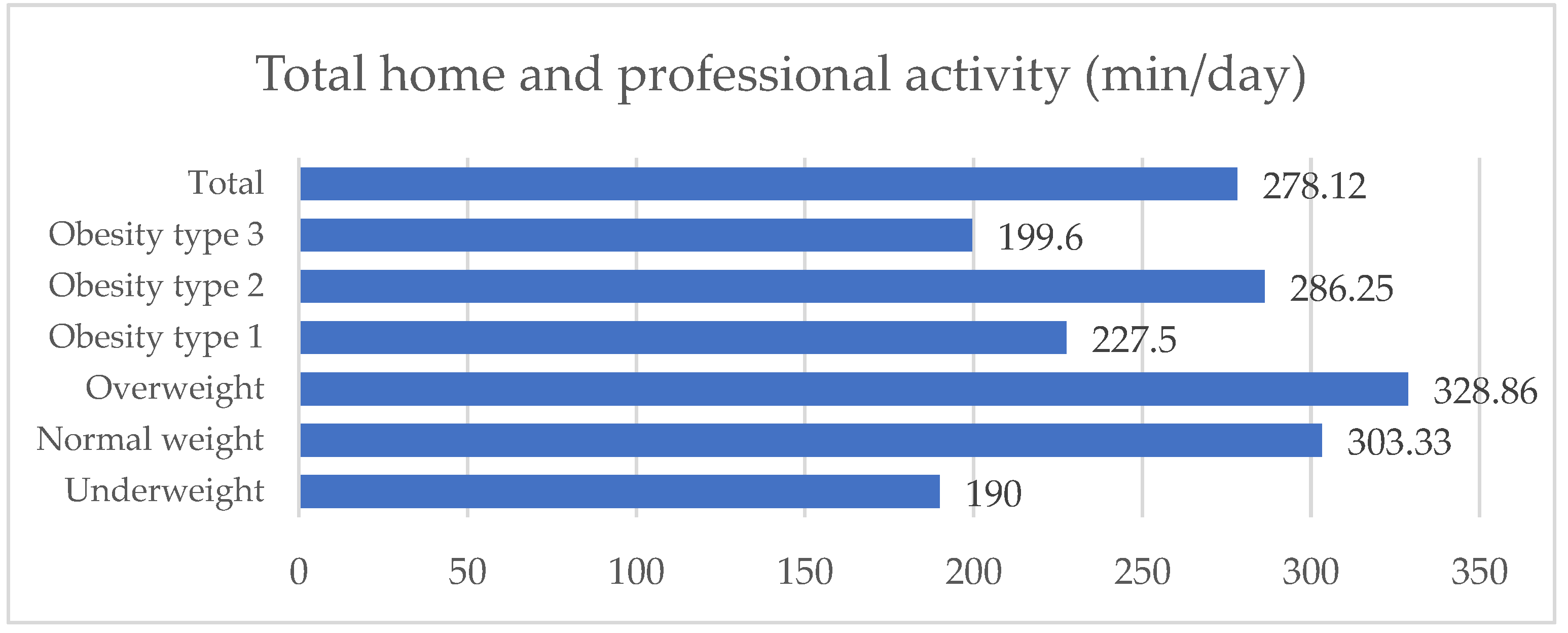
23] as duration, frequency, and intensity. Vigorous physical activities involve hard physical effort and render breathing much harder than usual. Moderate activities require moderate physical effort and render breathing harder than usual. We estimated the job-related physical activity, transportation, household physical activity, and leisure time. We calculated the total physical activity in minutes per day.
2.3. Statistical Analyses
The data were analyzed using the Statistical Package for the Social Sciences (SPSS), version 20. Descriptive statistics were calculated: means and standard deviations (SD) for continuous variables or frequencies and percentages for categorical variables. The sample size was calculated according to the Cochran formula, taking into account the prevalence of OA in the Romanian population (6.43% prevalence, the result was 92.45).
Multiple regression was run to predict the MDA, GSSH, and GSSG association with dietary vegetables and fruit intake.
4. Discussion
Our study aimed to assess the oxidative status (lipid peroxidation and antioxidant status) in relation to the diet and physical activity of patients with OA.
Obese individuals have shown markers indicative of oxidative stress: (1) elevated levels of reactive oxygen species and (2) diminished antioxidant defenses, which are associated with lower antioxidant enzymes [
24]. Our study showed that 89.9% of the investigated patients with OA were overweight or obese. The pathophysiology of the link between OA and obesity is related to the direct effect of excess mechanical loads being placed on the cartilage and an adipose tissue effect [
25]. Adipocytes produce and release adipokines (e.g., leptin). They are also a local inflammatory reaction site when the adipose tissue is ectopic. Each additional kilogram of body weight adds a six-kilogram load to each knee [
26,
27]. This excess weight can induce cartilage degeneration because of more significant mechanical stress on weight-bearing joints. Leptin is a cytokine produced by adipocytes in white adipose tissue (hence its name “adipokine”) [
28], which is released into the systemic circulation where it can reach the joints through the subchondral vascular network [
29,
30]. Chondrocytes have leptin receptors; the adipokines play an essential role in cartilage and bone homeostasis, but at overly high concentrations, they contribute to the appearance and progression of OA (destruction of cartilage) [
31]. A higher leptin concentration has been found in the synovial fluid of arthritic joints than that of non-arthritic joints [
32]. Visceral adipose tissue is significantly reduced with regular physical activity (following the health guidelines for 150 min of moderate physical activity per week), even if no weight is lost [
33]. Despite the existing literature data regarding the link between obesity, OA, and oxidative status, the present study cannot support these due to the small sample size, with a small percentage of normoweight women. Future studies, of larger sample sizes of Romanian patients with OA, are required to establish this relationship.
Low education, non-managerial occupation, and income level tend to predict pain, physical dysfunction, and disability among adults with osteoarthritis. These associations can be attributed to more significant strenuous physical activity among people with low social and economic status [
34]. Low education attainment and occupation are associated with low socioeconomic status (SES) and the development of other chronic illnesses. In the present study, 84.9% of the patients with osteoarthritis had primary or secondary education. Moreover, 52.1% were living in rural areas. The physical activity assessment of females with OA showed an active life with a level over the minimum recommendation for healthy adults. This level is attributed mainly to moderate home physical activity, evidenced by our study. Females with low education and the majority from the rural area may be involved in more physical activity (mainly at home, non-organized) than other females. People from rural areas are also more likely to be involved in physical activities such as gardening. There are no differences in the level of physical activity between the categories of obesity among patients with OA; PA could lead to a protective effect. The results also showed no differences in the levels of enzymes in patients with different degrees of obesity. Low socioeconomic status may influence their behaviors regarding seeking and access to medical care. In addition, these conditions may impact on health outcomes, quality of life, and other chronic conditions and mortality [
35].
Both aerobic and anaerobic activities possess the potential to result in increased ROS and RNS production and subsequent oxidative stress. While obesity has been shown to exacerbate the oxidative stress response, dietary manipulation and exercise training may serve as an effective intervention to ameliorate oxidative stress profiles. Whether exercise training improves oxidative stress and inflammatory profiles in the absence of weight loss remains unclear; however, strict calorie restriction alone or coupled with physical activity intervention demonstrates promise in alleviating oxidative stress in obese individuals when accompanied by weight reduction [
36,
37]. On the other hand, animal models showed that exercise modulates transcription in several metabolic pathways associated with extracellular matrix (ECM) biosynthesis and inflammation/immune responses in the normal cartilage of rats undergoing treadmill walking. Moreover, exercise was able to suppress the expression of genes involved in ECM degradation, bone formation, and initiation of pro-inflammatory cascades, which are known to be upregulated in OA (Mmp9, Mmp8, Igf1, ColIa1, Adamts3, Adamts14), highlighting a positive effect on cartilage preservation [
38].
Since our sample comprised mainly patients with obesity or overweight, we cannot draw clear conclusions regarding the relationship between the level of PA, obesity and oxidative status, despite other studies that showed that exercise improved the MDA oxidative stress marker [
39]. Exercise training improves the antioxidant enzyme activity with no telomere length changes [
40]. The results of the present study showed that the oxidative stress (oxidant and antioxidant balance) was not statistically different in patients with obesity and patients with different levels of physical activity.
Fruit and vegetables are sources of natural antioxidants such as vitamin C, vitamin E, carotenoids, and flavonoids. However, the protective effects against diseases may also be the result of unknown antioxidant compounds or the synergy of several different antioxidants in fruit and vegetables [
41,
42]. Fruit and vegetables are a source of glutathione and increase the erythrocytes’ glutathione peroxidase activity and resistance of plasma lipoproteins to oxidation [
43].
The results of our study showed a low intake of vegetables in the study group. The results are similar to other studies on different samples of the Romanian population, which showed that the vegetable intake is low compared with the recommendation [
44,
45]. On the other hand, fruit are consumed in higher amounts than vegetables.
Patients with OA may have a lower level of plasma antioxidant status due to the reduced intake of vegetables and fruit. The multivariate analyses showed no statistically significant relationship between vegetable and fruit intake and the body’s antioxidant status (GSH, GSSG, GSH/GSSG). A high-calorie diet also correlates with a need for a diet enriched with antioxidants.
Our study showed that only 11% of our patients were normal weight; the rest were overweight or obese. These results suggest that OA patients may have a high energy intake, taking into account the level of physical activity of the sample.
Increased consumption of vegetables and fruit would reduce oxidative stress and damage markers, but it is unknown whether these markers are related to dietary intake [
46,
47,
48].
ROS are known to be involved in biological events such as cell signaling, chondrocyte senescence, apoptosis, extracellular matrix synthesis and degradation, synovial inflammation, and dysfunction of subchondral bone. ROS have been recognized as signaling molecules that regulate a variety of physiological processes, for example, are required for cytokines, insulin, growth factor, and AP-1 and NF-kB signaling. The antioxidant property of GSH is mostly attributed to GSH peroxidase-catalyzed reactions, where hydrogen peroxide and lipid peroxide are reduced while GSH is oxidized to GSSG. GSSG is then reduced back to GSH by GSSG reductase via the utilization of nicotinamide adenin dinucleotide phosphate (NADPH) establishing the redox cycle. GSH largely determines the intracellular redox potential: the GSSG ratio and oxidative stress overcome the ability of the cell to reduce GSSG in GSH [
20].
A recent discovery showed that OA pathogenesis was linked with a form of regulated cell death named ferroptosis. Ferroptosis is characterized by the iron-dependent accumulation of lipid hydroperoxide that reaches lethal levels. Yao et al. [
49] first indicated that chondrocytes underwent ferroptosis under inflammatory and iron-overload conditions and that ferroptosis contributed to the progression of OA in vivo and promoted matrix metalloproteinase (MMP)-13 expression while inhibiting COL2 expression in chondrocytes cultured in vitro. Miao et al. [
50] found that iron accumulated in cartilage and synovial fluid during OA progression and that the expression of biomarkers of the peroxidation defense system, including glutathione peroxidase (GPX4) and glutathione (GSH) levels, was decreased in the patient samples. Moreover, as a characteristic change in ferroptosis, morphological changes in mitochondria have also been observed in OA cartilage by transmission electron microscopy, indicating that ferroptosis is closely associated with OA [
51].
The present study showed that MDA was significantly inversely associated with a low intake of vegetables and fruit consumption. Similar to our findings, studies from the literature showed that vegetables and fruit consumption enhanced antioxidant levels (SOD and GPX) and reduced oxidant levels (MDA) and genetic damage [
52,
53].
There are few studies in the literature on human data that investigated oxidative markers from blood samples [
10].
To the best of our knowledge, this is the first study in Romania to analyze the level of GSH and GSSG from blood samples of patients with OA. Unfortunately, our results cannot be compared to data on human models because the majority of the studies are preclinical. The ratio GSH:GSSG is a better marker of oxidative stress compared to antioxidant enzymes from blood samples that can be consumed in redox reaction [
54].
Our study showed the need for the embedding of public health policies in order to improve the lifestyle of patients with OA. Females must be educated to increase vegetable and fruit consumption. In addition, one must be educated to reduce caloric intake to prevent overweight and obesity. By having healthy food choices with an increased vegetable intake, patients with OA may reduce the caloric-dense food consumption. On the other hand, maintaining an active lifestyle will increase energy expenditure, reduce adipose tissue, and decrease adipokine. In addition, supplementation with GSH exogenously (as a precursor—N-acetyl-cysteine) by functional foods or supplements could be an opportunity to improve the antioxidant status in OA; however, further interventional studies are needed on this topic.
Limitations. Our study is subject to limitations. First is the size of the sample. Larger sample sizes would provide better information. However, according to statistical calculation, the sample represented the population. Another limitation of the study was the reported questionnaire—both diet and physical activity data were collected by interview. Bias can appear by overestimation or underestimation of different categories of food intake in the FFQ. Physical activity may also be overestimated; future objective methods may be used to estimate physical activity or diet better.