Remission of Acute Food Protein-Induced Enterocolitis Syndrome Confirmed by Oral Food Challenges in Japan
Abstract
1. Introduction
2. Methods
2.1. Study Design, Setting, and Participants
2.2. Variables and Data Source
2.3. Laboratory Tests
2.4. Skin Prick Test
2.5. Oral Food Challenge Protocol
2.6. Statistical Analysis
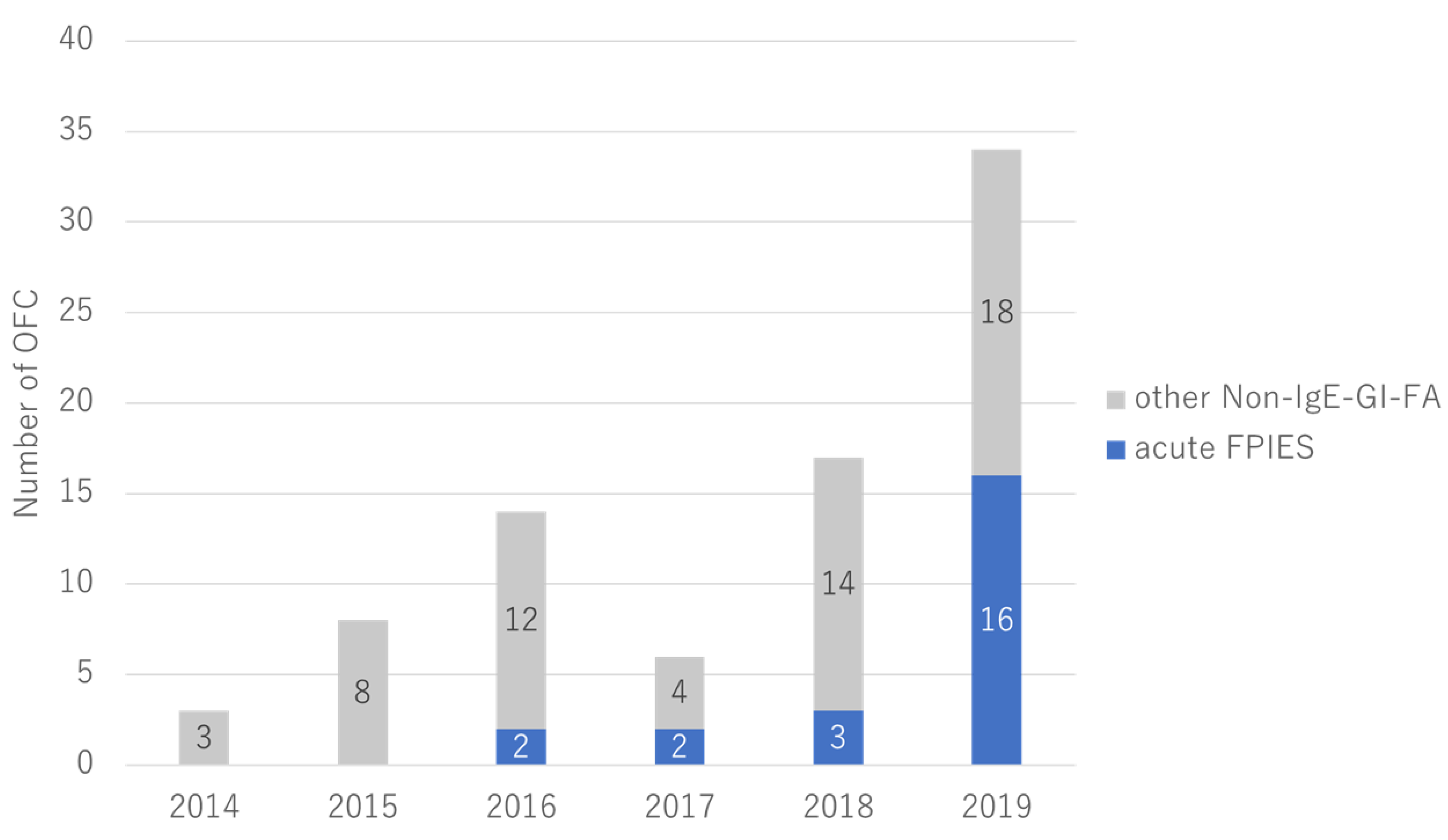
3. Results
3.1. Patients and Clinical Features
3.2. OFC Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar] [PubMed]
- Yamamoto-Hanada, K.; Pak, K.; Saito-Abe, M.; Yang, L.; Sato, M.; Irahara, M.; Mezawa, H.; Sasaki, H.; Nishizato, M.; Ishitsuka, K.; et al. Allergy and immunology in young children of Japan: The JECS cohort. World Allergy Organ. J. 2020, 13, 100479. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Hayashi, D.; Kajita, N.; Kinoshita, M.; Ishii, T.; Tsumura, Y.; Horimukai, K.; Yoshida, K.; Takahashi, T.; Morita, H.; et al. Recent dramatic increase in patients with food protein-induced enterocolitis syndrome (FPIES) provoked by hen’s egg in Japan. J. Allergy Clin. Immunol. Pract. 2022, 10, 1110–1112.e2. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Food protein-induced enterocolitis syndrome epidemiology. Ann. Allergy Asthma Immunol. 2021, 126, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Goldberg, M.R.; Rajuan, N.; Cohen, A.; Leshno, M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: A large-scale, prospective population-based study. J. Allergy Clin. Immunol. 2011, 127, 647–653.e1-3. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Pecora, V.; Fiocchi, A.G.; Nowak-Wegrzyn, A. Food protein-induced enterocolitis syndrome: A review of the new guidelines. World Allergy Organ J. 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Makita, E.; Sugawara, D.; Kuroda, S.; Itabashi, K.; Ichihashi, K. Usefulness of thymus and activation-regulated chemokine (TARC) for FPIES diagnosis. Pediatr. Allergy Immunol. 2022, 33, e13649. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, A.; Nowak-Wegrzyn, A. Food Protein-Induced Enterocolitis Syndrome: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2019, 57, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Giorgio, V.; Dello Iacono, I.; Novembre, E.; Mori, F.; Onesimo, R. A multicentre retrospective study of 66 Italian children with food protein-induced enterocolitis syndrome: Different management for different phenotypes. Clin. Exp. Allergy 2012, 42, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Xepapadaki, P.; Kitsioulis, N.A.; Manousakis, E.; Manolaraki, I.; Douladiris, N.; Papadopoulos, N.G. Remission Patterns of Food Protein-Induced Enterocolitis Syndrome in a Greek Pediatric Population. Int. Arch. Allergy Immunol. 2019, 180, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Caubet, J.C.; Szajewska, H.; Shamir, R.; Nowak-Wegrzyn, A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr. Allergy Immunol. 2017, 28, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Sultafa, J.; McKibbon, L.; Roberts, H.; Sarraj, J.; Kim, H. Modified oral food challenge protocol approach in the diagnosis of Food Protein-Induced Enterocolitis Syndrome. Allergy Asthma Clin. Immunol. 2022, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, L.M.; Nowak-Wegrzyn, A.; Schultz, F.; Phipatanakul, W.; Bingemann, T.A. The evolution of food protein-induced enterocolitis syndrome: From a diagnosis that did not exist to a condition in need of answers. Ann. Allergy Asthma Immunol. 2021, 126, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Maciag, M.C.; Bartnikas, L.M.; Sicherer, S.H.; Herbert, L.J.; Young, M.C.; Matney, F.; Westcott-Chavez, A.A.; Petty, C.R.; Phipatanakul, W.; Bingemann, T.A. A Slice of Food Protein-Induced Enterocolitis Syndrome (FPIES): Insights from 441 Children with FPIES as Provided by Caregivers in the International FPIES Association. J. Allergy Clin. Immunol. Pract. 2020, 8, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Mehr, S.; Frith, K.; Barnes, E.H.; Campbell, D.E.; Group, F.S. Food protein-induced enterocolitis syndrome in Australia: A population-based study, 2012–2014. J. Allergy Clin. Immunol. 2017, 140, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Zapatero Remón, L.; Alonso Lebrero, E.; Martín Fernández, E.; Martínez Molero, M.I. Food-protein-induced enterocolitis syndrome caused by fish. Allergol. Immunopathol. 2005, 33, 312–316. [Google Scholar] [CrossRef]
- Díaz, J.J.; Espín, B.; Segarra, O.; Domínguez-Ortega, G.; Blasco-Alonso, J.; Cano, B.; Rayo, A.; Moreno, A. Food Protein-induced Enterocolitis Syndrome: Data From a Multicenter Retrospective Study in Spain. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Toyama, Y.; Ishii, T.; Morita, K.; Tsumura, Y.; Takahashi, T.; Akashi, M.; Morita, H. Multicenter retrospective study of patients with food protein-induced enterocolitis syndrome provoked by hen’s egg. J. Allergy Clin. Immunol. Pract. 2021, 9, 547–549.e1. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Tanaka, H.; Meguro, T.; Kimura, M. Three cases of food protein-induced enterocolitis syndrome caused by egg yolk. Allergol. Int. 2019, 68, 110–111. [Google Scholar] [CrossRef] [PubMed]

| Major Criterion: |
|---|
| Vomiting in the 1 to 4 h period following the ingestion of the suspect food and absence of classic IgE-mediated allergic skin or respiratory symptoms. |
| Minor criteria: |
| 1. A second (or more) episode of repetitive vomiting after eating the same suspect food; 2. Repetitive vomiting episode 1–4 h after eating a different food; 3. Extreme lethargy with any suspected reaction; 4. Marked pallor with any suspected reaction; 5. Need for emergency department visit with any suspected reaction; 6. Need for intravenous fluid support with any suspected reaction; 7. Diarrhea in 24 h (usually 5–10 h); 8. Hypotension; 9. Hypothermia. |
| All (n = 23) | OFC-Positive (n = 8) | OFC-Negative (n = 15) | p-Value | |
|---|---|---|---|---|
| Male, no. (%) | 11 (47.8) | 5 (62.5) | 6 (40.0) | 0.555 * |
| Age at onset of initial reactions (month), median (IQR †), range | 7.0 (6.25–8.0), 0–15 | 8.0 (6.0–8.25), 0–14 | 7.0 (6.0–7.5), 0–15 | 0.324 ** |
| Age at diagnosis (month), median (IQR †) | 8.0 (6.25–11.5) | 11.5 (7.25–17.8) | 7.0 (6.0–9.0) | 0.217 ** |
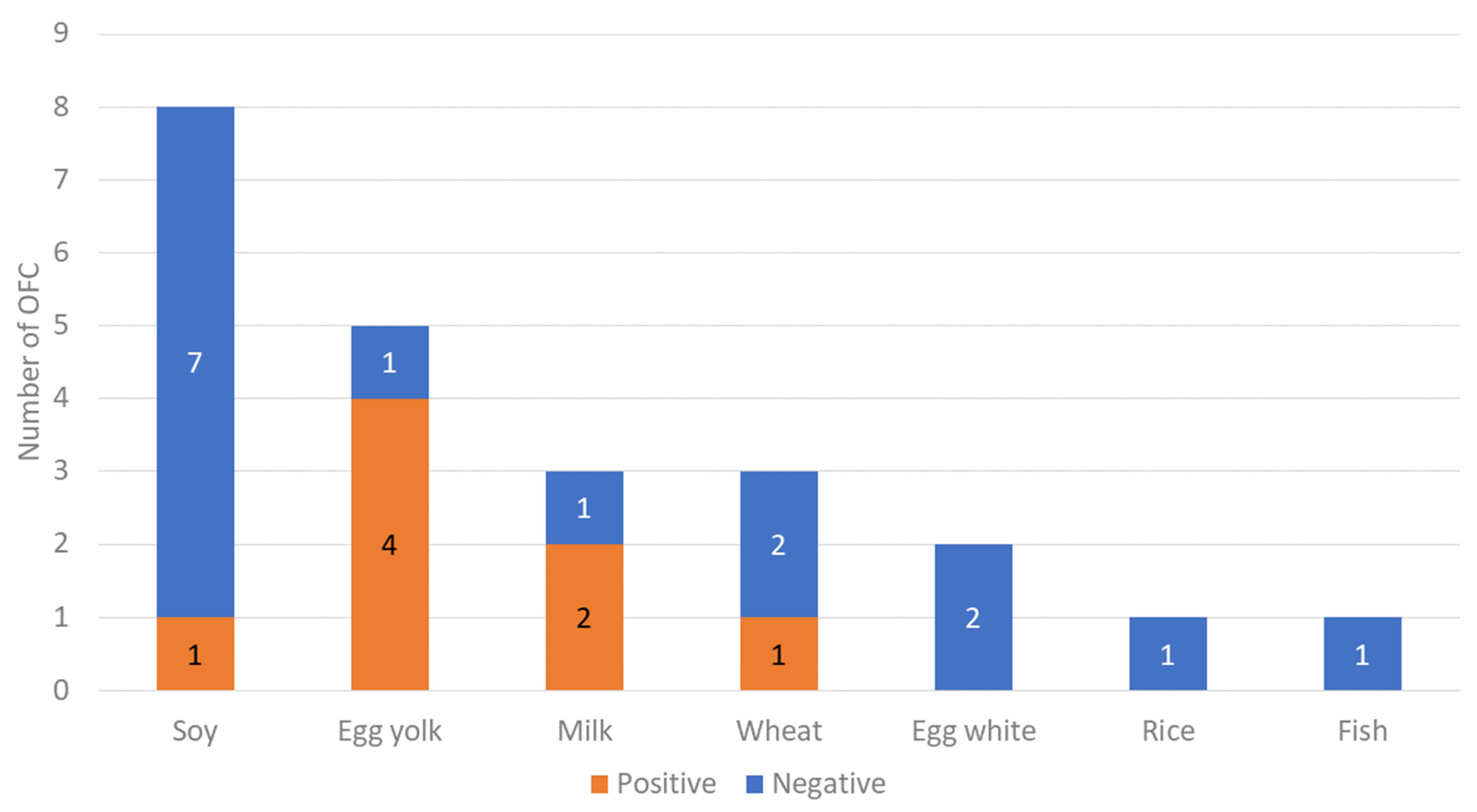
| Causative food, no. (%) | ||||
| Soy | 8 (34.8) | 1 (12.5) | 7 (46.7) | 0.136 * |
| Egg yolk | 5 (21.7) | 4 (50.0) | 1 (6.7) | |
| Milk | 3 (13.0) | 2 (25.0) | 1 (6.7) | |
| Wheat | 3 (13.0) | 1 (12.5) | 2 (13.3) | |
| Egg white | 2 (8.7) | 0 (0.0) | 2 (13.3) | |
| Rice | 1 (4.4) | 0 (0.0) | 1 (6.7) | |
| Fish | 1 (4.4) | 0 (0.0) | 1 (6.7) | |
| Skin prick test for trigger foods positivity, no. (%) §§ | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Specific IgE antibody positivity, no. (%) ‡ | 3 (13.0) | 1 (12.5) | 2 (13.3) | 0.550 * |
| Additional allergic conditions, no. (%) | ||||
| Atopic dermatitis | 9 (39.1) | 5 (62.5) | 4 (26.7) | 0.221 * |
| Asthma | 1 (4.4) | 0 (0.0) | 1 (6.7) | 0.742 * |
| Allergic rhinitis | 1 (4.4) | 1 (12.5) | 0 (0.0) | 0.742 * |
| IgE-food allergy | 2 (8.7) | 2 (25.0) | 0 (0.0) | 0.213 * |
| Birth history of cesarean section, no. (%) | 11 (47.8) | 5 (62.5) | 6 (40.0) | 0.551 * |
| Birth weight (g) § | 2963 ± 429 | 2956 ± 575 | 2996 ± 413 | 0.560 ** |
| Breastfeeding at onset, no. (%) | 17 (73.9) | 6 (75.0) | 11 (73.3) | 0.681 * |
| Family allergic disease history, no. (%) | 15 (65.2) | 5 (62.5) | 10 (66.7) | 0.795 * |
| Bodyweight at OFC (kg) ‡ | 11.1 ± 2.7 | 11.3 ± 2.95 | 10.8 ± 2.54 | 0.557 *** |
| White blood cell (/μL) § | 9631 ± 3290 | 8076 ± 1670 | 10,306 ± 3656 | 0.158 *** |
| Neutrophil (%) § | 31.8 ± 11.8 | 36.9 ± 9.97 | 29.5 ± 12.2 | 0.086 *** |
| Eosinophil (%) § | 2.80 ± 2.32 | 3.06 ± 2.82 | 2.69 ± 2.07 | 0.574 *** |
| CRP (mg/dL) § | 2.80 ± 2.32 | 1.25 ± 2.09 | 0.30 ± 0.91 | 0.297 *** |
| Total IgE (IU/mL) § | 80.9 ± 170 | 52.6 ± 62.3 | 94.8 ± 201 | 0.519 *** |
| Specific IgE antibody (UA/mL) § | 0.170 ± 0.194 | 0.14 ± 0.01 | 0.18 ± 0.23 | 0.543 *** |
| TARC (pg/mL) § | 718 ± 581 | 961 ± 857 | 589 ± 335 | 0.314 *** |
| Age in months OFC performed, median (IQR †), range | 21.0 (15.3–32.0), 6–56 | 23.5 (15.5–33.3), 7–56 | 19.0 (14.5–31.5), 6–45 | 0.722 ** |
| Duration between onset and OFC (months) | 14 (8–24) | 14 (7.75–20.75) | 13 (8.5–23.5) | 0.897 ** |
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Sex | Female | Male | Male | Male | Male | Female | Female | Male |
| Food | Milk | Milk | Egg yolk | Egg yolk | Egg yolk | Wheat | Egg yolk | Soy |
| Initial diagnosis | Acute FPIES | Acute FPIES or FPIAP | Acute FPIES | Acute FPIES | Acute FPIES | Acute FPIES | Acute FPIES | Acute FPIES |
| Age at onset (m) | 0 | 0 | 9 | 8 | 14 | 8 | 8 | 8 |
| Age at diagnosis(m) | 1 | 0 | 16 | 13 | 26 | 10 | 15 | 8 |
| Age at OFC (m) | 7 | 14 | 56 | 25 | 32 | 22 | 37 | 16 |
| First reaction of acute FPIES (times) | Vomiting (2) | Bloody stool Vomiting (2) | Vomiting (3) Diarrhea (2) | Vomiting (10) | Vomiting (frequent) | Vomiting (5) Diarrhea (1) | Vomiting (2) Lethargy | Vomiting (4) Diarrhea (1) |
| Time (h) | 3–5 | Unknown | 2 | 3 | 2 | 5 | 2 | 4 |
| Severity in the first reaction | Mild | Mild | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| OFC reaction (times) | Vomiting (4) | Vomiting (3) | Vomiting (2) Diarrhea (1) Abdominal pain | Vomiting (4) | Vomiting (3) Abdominal pain Lethargy | Vomiting (3) | Vomiting (2) Lethargy | Vomiting (1) Lethargy |
| Time (h) | 2.5 | 8 | 2 | 2.5 | 2 | 2 | 3 | 5 |
| Day (day) | 1 | 2 | 1 | 1 | 1 | 4 | 1 | 1 |
| Food protein of body weight at the reaction (g/kg) | 0.008 | 0.03 | 0.006 | 0.007 | 0.006 | 0.3 | 0.007 | 0.006 |
| Severity in OFC | Moderate | Moderate | Mild | Moderate | Moderate | Moderate | Moderate | Mild |
| Treatment | Intravenous normal saline bolus | Intravenous normal saline bolus | None | Intravenous normal saline bolus and corticosteroids | None | Intravenous normal saline bolus and corticosteroids | Intravenous normal saline bolus | None |
| Confirmatory diagnosis by OFC | Acute FPIES | Unclassifiable † | Acute FPIES | Acute FPIES | Acute FPIES | Acute FPIES | Acute FPIES | Acute FPIES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, K.; Yamamoto-Hanada, K.; Sato, M.; Toyokuni, K.; Ogita, H.; Kiguchi, T.; Miyagi, Y.; Inuzuka, Y.; Saito-Abe, M.; Irahara, M.; et al. Remission of Acute Food Protein-Induced Enterocolitis Syndrome Confirmed by Oral Food Challenges in Japan. Nutrients 2022, 14, 4158. https://doi.org/10.3390/nu14194158
Nishimura K, Yamamoto-Hanada K, Sato M, Toyokuni K, Ogita H, Kiguchi T, Miyagi Y, Inuzuka Y, Saito-Abe M, Irahara M, et al. Remission of Acute Food Protein-Induced Enterocolitis Syndrome Confirmed by Oral Food Challenges in Japan. Nutrients. 2022; 14(19):4158. https://doi.org/10.3390/nu14194158
Chicago/Turabian StyleNishimura, Koji, Kiwako Yamamoto-Hanada, Miori Sato, Kenji Toyokuni, Hiroya Ogita, Tomoyuki Kiguchi, Yoshitsune Miyagi, Yusuke Inuzuka, Mayako Saito-Abe, Makoto Irahara, and et al. 2022. "Remission of Acute Food Protein-Induced Enterocolitis Syndrome Confirmed by Oral Food Challenges in Japan" Nutrients 14, no. 19: 4158. https://doi.org/10.3390/nu14194158
APA StyleNishimura, K., Yamamoto-Hanada, K., Sato, M., Toyokuni, K., Ogita, H., Kiguchi, T., Miyagi, Y., Inuzuka, Y., Saito-Abe, M., Irahara, M., Ishikawa, F., Kabashima, S., Miyaji, Y., Fukuie, T., Nomura, I., & Ohya, Y. (2022). Remission of Acute Food Protein-Induced Enterocolitis Syndrome Confirmed by Oral Food Challenges in Japan. Nutrients, 14(19), 4158. https://doi.org/10.3390/nu14194158







