External Validation of Equations to Estimate Resting Energy Expenditure in Critically Ill Children and Adolescents with and without Malnutrition: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Data
2.3. Anthropometry
2.4. Indirect Calorimetry
2.5. Prediction Equations
2.6. Statistical Analysis
3. Results
3.1. Study Population
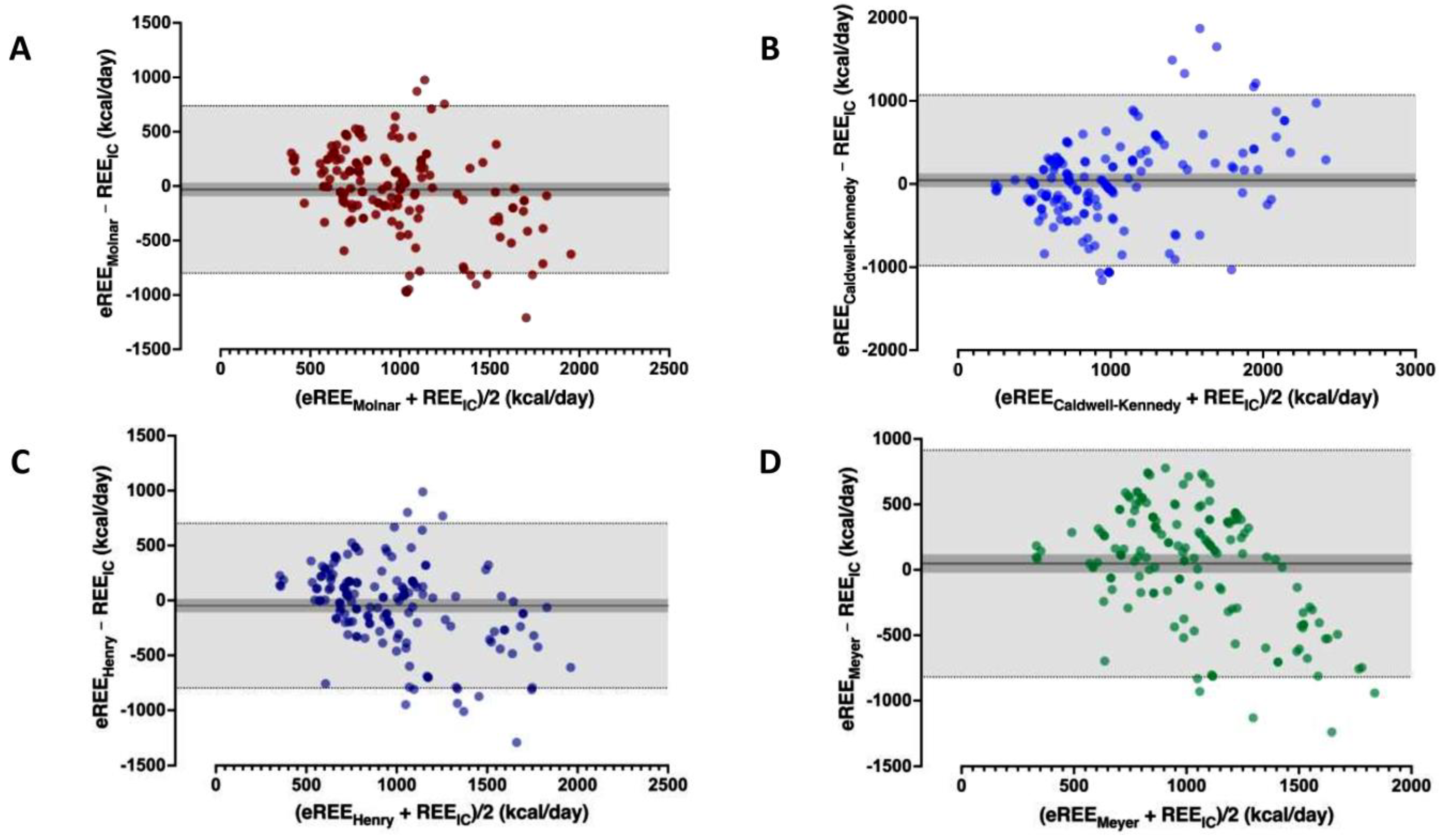
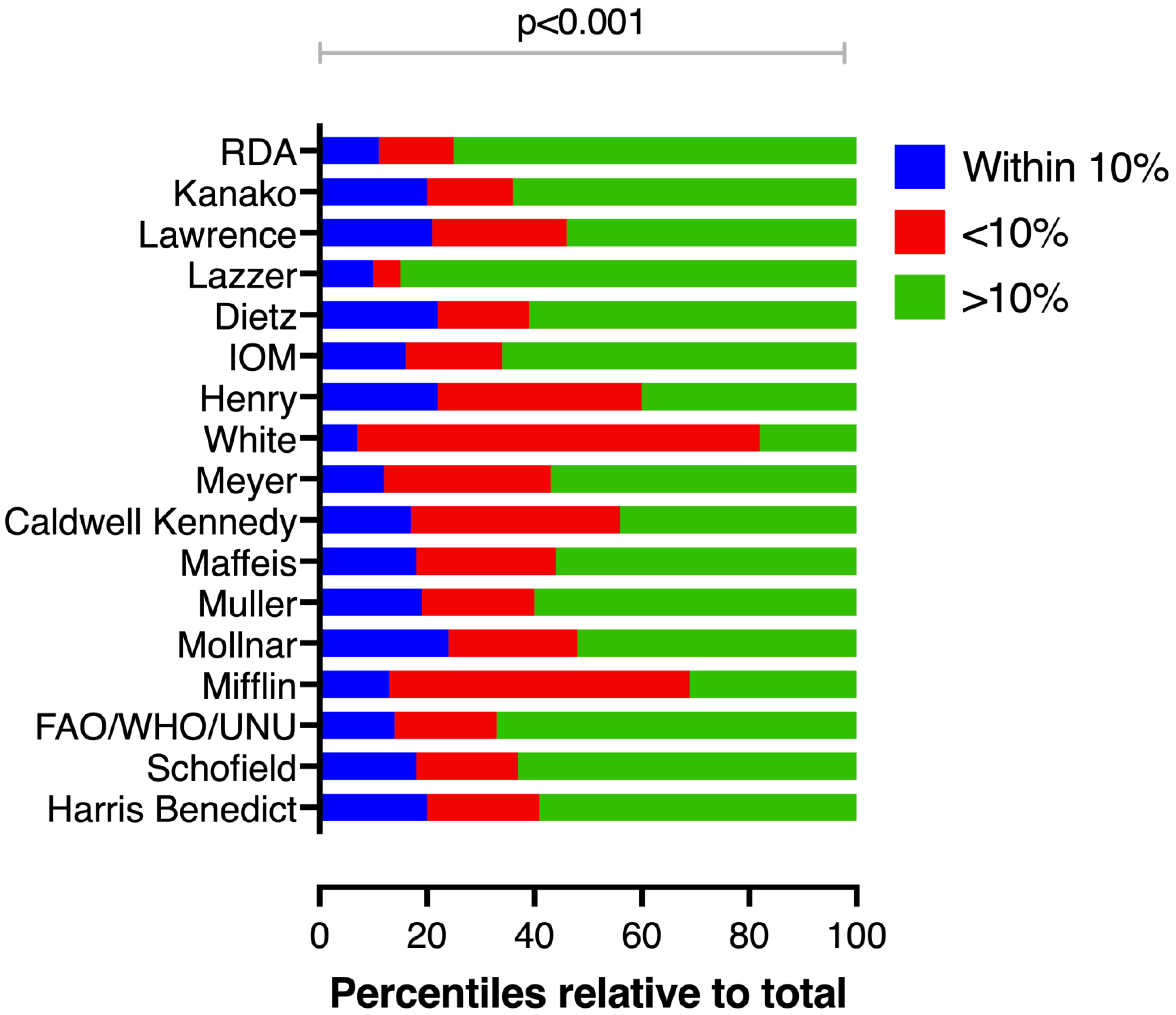
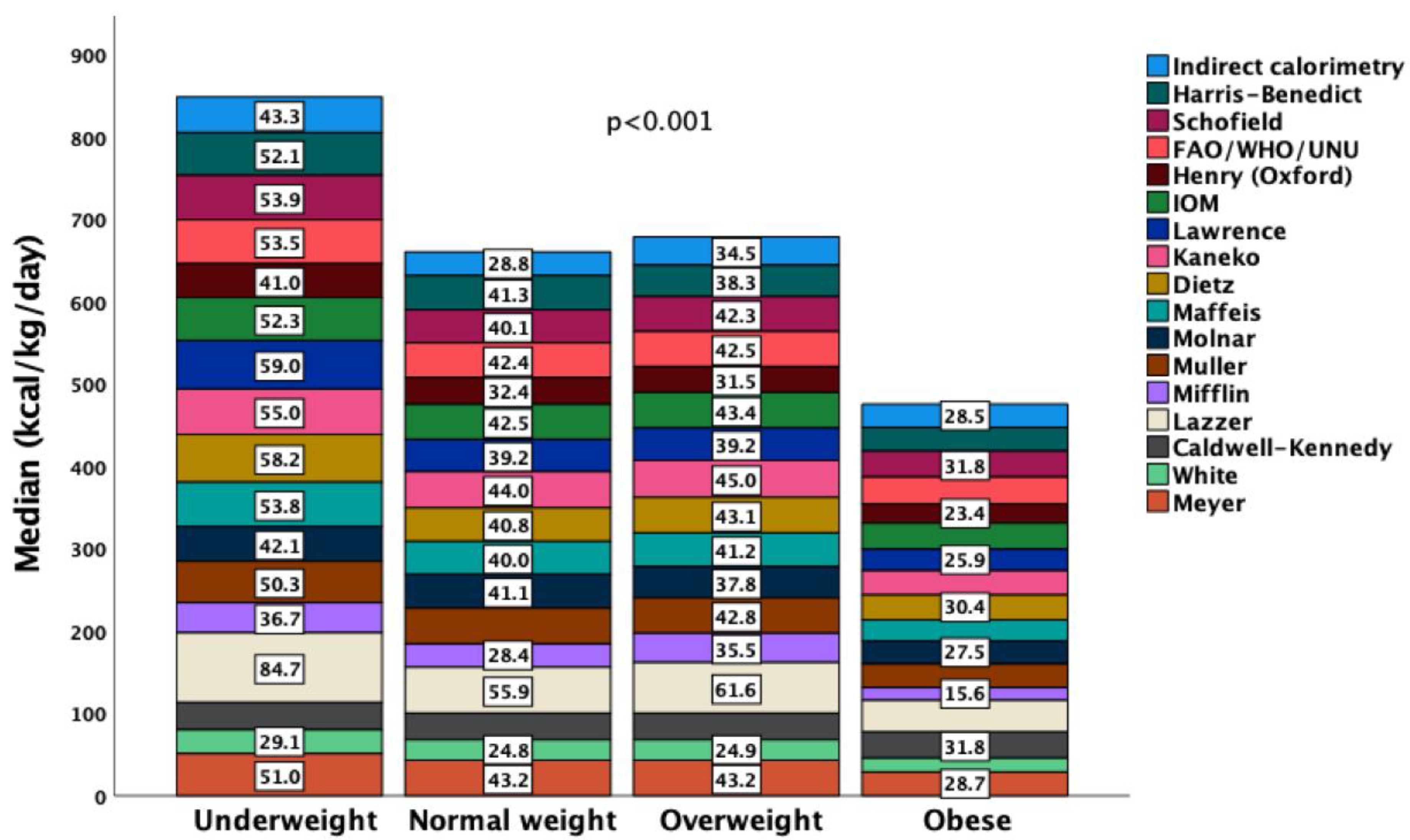
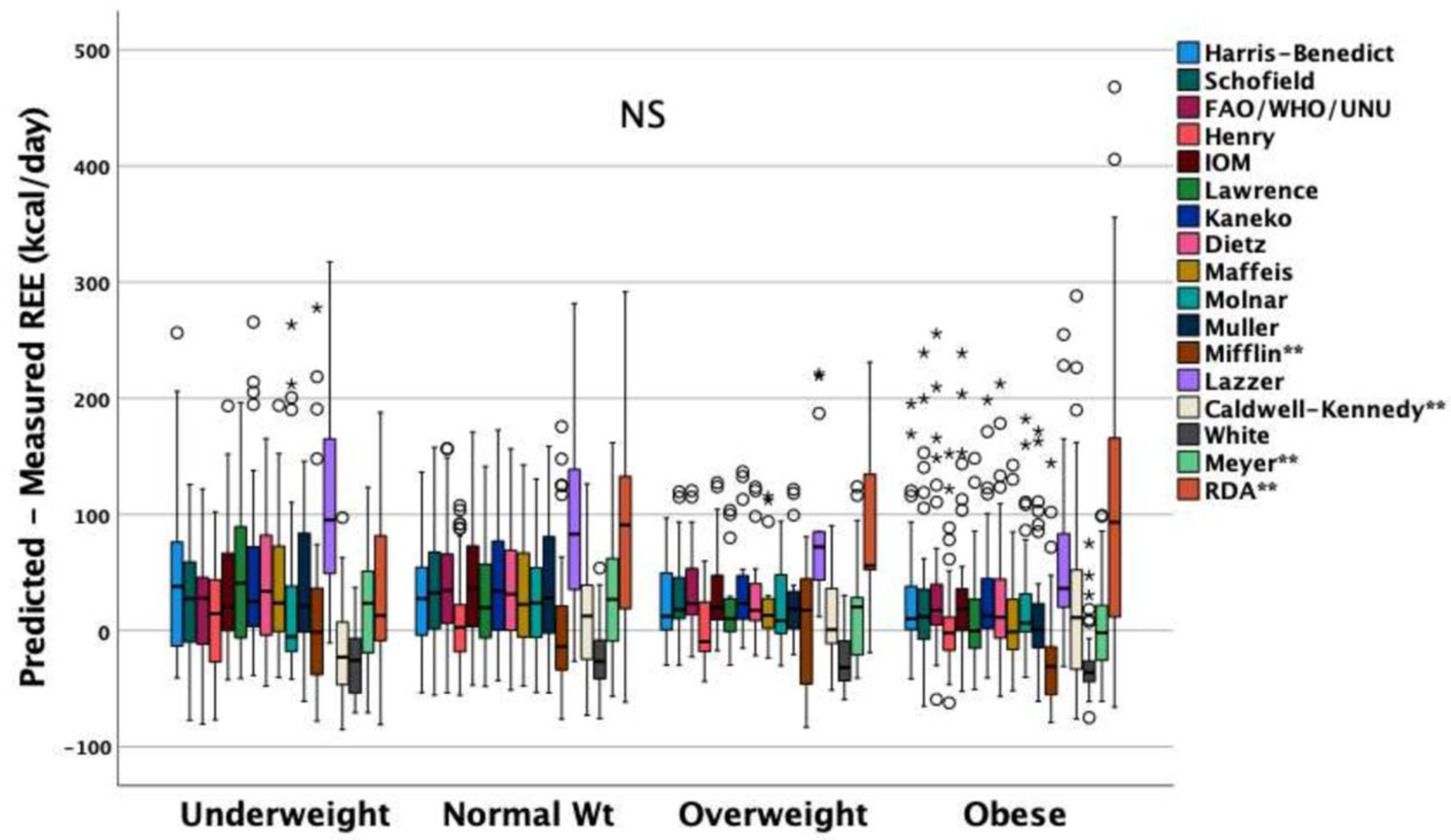
3.2. Performance of Predictive Equations
3.3. Malnutrition and Factors Independently Associated with REEIC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, R.M.; Cheeseman, P.; Preedy, V.; Baker, A.J.; Grimble, G. Can Energy Expenditure Be Predicted in Critically Ill Children? Pediatr. Crit. Care Med. 2003, 4, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Energy and Protein Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organ. Tech. Rep. Ser. 1985, 724, 1–206. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Food and Nutrition Board of the Institute of Medicine, The National Academies Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Schofield, W.N. Predicting Basal Metabolic Rate, New Standards and Review of Previous Work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar] [PubMed]
- Henry, C.J.K. Basal Metabolic Rate Studies in Humans: Measurement and Development of New Equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef]
- Lawrence, J.C.; Lee, H.-M.; Kim, J.-H.; Kim, E.-K. Variability in Results from Predicted Resting Energy Needs as Compared to Measured Resting Energy Expenditure in Korean Children. Nutr. Res. 2009, 29, 777–783. [Google Scholar] [CrossRef]
- Kaneko, K.; Ito, C.; Koizumi, K.; Watanabe, S.; Umeda, Y.; Ishikawa-Takata, K. Resting Energy Expenditure (REE) in Six- to Seventeen-Year-Old Japanese Children and Adolescents. J. Nutr. Sci. Vitam. 2013, 59, 299–309. [Google Scholar] [CrossRef][Green Version]
- Dietz, W.H.; Bandini, L.G.; Schoeller, D.A. Estimates of Metabolic Rate in Obese and Nonobese Adolescents. J. Pediatr. 1991, 118, 146–149. [Google Scholar] [CrossRef]
- Maffeis, C.; Schutz, Y.; Micciolo, R.; Zoccante, L.; Pinelli, L. Resting Metabolic Rate in Six- to Ten-Year-Old Obese and Nonobese Children. J. Pediatr. 1993, 122, 556–562. [Google Scholar] [CrossRef]
- Molnár, D.; Jeges, S.; Erhardt, E.; Schutz, Y. Measured and Predicted Resting Metabolic Rate in Obese and Nonobese Adolescents. J. Pediatr. 1995, 127, 571–577. [Google Scholar] [CrossRef]
- Müller, M.J.; Bosy-Westphal, A.; Klaus, S.; Kreymann, G.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; Selberg, O.; et al. World Health Organization Equations Have Shortcomings for Predicting Resting Energy Expenditure in Persons from a Modern, Affluent Population: Generation of a New Reference Standard from a Retrospective Analysis of a German Database of Resting Energy Expenditure. Am. J. Clin. Nutr. 2004, 80, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Lazzer, S.; Patrizi, A.; De Col, A.; Saezza, A.; Sartorio, A. Prediction of Basal Metabolic Rate in Obese Children and Adolescents Considering Pubertal Stages and Anthropometric Characteristics or Body Composition. Eur. J. Clin. Nutr. 2014, 68, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Servín, J.; Avila-Nava, A.; González-Salazar, L.E.; Pérez-González, O.A.; Servín-Rodas, M.D.C.; Serralde-Zuñiga, A.E.; Medina-Vera, I.; Guevara-Cruz, M. Resting Energy Expenditure Prediction Equations in the Pediatric Population: A Systematic Review. Front. Pediatr. 2021, 9, 795364. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Bedogni, G.; Bertoli, S.; De Amicis, R.; Foppiani, A.; De Col, A.; Tringali, G.; Marazzi, N.; De Cosmi, V.; Agostoni, C.; Battezzati, A.; et al. External Validation of Equations to Estimate Resting Energy Expenditure in 2037 Children and Adolescents with and 389 without Obesity: A Cross-Sectional Study. Nutrients 2020, 12, 1421. [Google Scholar] [CrossRef]
- Vazquez Martinez, J.L.; Martinez-Romillo, P.D.; Diez Sebastian, J.; Ruza Tarrio, F. Predicted versus Measured Energy Expenditure by Continuous, Online Indirect Calorimetry in Ventilated, Critically Ill Children during the Early Postinjury Period. Pediatr. Crit. Care Med. 2004, 5, 19–27. [Google Scholar] [CrossRef]
- Caldwell, M.D.; Kennedy-Caldwell, C. Normal Nutritional Requirements. Surg. Clin. N. Am. 1981, 61, 489–507. [Google Scholar] [CrossRef]
- White, M.S.; Shepherd, R.W.; McEniery, J.A. Energy Expenditure in 100 Ventilated, Critically Ill Children: Improving the Accuracy of Predictive Equations. Crit. Care Med. 2000, 28, 2307–2312. [Google Scholar] [CrossRef]
- Meyer, R.; Kulinskaya, E.; Briassoulis, G.; Taylor, R.M.; Cooper, M.; Pathan, N.; Habibi, P. The Challenge of Developing a New Predictive Formula to Estimate Energy Requirements in Ventilated Critically Ill Children. Nutr. Clin. Pract. 2012, 27, 669–676. [Google Scholar] [CrossRef]
- National Research Council (US). Subcommittee on the Tenth Edition of the Recommended Dietary Allowances Recommended Dietary Allowances, 10th ed.; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 1989; ISBN 978-0-309-04633-6. [Google Scholar]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Venkataraman, S.; Thompson, A.E. Energy Expenditure in Critically Ill Children. Crit. Care Med. 2000, 28, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.M.; Dwyer, J.; Snelling, L.K.; Dallal, G.E.; Adelson, J.W. Pitfalls in Predicting Resting Energy Requirements in Critically Ill Children: A Comparison of Predictive Methods to Indirect Calorimetry. Nutr. Clin. Pract. 2002, 17, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.K.; Thomsen, L.P.; Kjaergaard, S.; Rees, S.E.; Karbing, D.S. Reliability of, and Agreement Between, Two Breath-by-Breath Indirect Calorimeters at Varying Levels of Inspiratory Oxygen. Nutr. Clin. Pract. 2019, 34, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Briassoulis, P.; Michaeloudi, E.; Fitrolaki, D.-M.; Spanaki, A.-M.; Briassouli, E. The Effects of Endotracheal Suctioning on the Accuracy of Oxygen Consumption and Carbon Dioxide Production Measurements and Pulmonary Mechanics Calculated by a Compact Metabolic Monitor. Anesth. Analg. 2009, 109, 873–879. [Google Scholar] [CrossRef]
- Briassoulis, G.; Michaeloudi, E.; Fitrolaki, D.-M.; Spanaki, A.-M.; Briassouli, E. Influence of Different Ventilator Modes on Vo(2) and Vco(2) Measurements Using a Compact Metabolic Monitor. Nutrition 2009, 25, 1106–1114. [Google Scholar] [CrossRef]
- Jhang, W.K.; Park, S.J. Energy Expenditure in Mechanically Ventilated Korean Children: Single-Center Evaluation of a New Estimation Equation. Pediatr. Crit. Care Med. 2020, 21, e522–e529. [Google Scholar] [CrossRef]
- Verhoeven, J.J.; Hazelzet, J.A.; van der Voort, E.; Joosten, K.F. Comparison of Measured and Predicted Energy Expenditure in Mechanically Ventilated Children. Intensive Care Med. 1998, 24, 464–468. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, J. Meta-Analysis for the Prediction of Mortality Rates in a Pediatric Intensive Care Unit Using Different Scores: PRISM-III/IV, PIM-3, and PELOD-2. Front. Pediatr. 2021, 9, 712276. [Google Scholar] [CrossRef]
- Trope, R.; Vaz, S.; Zinger, M.; Sagy, M. An Updated Therapeutic Intervention Scoring System for Critically Ill Children Enables Nursing Workload Assessment with Insight Into Potential Untoward Events. J. Intensive Care Med. 2015, 30, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- CDC BMI Calculator for Child and Teen. Available online: https://www.cdc.gov/healthyweight/bmi/calculator.html (accessed on 3 August 2022).
- Coss-Bu, J.A.; Klish, W.J.; Walding, D.; Stein, F.; Smith, E.O.; Jefferson, L.S. Energy Metabolism, Nitrogen Balance, and Substrate Utilization in Critically Ill Children. Am. J. Clin. Nutr. 2001, 74, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Venkataraman, S.; Thompson, A. Cytokines and Metabolic Patterns in Pediatric Patients with Critical Illness. Clin. Dev. Immunol. 2010, 2010, 354047. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Smallwood, C.D.; Joosten, K.F.M.; Hulst, J.M.; Tasker, R.C.; Duggan, C.P. Accuracy of a Simplified Equation for Energy Expenditure Based on Bedside Volumetric Carbon Dioxide Elimination Measurement–A Two-Center Study. Clin. Nutr. 2015, 34, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Rodríguez, G.; Moreno, L.A.; Sarría, A.; Fleta, J.; Bueno, M. Resting Energy Expenditure in Children and Adolescents: Agreement between Calorimetry and Prediction Equations. Clin. Nutr. 2002, 21, 255–260. [Google Scholar] [CrossRef]
- Mehta, N.M.; Bechard, L.J.; Dolan, M.; Ariagno, K.; Jiang, H.; Duggan, C. Energy Imbalance and the Risk of Overfeeding in Critically Ill Children. Pediatr. Crit. Care Med. 2011, 12, 398–405. [Google Scholar] [CrossRef]
- Spanaki, A.M.; Tavladaki, T.; Dimitriou, H.; Kozlov, A.V.; Duvigneau, J.C.; Meleti, E.; Weidinger, A.; Papakonstantinou, E.; Briassoulis, G. Longitudinal Profiles of Metabolism and Bioenergetics Associated with Innate Immune Hormonal Inflammatory Responses and Amino-Acid Kinetics in Severe Sepsis and Systemic Inflammatory Response Syndrome in Children. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1061–1074. [Google Scholar] [CrossRef]
- Briassoulis, G.; Briassouli, E.; Tavladaki, T.; Ilia, S.; Fitrolaki, D.M.; Spanaki, A.M. Unpredictable Combination of Metabolic and Feeding Patterns in Malnourished Critically Ill Children: The Malnutrition-Energy Assessment Question. Intensive Care Med. 2014, 40, 120–122. [Google Scholar] [CrossRef]
- Tavladaki, T.; Spanaki, A.M.; Dimitriou, H.; Kondili, E.; Choulaki, C.; Georgopoulos, D.; Briassoulis, G. Similar Metabolic, Innate Immunity, and Adipokine Profiles in Adult and Pediatric Sepsis Versus Systemic Inflammatory Response Syndrome-A Pilot Study. Pediatr. Crit. Care Med. 2017, 18, e494–e505. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting Energy Expenditure, Calorie and Protein Consumption in Critically Ill Patients: A Retrospective Cohort Study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.-Y.; Zheng, W.-H.; Zhou, H.; Xu, Y.; Huang, H.-B. Energy Delivery Guided by Indirect Calorimetry in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit. Care 2021, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Pertzov, B.; Bar-Yoseph, H.; Menndel, Y.; Bendavid, I.; Kagan, I.; Glass, Y.D.; Singer, P. The Effect of Indirect Calorimetry Guided Isocaloric Nutrition on Mortality in Critically Ill Patients-a Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2022, 76, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Tume, L.N.; Valla, F.V.; Joosten, K.; Jotterand Chaparro, C.; Latten, L.; Marino, L.V.; Macleod, I.; Moullet, C.; Pathan, N.; Rooze, S.; et al. Nutritional Support for Children during Critical Illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) Metabolism, Endocrine and Nutrition Section Position Statement and Clinical Recommendations. Intensive Care Med. 2020, 46, 411–425. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- De Waele, E.; van Zanten, A.R.H. Routine Use of Indirect Calorimetry in Critically Ill Patients: Pros and Cons. Crit. Care 2022, 26, 123. [Google Scholar] [CrossRef]
- Moonen, H.P.F.X.; Beckers, K.J.H.; van Zanten, A.R.H. Energy Expenditure and Indirect Calorimetry in Critical Illness and Convalescence: Current Evidence and Practical Considerations. J. Intensive Care 2021, 9, 8. [Google Scholar] [CrossRef]
- Kyle, U.G.; Arriaza, A.; Esposito, M.; Coss-Bu, J.A. Is Indirect Calorimetry a Necessity or a Luxury in the Pediatric Intensive Care Unit? JPEN J. Parenter. Enter. Nutr. 2012, 36, 177–182. [Google Scholar] [CrossRef]
- Jotterand Chaparro, C.; Moullet, C.; Taffé, P.; Laure Depeyre, J.; Perez, M.-H.; Longchamp, D.; Cotting, J. Estimation of Resting Energy Expenditure Using Predictive Equations in Critically Ill Children: Results of a Systematic Review. JPEN J. Parenter. Enter. Nutr. 2018, 42, 976–986. [Google Scholar] [CrossRef]
- Wewalka, M.; Schneeweiss, M.; Haselwanter, P.; Schneeweiss, B.; Zauner, C. Age-Dependent Differences in Energy Metabolism in the Acute Phase of Critical Illness. Nutrition 2022, 101, 111684. [Google Scholar] [CrossRef] [PubMed]
- Singer, P. Preserving the Quality of Life: Nutrition in the ICU. Crit. Care 2019, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Tume, L.N.; Ista, E.; Verbruggen, S.; Jotterand Chaparro, C.; Moullet, C.; Latten, L.; Marino, L.V.; Valla, F.V. Practical Strategies to Implement the ESPNIC Nutrition Clinical Recommendations into PICU Practice. Clin. Nutr. ESPEN 2021, 42, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.C.; Zavras, N.J.; Hatzis MD, T.D. Effectiveness and Safety of a Protocol for Promotion of Early Intragastric Feeding in Critically Ill Children. Pediatr. Crit. Care Med. 2001, 2, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Whittle, J.; Molinger, J.; MacLeod, D.; Haines, K.; Wischmeyer, P.E. LEEP-COVID Study Group Persistent Hypermetabolism and Longitudinal Energy Expenditure in Critically Ill Patients with COVID-19. Crit. Care 2020, 24, 581. [Google Scholar] [CrossRef] [PubMed]
- De Waele, E.; Jonckheer, J.; Wischmeyer, P. Indirect Calorimetry In Critical Illness: A New Standard of Care? Curr. Opin. Crit. Care 2021, 27, 334–343. [Google Scholar] [CrossRef] [PubMed]
| N = 153 | |||||
|---|---|---|---|---|---|
| Demographic | Clinical Data | Indirect Calorimetry | |||
| Age (years) | 7.5 (5; 12.5) | PRISM score | 9 (6; 15) | REE (kcal/day) | 928 (651; 1238) |
| Sex (male/female) | 108/45, (70.6%/29.4%) | TISS score | 41 (36; 46) | REE (kcal/kg/day) | 32.3 (23.0; 48.3) |
| Anthropometric | PELOD score | 7 (2; 18) | VO2 (mL/min) | 134 (95.5; 176.8) | |
| Body weight (kg) | 25 (16.5; 41.5) | FiO2 (%) | 35 (30; 50) | VCO2 (mL/min) | 111 (74.6; 153.2) |
| Height (cm) | 130 (111; 148) | pH | 7.39 (7.35; 7.43) | Respiratory Quotient | 0.85 (0.77; 0.91) |
| BMI (kg/m2) | 16.6 (15.2; 20.6) | pO2 (mmHg) | 96 (87; 111) | Metabolic state * (kcal/day) | 88.5 (69.7; 106.7) |
| z-score weight for age | 0.42 (−1.2; 1.2) | pCO2 (mmHg) | 36 (33.5; 39.1) | Metabolic pattern ** | |
| z-score height for age | −0.03 (−0.54; 0.55) | HCO3 (mEq/L) | 22.3 (19.6; 24.5) | Normometabolic | 42 (27.5%) |
| z-score BMI for age | 0.47 (−0.98; 1.65) | Heart Rate (bpm) | 100 (80: 119) | Hypometabolic | 82 (53.6%) |
| BMI nutrition status | Respiratory rate (bpm) | 22 (18; 25.8) | Hypermetabolic | 29 (19%) | |
| Underweight | 30 (19.6%) | Systolic Blood Pressure (mmHg) | 97 (78; 107) | Nutrition day 3 | |
| Normal BMI | 69 (45.1%) | Body Temperature (°C) | 37.2 (36.8; 37.8) | Energy intake (kcal/day) | 720 (480; 1000) |
| Overweight | 16 (10.5%) | Neuromuscular blockade, yes | 11/66 (16.7%) | Energy intake (kcal/kg/day) | 27.4 (16; 41.7) |
| Obese | 38 (24.8%) | Vasoactive, yes | 40/82 (56.3%) | Energy intake/REE ratio | |
| Clinical diagnosis | Lactate (mg/dL) | 10.8 (6.3; 18) | Energy intake/REE (%) | 88.2 (47.7; 112.9) | |
| Respiratory failure | 40 (26.2%) | Glucose (mg/dL) | 103 (93; 121) | Feeding status | |
| Sepsis | 27 (17.6%) | Albumin (mg/dL) | 3.1 (2.7; 3.4) | Adequate | 40/123 (32.5%) |
| Surgical | 11 (7.2%) | C-Reactive Protein (mg/dL) | 8 (1.3; 16) | Underfeeding | 49/123 (39.8%) |
| Organ failure | 4 (2.6%) | Length of Stay (days) | 14 (6.5; 23.5) | Overfeeding | 34/123 (27.6%) |
| Trauma | 41 (26.8%) | Mechanical Ventilation (days) | 12 (5; 18) | Underfeeding/Obese | 15/27 (55.6%) |
| Neurologic | 30 (19.6%) | Mortality | 6 (3.9%) | Overfeeding/Underweight | 9/25 (36%) |
| REE (kcal/Day) | Agreement-Precision * | Paired Differences-Variability # | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compared Equation | IQR 25th | Median | IQR 75th | Mean Bias | SD | Limits of Agreement | Medan of Differences | IQR 25th; 75th | CV (%) | p Value |
| n = 153 | ||||||||||
| Indirect Calorimetry | 651.35 | 928.30 | 1238.39 | |||||||
| Harris–Benedict | 920.17 | 1083.41 | 1263.46 | 142 | 391 | −624; 908 | 174 | −48; 388 | 275 | <0.001 |
| Schofield H-W | 864.58 | 1057.30 | 1439.47 | 185 | 427 | −652; 1021 | 191 | −41; 469 | 231 | <0.001 |
| FAO/WHO/UNU | 727.13 | 935.25 | 1216.50 | 146 | 398 | −634; 926 | 142 | −32; 430 | 273 | <0.001 |
| Henry (Oxford) | 739.37 | 860.654 | 1172.30 | −47 | 383 | −798; 703 | 5 | −236; 176 | 809 | 0.421 |
| IOM | 937.55 | 1090.30 | 1404.64 | 209 | 409 | −593; 1011 | 205 | 21; 481 | 196 | <0.001 |
| Lawrence | 885.64 | 995.93 | 1296.82 | 81 | 384 | −672; 834 | 130 | −119; 342 | 475 | <0.002 |
| Kaneko | 1016.62 | 1122.27 | 1357.78 | 209 | 387 | −549; 967 | 211 | 21; 468 | 185 | <0.001 |
| Dietz | 919.61 | 1072.04 | 1393.22 | 181 | 397 | −598; 959 | 219 | −25; 434 | 220 | <0.001 |
| Maffeis | 921.37 | 1048.95 | 1215.10 | 87 | 388 | −673; 846 | 127 | −134; 396 | 448 | <0.002 |
| Molnar | 929.43 | 1126.62 | 1339.89 | −32 | 393 | −802; 739 | −8 | −207; 235 | 1242 | 0.843 |
| Muller | 869.15 | 1062.50 | 1471.10 | 96 | 393 | −674; 866 | 111 | −120; 352 | 410 | <0.001 |
| Mifflin | 561.30 | 769.90 | 1050.96 | −159.9 | 393.3 | −966.8; 575 | −926 | −1235; −650 | 201 | <0.001 |
| Lazzer (equation 1) | 1346.00 | 1548.00 | 1831.00 | 592 | 402 | −196; 1380 | 627 | 385; 869 | 68 | <0.001 |
| Caldwell–Kennedy | 539.55 | 806.37 | 1378.03 | 44 | 524 | −983; 1071 | 35 | −213; 291 | 1187 | 0.358 |
| White (equation 2) | 512.07 | 606.06 | 784.21 | −342 | 390 | −1107; 422 | −282 | −520; −69 | 114 | <0.001 |
| Meyer (equation C) | 800.56 | 1054.00 | 1302.86 | 47 | 442 | −820; 915 | 137 | −264; 382 | 935 | 0.058 |
| RDA | 880.00 | 1320.00 | 2365.00 | 742 | 940 | −1101; 2585 | 568 | 58; 1210 | 127 | <0.001 |
| Reliability ^ | Accuracy # | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compared Equation | All n = 153 | Underweight n = 30 | Normal Weight n = 69 | Overweight n = 16 | Obese n = 38 | |||||||||
| ICC (Average Measures) | p Value | Within ±10% | <−10% | >+10% | p Value * | p Value* | p Value * | p Value * | p Value * | |||||
| Harris–Benedict | 0.699 (0.58; 0.78) | <0.001 | 20.3 | 20.9 | 58.8 | <0.001 | 2/28 | <0.001 | 13/56 | <0.001 | 5/11 | 0.134 | 11/27 | <0.01 |
| Schofield | 0.73 (0.63; 0.80) | <0.001 | 17.6 | 19 | 63.4 | <0.001 | 5/25 | <0.001 | 13/56 | <0.001 | 2/14 | 0.003 | 8/30 | <0.001 |
| FAO/WHO/UNU | 0.74 (0.64; 0.81) | <0.001 | 14.4 | 19 | 66.7 | <0.001 | 3/27 | <0.001 | 12/57 | <0.001 | 1/15 | <0.001 | 7/31 | <0.001 |
| Henry (Oxford) | 0.70 (0.59; 0.78) | <0.001 | 21.6 | 37.9 | 40.5 | 0.008 | 3/27 | <0.001 | 17/52 | <0.001 | 2/14 | 0.003 | 12/26 | 0.023 |
| IOM | 0.72 (0.61; 0.79) | <0.001 | 15.7 | 17.6 | 66.7 | <0.001 | 4/26 | <0.001 | 12/57 | <0.001 | 3/13 | 0.012 | 7/31 | <0.001 |
| Lawrence | 0.65 (0.52; 0.75) | <0.001 | 20.9 | 24.8 | 54.2 | <0.001 | 6/24 | <0.001 | 12/57 | <0.001 | 5/11 | 0.134 | 9/329 | <0.001 |
| Kaneko | 0.67 (0.55; 0.76) | <0.001 | 20.3 | 15.7 | 64.1 | <0.001 | 6/24 | <0.001 | 10/59 | <0.001 | 3/13 | 0.012 | 12/26 | 0.023 |
| Dietz | 0.72 (0.61; 0.79) | <0.001 | 21.6 | 17 | 61.4 | <0.001 | 5/25 | <0.001 | 10/59 | <0.001 | 5/11 | 0.134 | 11/27 | 0.009 |
| Maffeis | 0.62 (0.47; 0.72) | <0.001 | 17.6 | 26.1 | 56.2 | <0.001 | 5/25 | <0.001 | 12/57 | <0.001 | 4/12 | 0.046 | 6/32 | <0.001 |
| Molnar | 0.68 (0.56; 0.77) | <0.001 | 24.2 | 23.5 | 52.3 | <0.001 | 6/24 | <0.001 | 14/55 | <0.001 | 5/11 | 0.134 | 12/26 | 0.023 |
| Muller | 0.67 (0.55; 0.76) | <0.001 | 19 | 20.9 | 60.1 | <0.001 | 3/27 | <0.001 | 11/58 | <0.001 | 3/13 | 0.012 | 12/26 | 0.023 |
| Mifflin | 0.68 (0.57; 0.77) | <0.001 | 13.1 | 55.6 | 31.4 | <0.001 | 5/25 | <0.001 | 10/59 | <0.001 | 2/14 | 0.003 | 3/35 | <0.001 |
| Lazzer (equation 1) | 0.69 (0.58; 0.77) | <0.001 | 9.8 | 4.6 | 85.6 | <0.001 | 4/26 | <0.001 | 7/62 | <0.001 | 0/16 | - | 4/34 | <0.001 |
| Caldwell–Kennedy | 0.72 (0.61; 0.79) | <0.001 | 17 | 38.6 | 44.4 | <0.001 | 7/23 | <0.001 | 7/62 | <0.001 | 5/11 | 0.003 | 7/31 | <0.001 |
| White (equation 2) | 0.60 (0.46; 0.71) | <0.001 | 6.5 | 75.2 | 18.3 | <0.001 | 2/28 | <0.001 | 4/65 | <0.001 | 1/15 | <0.001 | 3/35 | <0.001 |
| Meyer (equation C) | 0.51 (0.32; 0.64) | <0.001 | 12.4 | 30.7 | 56.9 | <0.001 | 2/28 | <0.001 | 11/58 | <0.001 | 0/16 | - | 6/32 | <0.001 |
| RDA | 0.58 (0.42; 0.69) | <0.001 | 10.5 | 14.4 | 75.2 | <0.001 | 8/22 | 0.011 | 4/65 | <0.001 | 1/15 | <0.001 | 3/35 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briassoulis, G.; Briassouli, E.; Ilia, S.; Briassoulis, P. External Validation of Equations to Estimate Resting Energy Expenditure in Critically Ill Children and Adolescents with and without Malnutrition: A Cross-Sectional Study. Nutrients 2022, 14, 4149. https://doi.org/10.3390/nu14194149
Briassoulis G, Briassouli E, Ilia S, Briassoulis P. External Validation of Equations to Estimate Resting Energy Expenditure in Critically Ill Children and Adolescents with and without Malnutrition: A Cross-Sectional Study. Nutrients. 2022; 14(19):4149. https://doi.org/10.3390/nu14194149
Chicago/Turabian StyleBriassoulis, George, Efrossini Briassouli, Stavroula Ilia, and Panagiotis Briassoulis. 2022. "External Validation of Equations to Estimate Resting Energy Expenditure in Critically Ill Children and Adolescents with and without Malnutrition: A Cross-Sectional Study" Nutrients 14, no. 19: 4149. https://doi.org/10.3390/nu14194149
APA StyleBriassoulis, G., Briassouli, E., Ilia, S., & Briassoulis, P. (2022). External Validation of Equations to Estimate Resting Energy Expenditure in Critically Ill Children and Adolescents with and without Malnutrition: A Cross-Sectional Study. Nutrients, 14(19), 4149. https://doi.org/10.3390/nu14194149







