1. Introduction
Polycystic ovary syndrome (PCOS), one of the most common gynecological endocrinopathy, affecting approximately 8% to 18% women in the premenopausal period [
1]. According to the Rotterdam conference [
2], PCOS is mainly characterized by androgen excess and ovarian dysfunction (oligo-anovulation or polycystic ovarian morphology). The pathological mechanism of PCOS is generally associated with the hypothalamic–pituitary–ovarian axis [
3]. In PCOS, hypothalamic gonadotropin-releasing hormone (GnRH) pulses are activated, and the release of the luteinizing hormone (LH) is enhanced relative to the follicle stimulating hormone (FSH), which makes theca cells preferentially secrete more androgens [
4]. Hyperandrogenism affects follicular development via a complex mechanism, such as insulin resistance and dyslipidemia. It is difficult to distinguish the causal relationship between these pathological factors, as they jointly form a vicious cycle of aggravating PCOS [
5]. Additionally, various studies have indicated that systemic low-grade inflammation and oxidative stress are associated with the development of PCOS [
6,
7].
Despite the substantial burden PCOS has caused, no drug has been approved specifically for it by the Food and Drug Administration nor the European Medicines Agency [
8,
9]. Current medication treatments for PCOS, including letrozole, oral contraceptives, anti-androgens, and clomiphene are suboptimal. The side effects of these drugs, including clinical resistance and nausea, along with ovarian hyperstimulation syndrome, are prone to occur with long-term treatments [
10,
11,
12]. Non-invasive, symptom-oriented, and preventative treatments are sorely needed.
The first-line therapy for PCOS patients with mild symptoms is lifestyle modification [
13,
14]. Dietary intervention is a promising strategy for the treatment of PCOS that has been widely recognized [
15,
16]. Among the phytonutrients in dietary macronutrients, flavonoids have attracted considerable attention for their potential antioxidant and free-radical scavenging effects against metabolism and endocrine-related diseases [
17,
18]. The basic structure of flavonoids includes the common C6C3C6 skeleton and consists of two phenyl rings and one oxygenated heterocyclic ring (
Figure S1) [
19]. Based upon variations in the heterocyclic ring, flavonoids can be divided into anthocyanidins, flavanols (flavan-3-ols or catechins), flavanones, flavones, flavonols, and isoflavones [
20]. Accumulating studies suggest that the bioavailability of flavonoids is higher than previously thought [
21]. In recent years, clinical trials using specific flavonoids to manage PCOS have been carried out on a small scale. Previous studies explored the efficacy of soy isoflavones in PCOS patients. The results indicated that after the 12-week intervention of 50 mg/day soy isoflavones, markers of insulin resistance, hormonal status, lipid profiles, and oxidative stress were partly alleviated in women with PCOS [
22]. Another study assessed the therapeutic effects of puerarin in patients with PCOS. Compared with before the treatment, significantly improved levels of sex hormone binding globulin and superoxide dismutase were observed [
23]. A systematic review including five experimental studies and three clinical trials showed the beneficial effects of quercetin on ovarian histomorphology, hormone disorders, and dyslipidemia, while no significant effect was reported for weight loss [
24]. To date, there is still a lack of rigorous clinical trials to investigate the efficacy of flavonoids on PCOS patients. Among the published randomized controlled trials, a small number of biases related to health status, genetic background, or methodology have been observed [
24]. Reviews based on preclinical studies may assist in offering solid evidence and informing future experimental and clinical trials. Additionally, mice and rats are ideal animal models for PCOS because they are sensitive to hormone stimulation and possess a stable estrous cycle [
25].
Herein, we report on a systematic review and meta-analysis of data from studies testing the efficacy of flavonoids on animal models of PCOS. The changes in ovarian histomorphology and hormonal status were included as observation parameters. In addition, we evaluated whether the effects differ in terms of the type of flavonoid, dose, treatment duration, and PCOS induction drug by subgroup analysis.
2. Methods
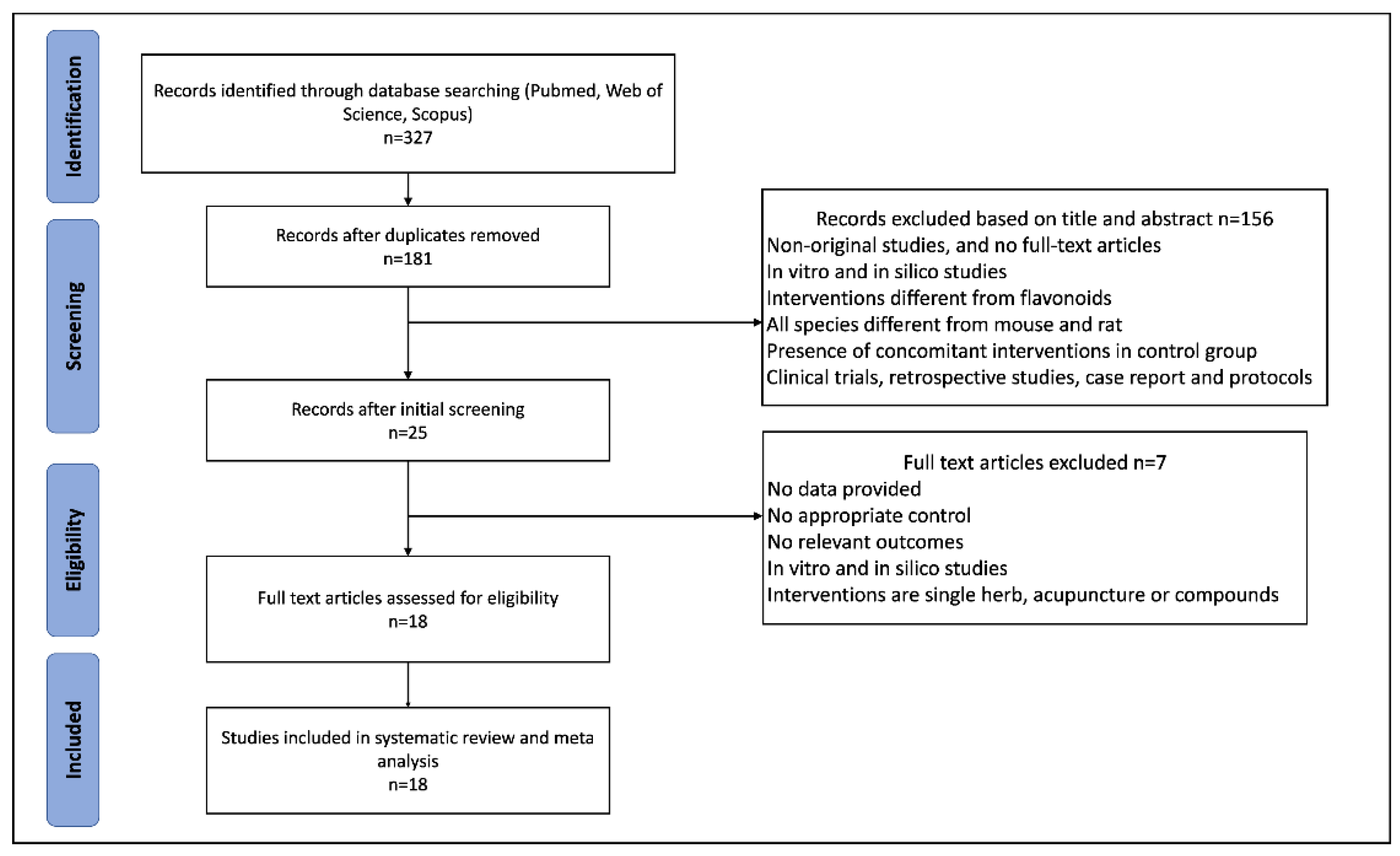
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [
26]. The protocol based on SYRCLE’s tool for animal studies [
27] was registered in PROSPERO (registration number: CRD42022328355).
2.1. Search Strategy
We conducted a systematic search of PubMed, Web of Science, and Scopus from inception to March 2022. The language of publications was limited to English. The specific search items included (“anthocyanidins” OR “flavanols” OR “flavan-3-ols” OR “catechins” OR “flavanones” OR “flavones” OR “flavonols” OR “isoflavones”) AND (“polycystic ovary syndrome” OR “polycystic ovarian syndrome” OR “PCOS”) AND (“mice” OR “mouse” OR “rat” OR “rats” OR “animal”). Additionally, a manual search was conducted to check the relevant publications by two authors.
2.2. Inclusion and Exclusion Criteria
Studies were considered eligible based on the following inclusion criteria: (1) The participants were animal models of PCOS; (2) the intervention drugs were flavonoids (pure flavonoids or flavonoids extracts) and the type of flavonoid and dose were clarified; (3) the comparison was the PCOS induction group with no treatment; (4) the outcomes included the effects of flavonoids on the development of PCOS, histomorphology, and hormonal alternations in animal models, and the original trials should report one or more following outcomes: the count of atretic follicles, the count of cystic follicles, the count of corpus luteum, LH, FSH, LH/FSH, free testosterone (FT), estradiol, and progesterone; and (5) studies used animal models and were published in English.
Two reviewers examined the titles and abstracts of retrieved studies. The exclusion criteria were as follows: (1) Non-original full research articles; (2) clinical trials, in vitro models, retrospective studies, case reports, and protocols; (3) interventions different from flavonoids or without precise dose and duration of administration; and (4) the presence of concomitant interventions in the PCOS group. Further, full texts were assessed by two reviewers, and publications without relevant outcomes were excluded.
2.3. Dara Extraction
Two reviewers independently assessed the extraction of data from selected literature. Any difference was resolved by discussion with the third reviewer. The following essential details were summarized as the baseline characteristics of the studies: (1) Publication details (author and year); (2) intervention performed (type of flavonoid, dose, route, and duration of administration); (3) PCOS induction drug and methods; (4) animal used (species, strain, age, and weight); (5) outcomes included.
All the data of outcome measures were continuous. We extracted data reporting the sample size per group (
N), mean values, and variance [standard deviation (SD) or standard error of mean (SEM)]. SEM was converted to SD by using the formula (
). When treatment was administrated in multiple doses, the group using the highest dose was recorded [
28]. In case the outcomes were only presented graphically, the reviewers used the ImageJ software to quantify the results.
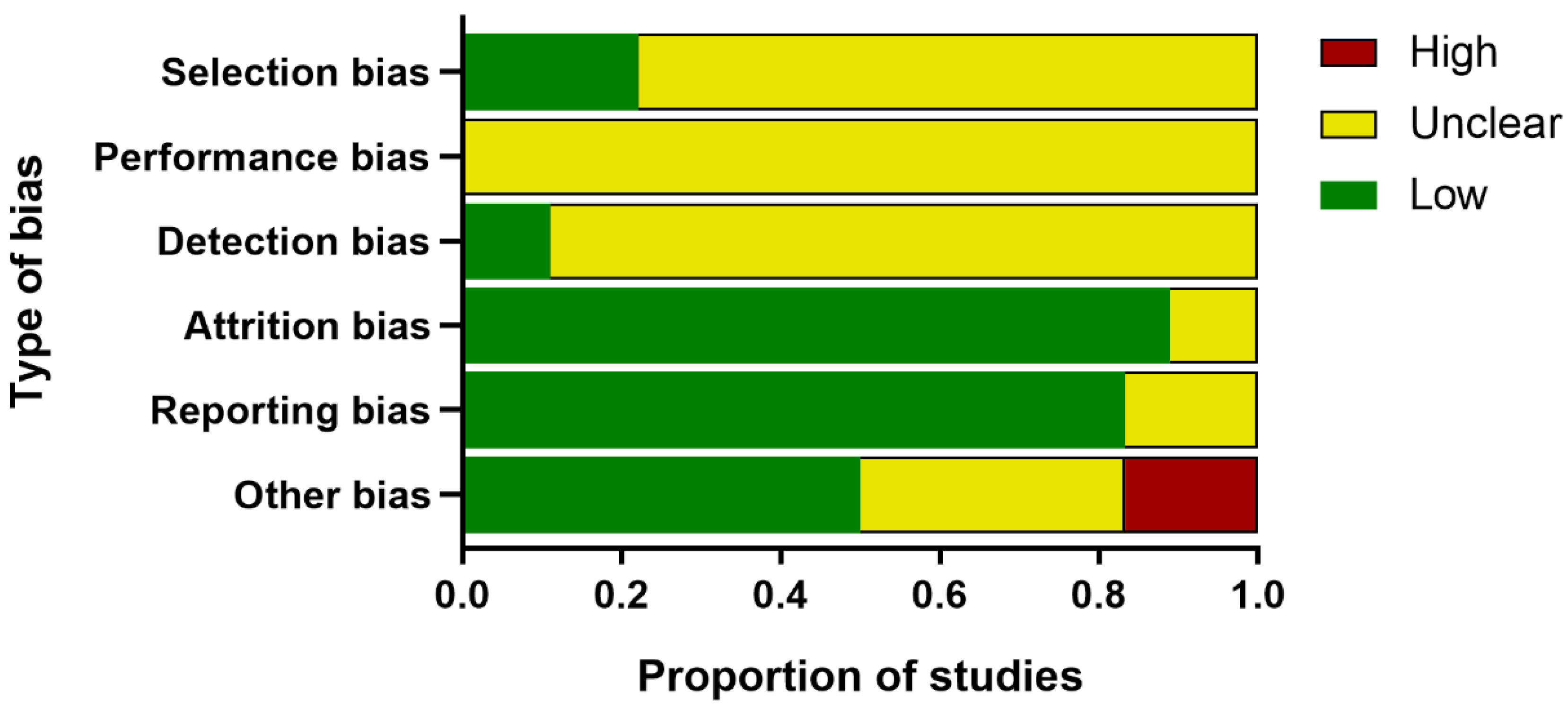
2.4. Quality Assessment
Two reviewers independently assessed the internal validity of the included publications, referencing the SYCLE’s risk of bias tool for animal experiments [
27]. This six-part checklist of evaluation included: (1) Selection bias (sequence generation, baseline characteristics, and allocation concealment); (2) performance bias (random housing and blinding of trial caregivers); (3) detection bias (random outcome assessment and blinding of outcome assessors); (4) attrition bias (incomplete outcome data); (5) reporting bias (selective outcome reporting); and (6) other bias (assessment of PCOS model, temperature control, drug production institutions, conflict of interest, et al.). Any discrepancy was discussed with the third reviewer.
2.5. Statistical Analysis
R software (V4.1.3) was adopted for data analysis and visualization (package meta and dmetar). All the outcome measures were continuous, and the standardized mean difference (SMD) was calculated with 95% confidence intervals (CIs) as the overall effects. A random-effect model was performed. Heterogeneity was assessed by the Q statistic and quantified using the
results [
29].
was considered statistically significant. When
, subgroup analyses were conducted to explore the sources of heterogeneity. The type of flavonoid, dose, duration of administration, and PCOS induction drug were considered as the potential subgroup basis. Sensitivity analyses were performed to confirm the robustness of the results by removing one study and repeating the meta-analysis. Publication bias was assessed with the trim-and-fill method, and the Egger’s bias test was performed if the results contained at least ten studies.
4. Discussion
Flavonoids are widely distributed plant secondary metabolites which are found in various fruit, vegetables, and herbal medicines. They play important roles in human health through the consumption of plant-derived foods by scavenging free radicals and inhibiting metal-ion chelators for their powerful antioxidant properties [
48]. Flavonoids are also associated with the modulation of the immune system, despite that they represent ancillary ingredients with immunomodulatory properties that require more evidence [
49]. Nevertheless, accumulating evidence demonstrates that flavonoids can inhibit regulatory enzymes and transcription factors involved in inflammation [
50,
51]. On account of their diverse bioavailability, flavonoids have been applied to prevent degenerative diseases, such as diabetes, cardiovascular complications, cancer, and hypoglycemia [
52,
53]. Additionally, significant applications of flavonoids have been unveiled in reproductive endocrine diseases, such as menopausal syndrome and endometriosis [
54,
55]. PCOS is an inflammatory, systematic, and autoimmune endocrinopathy [
56]. In PCOS patients, systematic low-grade inflammation compromises multiple aspects of fertility and is associated with hyperandrogenism and insulin resistance [
57]. Therefore, we speculate that flavonoids can ameliorate symptoms of PCOS. Our results demonstrate the efficacy of flavonoids on PCOS animal models.
The ovarian follicles of PCOS patients are manifested with a thickened theca cell layer and cyst formation. Most studies purport that histological changes such as follicular atresia are the cause of PCOS infertility [
58]. Inconsistent with previous PCOS-related meta-analysis which were based on clinical trials, histopathological changes were taken as the primary outcomes in our study. The convenient collection and observation of ovarian tissue is a major advantage of preclinical studies over clinical trials. Although the results of histomorphology were statistically positive, substantial heterogeneity was detected regarding the count of cystic follicles and the count of corpus luteum. Results from the subgroup analyses suggest that only one study which used soy isoflavone did not show a statistic reduction of the count of cystic follicles. In this study, the treatments were divided into three groups: the soy isoflavone group, the resistant starch group, and the soy isoflavone combined with resistant starch group, which individually showed a significant reduction in cyst formation [
31]. We only included the soy isoflavone group as the intervention method, which may have led to the heterogeneity. Similar results were obtained in measurements of the count of corpus luteum. In the letrozole-induced rats, a larger number of cystic follicles and a smaller number of corpus luteum were observed. Abnormalities in terms of follicle development occurred not only in the later, antral stages of follicles which are gonadotrophin dependent but also in the very earliest stages of folliculogenesis [
59]. The changes were related to LH and FSH disorders and a lack of interplay between granulosa cells [
60]. Soy isoflavones modulated hormone levels by binding to estrogen receptors, and the property may be enhanced by butyric acid, which was elevated by resistant starch intake [
61]. Additionally, the studies which applied TE and TP as PCOS induction drugs did not show significant improvements in terms of the corpus luteum count. A systematic review of PCOS animal models demonstrated that hormonal interventions using androgens promote the most consistent features of PCOS morphological phenotypes [
62]. Contrary to our expectations, the dose and duration of treatments were irrelevant to the efficacy of flavonoids, which revealed that the morphological changes of PCOS models are relatively fixed.
In addition, most of the included studies indicated a role for flavonoids in modulating hormonal status. The increased levels of LH/FSH and testosterone were due to the impaired hypothalamic–pituitary axis [
63]. LH is a central actor in theca cell dysregulation, which follows ovarian hyperandrogenism. Previous studies demonstrated that estrogen stimulated by LH is beneficial to the maturation of oocyte cytoplasm and membrane, which revealed the importance of LH [
64]. Additionally, in the middle and later stage of follicle development, granulosa cells begin to express luteinizing hormone receptor (LHCGR), and LH reaches the peak [
65]. Meanwhile, LH and FSH cooperate to stimulate ovulation and promote granulosa cell luteinization [
66]. Meanwhile, in PCOS patients, the expression of LHCGR is premature in granulosa cells [
67]. Continuous estrogen enhances the sensitivity of the pituitary gland to GnRH secreted by the hypothalamus, which increases the frequency and amplitude of GnRH pulse secretion and increases the level of LH. Because the negative feedback of hormones on FSH is greater than that on LH, the ratio of LH to FSH is higher [
68,
69]. Our meta-analysis revealed the downregulation of LH, LH/FSH, and FT with the administration of flavonoids. The dose, duration of administration, and PCOS induction drug were the main sources of heterogeneity in LH reduction. As we expected, with higher dose of flavonoids, there were lower levels of LH. Only the studies which applied letrozole as the induction drug did not show significant a reduction in LH. As for FT, the effects were not statistically different in studies using baicalin or rutin as the treatments. However, we did not find the sources of heterogeneity in the LH/FSH studies. Considering each study separately, we found the studies which used a medium dose showed better a reduction in terms of LH/FSH than those which used a low dose.
Among the studies included, PCOS induction drugs were divided into androgens (TP, TE, and DHEA), estrogens, aromatase inhibitors (letrozole), and insulin combined with hCG. Androgen induction may promote continuous high blood-free testosterone and a pathologic elevation in FSH that induces cystic formation [
70,
71]. High estrogen stimulation leads to the degeneration of hypothalamic neurons and the compensatory hyperplasia of the pituitary gland, which increases the sensitivity of the pituitary gland to GnRH. The level of LH increases and the secretion of FSH is inhibited, resulting in the typical characteristics of PCOS [
72]. Letrozole, as a nonsteroidal aromatase inhibitor, restrains the conversion of androgen to estrogen, leading to androgen accumulation [
73]. Insulin combined with hCG may destroy the normal pulse secretion mode of endogenous LH and is characterized by hyperandrogenism and insulin resistance [
74]. As PCOS is a highly heterogeneous disease, a model that fully simulates the characteristics of PCOS does not exist. The appropriate modeling methods should be applied according to the aim of the study.
To date, there have been only a small number of meta-analyses of PCOS in animal models. This might be explained by doubts about the substantial heterogeneity caused by the diversity of modeling methods and applied drugs. In order to better take advantage of the systematic review of preclinical studies and explain the changes induced by flavonoids on different parameters of PCOS, it is important to consider the molecular mechanisms by which flavonoids may produce effects. The literature reports that soy isoflavones were demonstrated to enhance the antioxidant capacity of rats and inhibit the activation of the nuclear factor-kappa beta (NF-κB) signaling pathway, hence reducing inflammatory cytokines [
32]. Similar results were reported in studies using catechins as treatments, with NF-κB-mediated inflammation and matrix metallopeptidase 2 and matrix metallopeptidase 9-mediated damage being ameliorated. Additionally, the signal transducer and activator of transcription 3 signaling was inhibited [
35]. Quercetin, a flavonol, was reported to decrease the expression of the CYP17A1 gene by inhibiting phosphatidylinositol 3-kinase (PI3K), leading to the regulation of ovarian steroidogenesis [
37]. Furthermore, another study suggested that quercetin has an apoptosis-inhibiting effect through increasing B-cell lymphoma-2 (Bcl2) and decreasing the Bcl2-Associated X (BAX) to Bcl2 ratio [
38]. The ability to restore the maturation of oocyte and regulate energy homeostasis was emphasized after the administration of quercetin [
39,
40]. The literature reports that the upregulation of adenosine 5′-monophosphate-activated kinase and activation of PI3K signaling contributed to the beneficial effect of baicalin on PCOS [
42]. Another publication revealed that GATA1 is one of the key genes affected by baicalin. PCOS models reversed the hyperandrogenic status after baicalin treatments [
43]. Rutin has metformin-like properties which play an important role in reducing reactive oxygen species and boosting the antioxidant status [
44]. The potential molecular mechanisms of various flavonoid effects on PCOS are illustrated in
Figure S13.
Our study also has many limitations. First, the language of publications was limited in English, so databases in other languages were excluded. The gray literature and negative results were also relatively lacking. Second, few studies measured pregnancy outcomes, which means the effect of flavonoids on infertility in animal models could not be assessed. Furthermore, metabolic disorders and insulin resistance were not evaluated. Third, although some aspects of heterogeneity were explained by different experimental designs, the remaining heterogeneity and publication bias should be valued. Finally, considering the great difference between species, the ovulation characteristics of humans and rodents should be considered. When our results are referenced by clinical protocols, the standard dose conversion should be conducted.