Abstract
Helicobacter pylori (H. pylori) is the most prevalent etiology of gastritis worldwide. H. pylori management depends mainly on antibiotics, especially the triple therapy formed of clarithromycin, amoxicillin, and proton pump inhibitors. Lately, many antibiotic-resistant strains have emerged, leading to a decrease in the eradication rates of H. pylori. Polaprezinc (PZN), a mucosal protective zinc-L-carnosine complex, may be a non-antibiotic agent to treat H. pylori without the risk of resistance. We performed a systematic review and meta-analysis to evaluate the efficacy and safety of a PZN-based regimen for the eradication of H. pylori. This study used a systematic review and meta-analysis synthesizing randomized controlled trials (RCTs) from WOS, SCOPUS, EMBASE, PubMed, and Google Scholar until 25 July 2022. We used the odds ratio (OR) for dichotomous outcomes presented with the corresponding 95% confidence interval (CI). We registered our protocol in PROSPERO with ID: CRD42022349231. We included 3 trials with a total of 396 participants who were randomized to either PZN plus triple therapy (n = 199) or triple therapy alone (control) (n = 197). Pooled OR found a statistical difference favoring the PZN arm in the intention to treat and per protocol H. pylori eradication rates (OR: 2.01 with 95% CI [1.27, 3.21], p = 0.003) and (OR: 2.65 with 95% CI [1.55, 4.54], p = 0.0004), respectively. We found no statistical difference between the two groups regarding the total adverse events (OR: 1.06 with 95% CI [0.55, 2.06], p = 0.85). PZN, when added to the triple therapy, yielded a better effect concerning the eradication rates of H. pylori with no difference in adverse event rates, and thus can be considered a valuable adjuvant for the management of H. pylori. However, the evidence is still scarce, and larger trials are needed to confirm or refute our findings.
1. Introduction
Helicobacter pylori (H. pylori), a virulent Gram-negative organism infecting mainly the human gastric mucosa, afflicted nearly 4.4 billion of the world’s population in 2015 [1]. Chronic infection with H. pylori can lead to the emergence of some serious alimentary complications, such as chronic gastritis, irritable bowel syndrome, peptic ulcer, and gastric cancer, the third most prevalent etiology of cancer-associated mortality around the world, ending the lives of over 850,000 humans every year [2,3,4,5]. In particular, H. pylori infection is associated with multiple possible etiologies of irritable bowel syndrome, including post-infectious responsiveness, inflammation, and alteration of the gut microorganisms [3,6,7]. Accepted extra-gastric manifestations of H. pylori infection are iron deficiency anemia, immune thrombocytopenic purpura, and vitamin B12 deficiency [8]. Moreover, it may increase the risk of acute coronary syndrome [9,10], cerebrovascular disease [11], and neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease [12,13]. The eradication of H. pylori plays a key role in decreasing the incidence of these complications.
The current frontline recommended regimen includes typical triple therapy (proton pump inhibitor (PPI), clarithromycin, and amoxicillin or metronidazole) or bismuth-based quadruple therapy (PPI or H2 receptor antagonists, metronidazole, tetracycline, and bismuth) and other antibiotic-based options [14,15,16]. With the global development of antibiotic resistance, the diminished efficacy of clarithromycin, metronidazole, and levofloxacin is reaching an alarming level of 15% [17,18,19].
Therefore, we need to widen our scope, find new innovative solutions, and decrease our dependence on antibiotics. Variable gastric mucosal protective agents have been proposed to help in peptic ulcer healing and in the eradication of H. pylori, such as rebamipide [20], sofalcone [21], and sucralfate [22], which have the advantage of being unaffected by drug resistance.
Moreover, polaprezinc (PZN), a zinc-L-carnosine complex (Figure 1) [23], has promising properties as an antioxidant promoting ulcer healing and mucosal protective agent to counteract various clinical conditions in animals and human studies [24,25,26,27,28,29,30,31,32]. Furthermore, PZN can function by ameliorating inflammation [33], preventing apoptosis [34], and protecting tight junctions. Additionally, PZN was reported to decrease the indomethacin-induced increase in the gut permeability [35,36], indicating a small bowel protective effect [37,38]. Vascularly, PZN was reported to activate the mesenchymal stem cells and increase the expression of insulin-like growth factor in endothelial tissue protecting the injured gastric and skin lesions [39,40]. Accordingly, PZN is a promising agent that can be implemented within the H. pylori treatment protocol; however, strong synthesized evidence is still lacking. Hence, our study’s goal is to assess the effectiveness and safety of PZN as a supportive agent to triple therapy (PPI + clarithromycin + amoxicillin) for the management of patients with H. pylori infection.
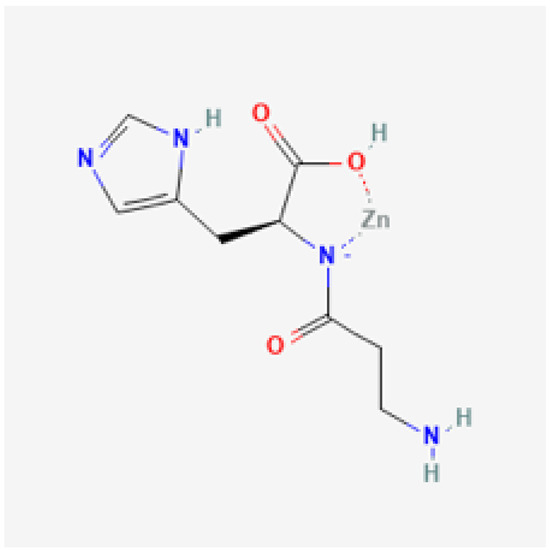
Figure 1.
Chemical structure of polaprezinc. Courtesy of the U.S. National Library of Medicine [23].
2. Materials and Methods
2.1. Protocol Registration
Our review was prospectively registered and published in an international prospective register of health-related systematic reviews (PROSPERO) with ID: CRD42022349231. We performed a systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [41,42,43] and the Cochrane Handbook of Systematic reviews and meta-analysis [44]. The process is documented in a PRISMA 2020 checklist (Appendix A).
2.2. Data Sources and Search Strategy
Web of Science, SCOPUS, EMBASE, PubMed (MEDLINE), Google Scholar, and Cochrane Central Register of Controlled Trials (CENTRAL) were comprehensively searched by two reviewers (A.M. and M.A.) until 25 July 2022. We used no filters. The thorough selection procedure is illustrated in (Table 1).

Table 1.
Search terms and results in different databases.
2.3. Eligibility Criteria
We included randomized controlled trials (RCTs) with the following PICO criteria: population (P): patients with H. pylori infection; intervention (I): PZN 150 mg plus triple therapy (amoxicillin, clarithromycin, and PPI), control (C) triple therapy only and outcome (O): the primary outcome of this study is to evaluate the eradication rate of H. pylori (patients who achieved H. pylori clearance) according to intention to treat or per protocol analysis. The secondary outcome is the safety, defined as any reported adverse events. The exclusion criteria involved animal studies, cohort, retrospective, case reports, case reports, non-randomized trials, laboratory studies, and conference abstracts.
2.4. Study Selection
After duplicates removal using the Covidence online tool [45], two investigators (A.M. and H.A.) independently checked the eligibility of titles and abstracts of the obtained records. Then, they evaluated the full texts of the relevant studies according to the previously mentioned eligibility criteria. Any discrepancies were solved via discussion to reach a consensus.
2.5. Data Extraction
Using a pilot-tested extraction form, two reviewers (A.A.S.A. and H.A.) separately extracted the following data from the included articles: study characteristics (year of publication, country, study design, total participants, used triple therapy, frequency, and dose of PZN and method by which H. pylori was diagnosed); baseline information (age, sex, number of patients in each group, and number and location of ulcers); and efficacy outcomes data (intention-to-treat H. pylori eradication rate, per-protocol H. pylori eradication rate, and adverse events including (nausea, vomiting, heartburn, diarrhea, skin rash, and total adverse events). Disagreements were resolved by another investigator (A.M.).
2.6. Risk of Bias and Quality Assessment
The Cochrane Collaboration’s technique was our guide to evaluate the risk of bias in randomized trials; two reviewers (A.A.S.A. and H.A.) separately evaluated the included studies for risk of bias (ROB) [46], based on the following six items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias. Disagreements were settled through discussion. Two reviewers (M.T. and B.A.) employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines to appraise the quality of the evidence [47,48,49]. Imprecision, indirectness, inconsistency, publication bias, and bias risk were evaluated. Our results about the quality of evidence were justified, written, and included in each outcome. Any discrepancies were handled through discussion.
2.7. Statistical Analysis
The statistical analysis was carried out with Revman software version 5.4 [50]. We used odds ratio to pool dichotomous outcomes presented with the corresponding 95% confidence interval (CI). We utilized the I-square and Chi-square tests to assess heterogeneity; while the Chi-square test tells whether there is heterogeneity, the I-square determines the depth of heterogeneity. A grand heterogeneity (for the Chi-square test) is named as an alpha level below 0.1, in accordance with the Cochrane Handbook (chapter nine) [46], while the I-square test is interpreted as: (0–40 percent: not significant; 30–60 percent: moderate heterogeneity; 50–90 percent: substantial heterogeneity). We used the fixed-effects model. We calculated the number needed to treat (NNT) via the next equation, Absolute risk reduction (ARR) = (control event rate) − (experimental event rate) and the NNT equals the inverse of the ARR.
3. Results
3.1. Search Results and Study Selection
We identified 1496 records after searching the databases, then 635 duplicates were excluded. Title and abstract screening excluded 841 irrelevant records. We moved to full-text screening with 20 articles, and 17 articles were excluded. Finally, only three articles met our inclusion criteria. The PRISMA flow chart of the detailed selection process is demonstrated in (Figure 2).
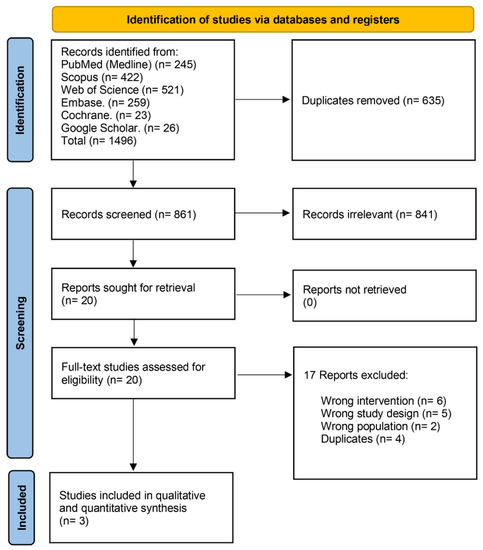
Figure 2.
PRISMA flow chart of the screening process.
3.2. Characteristics of Included Studies
We included 3 trials with a total of 396 participants who were randomized to either PZN plus triple therapy (n = 199) or triple therapy alone (control) (n = 197). Further included trials’ characteristics are presented in (Table 2). PZN dose was 150 mg twice daily for seven days in two trials [26,27] and for fourteen days in one trial [28]. Male participants were a total of 122 (61.3%) in the PZN group and 124 (62.94%) in the control group. Further baseline characteristics of the participants are presented in (Table 3).

Table 2.
Characteristics of the included studies.

Table 3.
Baseline characteristics of the participants.
3.3. Risk of Bias and Quality of Evidence
We appraised the quality of the included studies according to the Cochrane risk of bias tool [46], as shown in Figure 3. Regarding the selection bias, Isomoto et al. [26] had low risk in the random sequence generation and unclear risk in the allocation concealment, Kashimura et al. [27] had unclear risk in both domains, and Tan et al. [28] had low risk in both domains. Moreover, the included trials had a high risk of performance and detection biases, except Kashimura et al. [27], with a low risk of performance and detection biases. Additionally, the included trials had a low risk of attrition bias. Furthermore, all included trials had an unclear risk of reporting bias. Finally, the included trials had a low risk of other bias. Author judgments are furtherly clarified in the Appendix (Appendix B). Using the GRADE system, the included primary outcomes yielded very-low-quality evidence. Details and explanations are clarified in Table 4.
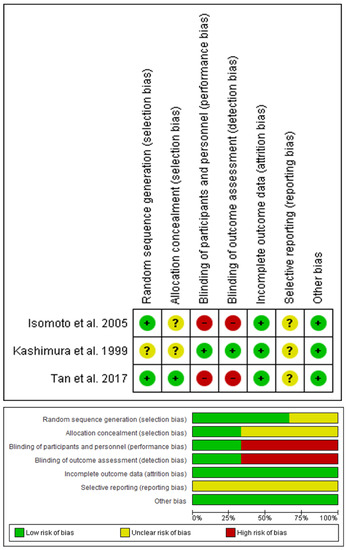
Figure 3.
Quality assessment of risk of bias in the studies in the meta-analysis. The upper panel presents a schematic representation of risks (low = red, unclear = yellow, and high = red) for specific types of biases of each of the studies in the review. The lower panel presents risks (low = red, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review [50].

Table 4.
GRADE evidence profile.
3.4. Primary Outcomes
3.4.1. H. pylori Eradication Rates Based on Intention to Treat Analysis
The pooled analysis favored the PZN group (OR: 2.01 with 95% CI [1.27, 3.21], p = 0.003) (very-low-quality evidence) (Figure 4A, Table 4). The pooled studies were homogenous (p = 0.27, I-square = 24%). From our calculation of the NNT on average, 7.5 patients would have to receive PZN treatment (instead of control treatment) for one additional patient to have the outcome, ARR = 0.67 − 0.804 = − 0.134. NNT = 1/ARR = 1/− 0.134 = −7.5.
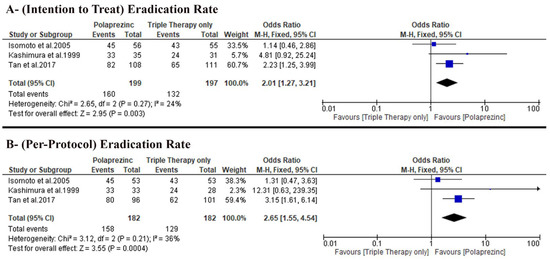
Figure 4.
Forest plot of the primary outcome (A) H. pylori eradication rates based on intention to treat analysis, (B) H. pylori eradication rate based on per protocol analysis [50]. OR: odds ratio, CI: confidence interval.
3.4.2. H. pylori Eradication Rates Based on per Protocol Analysis
The pooled analysis favored the PZN group (OR: 2.65 with 95% CI [1.55, 4.54], p = 0.0004) (very-low-quality evidence) (Figure 4B, Table 4). The pooled studies were homogenous (p = 0.21, I-square = 36%). On average, 6.3 patients would have to receive PZN treatment (instead of control treatment) for one additional patient to have the outcome, ARR = 0.70.88 − 0.86.81 = −0.1593. NNT = 1/ARR = 1/−0.1593 = −6.3.
3.5. Secondary Outcomes
3.5.1. Total Patients with Adverse Events
We found no difference between the two groups (OR: 1.06 with 95% CI [0.55, 2.06], p = 0.85) under the fixed-effects model (very-low-quality evidence). The pooled studies were homogenous (p = 0.35, I-square = 5%) (Figure 5A).
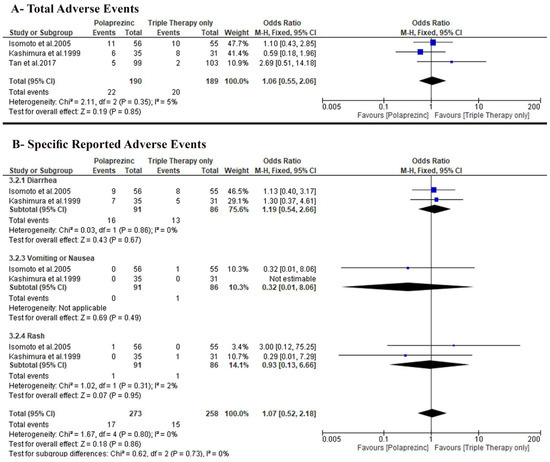
Figure 5.
Forest plot of the secondary outcomes (A) total adverse events, (B) specific reported adverse events [50]. OR: odds ratio, CI: confidence interval.
3.5.2. Specific Adverse Events
Only two trials, Isomoto et al. [26] and Kashimura et al. [27], reported specific adverse events incidence, and we found no difference between the two groups regarding the incidence of diarrhea (OR: 1.19 with 95% CI [0.54, 2.66], p = 0.67), vomiting or nausea (OR: 0.32 with 95% CI [0.01, 8.06], p = 0.49), and rash (OR: 0.93 with 95% CI [0.13, 6.66], p = 0.95) (Figure 5B).
4. Discussion
H. pylori infection and colonization of the human gastric mucosa are prevalent in over 50% of the world’s population [51]. Although most cases are asymptomatic, H. pylori can lead to significant complications, including peptic ulcer disease, gastric adenocarcinoma, and mucousa-associated lymphoma [52,53] Specifically, the incidence of peptic ulcer disease is about 10 to 20% of H. pylori patients with about 1 to 3% cases complicated by gastric cancer [4]. Accordingly, the burden of H. pylori is overwhelming, and an effective H. pylori eradication strategy is required. Therefore, we evaluated the efficacy and safety of PZN as an adjuvant muco-protective agent in adjuvant with the standard triple therapy to eradicate H. pylori.
Regarding the H. pylori eradication rate, our pooled analysis favored PZN over triple therapy alone in both ITT analysis (80.4% versus 67.01%) and per-protocol analysis (86.8% versus 70.9%), respectively. Moreover, the incidence of adverse events was similar in both groups.
The specific mechanism of the PZN role in enhancing the eradication of H. pylori is still to be investigated, with several proposed theories: first, zinc can inhibit the urease activity leading to H. pylori’s growth retardation by replacing the nickel ions at the active site of urease hindering the two metal ions from the complex formation [54]. Second, zinc can decrease the expression of interleukin 1 beta (IL-1β) by the gastric mucosa, further inhibiting H. pylori growth [55]. Third, PZN has shown to scavenge the monochloramine in H. pylori-infected Mongolian gerbils [25]. Finally, zinc has been shown to form a complex with famotidine inhibiting the urease enzyme and, subsequently, H. pylori growth, which was evident in both the antibiotic-resistant and sensitive strains [56]
Recently, in comparative transcriptome analysis, Fan et al. proposed multiple potential anti-H. pylori effects of zinc [57]. First, zinc can alter the composition, structure, and function of the H. pylori type IV secretion system by the downregulation of cagI gene; hence, zinc can partially block the pathogenicity of H. pylori. Second, zinc can alter the synthesis process of lipopolysaccharide (LPS), a significant virulent factor of H. pylori, by altering the biosynthesis of lipid A (a significant hydrophobic part of LPS). H. pylori’s surface LPS is a significant part of its cell wall contributing to the adhesion and infection of the gastric mucosa [57,58]. Therefore, disrupting LPS synthesis can subsequently affect the infectivity and adaptability of H. pylori [57]. Third, zinc upregulated the H. pylori translation and transcription genes, subsequently leading to increased protein biosynthesis, which can be an adaptation mechanism of H. pylori; however, Fan et al. argue that the synthesis of large amounts of in vivo proteins without the help of enough chaperones can lead to accumulation of mis- and unfolded proteins, subsequently disturbing the proteostasis and hindering H. pylori growth and even cell death [57]. Finally, zinc disrupted the flagellar protein assembly, disrupting H. pylori cell motility [57].
Regarding the status of high antimicrobial agents’ resistance, implementing PZN into H. pylori can be beneficial. To clarify, the H. pylori resistance to clarithromycin and metronidazole is currently reported to be ≥ 15% [18,19], leading to a significant drop in the H. pylori eradication rates of triple therapy between 50% and 70% [18,19], which is significantly lower than the recommended ITT Maastricht H. pylori eradication rate of >80% [15]. Accordingly, PZN regimen can be effectively used for H. pylori with an ITT H. pylori eradication rate of 80.4%. Moreover, in a recent RCT, PZN was adjunctly used with the bismuth quadruple therapy achieving an H. pylori eradication rate of 93.5%, which was statistically significant in comparison with the triple therapy [24].
Regarding safety, PZN was safe and well tolerable in comparison with the triple therapy. The typical PZN dose is 150 mg, containing 34 mg zinc and 116 mg L-carnosine [59]. All the included trials used the typical dose with no crucial adverse events, and the reported adverse events were minor and faded spontaneously or managed feasibly [26,27,28]. However, Tan et al. observed more adverse events associated with the high-dose PZN (300 mg); they attributed this effect to either the toxic effect of the high dosage or patients’ self-hypersensitivity [28]. Accordingly, the standard dose of PZN (150 mg) can be used safely with triple therapy.
4.1. Strengths
To the best of our awareness, this is the first systematic review and meta-analysis synthesizing evidence on the efficacy and safety of PZN for H. pylori eradication; hence, this study constitutes gold standard evidence in this regard. Moreover, our review was executed and fulfilled via the guidance of the PRISMA recommendations [42,43].
4.2. Limitations
Our review has a few limitations. First, we only included three RCTs with a small sample size and limited population distribution confined to the Far East [26,27,28]. Second, the proton pump inhibitor component of the triple therapy varied across the included trials; hence, this can affect our findings. Third, multiple confounding variables can significantly affect our findings, including smoking habits, genetic predisposition of cytochrome p450 2C19, the physical status of the participants, and H. pylori strain resistance. Fourth, all the included trials had a relatively short follow-up duration ranging from one to two months only [26,27,28]. Finally, the GRADE assessment yielded very-low-quality evidence; hence, the extrapolation and the generalization of our findings is limited.
4.3. Implications for Future Research
Future trials are required to address: first, the comparative efficacy of PZN adjunctly with the bismuth quadruple therapy versus the bismuth quadruple therapy alone is still to be investigated. To clarify, bismuth quadrable therapy is currently recommended as the first-line regimen in areas with a significant prevalence of ciprofloxacin and metronidazole resistance. As such, investigating the efficacy of PZN in the settings with significant resistance is still required [60]. Second, future trials should determine the baseline clarithromycin resistance to enable health authorities to predict the H. pylori eradication rate of PZN-based regimen in areas with known rates of clarithromycin resistance using the H. pylori-nomogram [28,61]. Finally, future trials should expand the follow-up duration up to 6 or 12 months to properly investigate the improvement in the gastrointestinal symptoms [28].
5. Conclusions
The addition of PZN to the triple therapy yielded greater eradication rates of H. pylori with no difference in adverse event rates and thus constitutes a valuable adjuvant for the management of H. pylori. However, the evidence is still scarce, and larger trials are needed to confirm or refute our findings. As such more high-quality, multicenter randomized controlled trials are warranted to ascertain its efficacy and yield generalizable findings.
Author Contributions
Conceptualization, A.M.; methodology, A.M., M.A., B.A. and A.A.S.A.; software, A.M., M.A. and B.A.; validation, A.M., M.A., B.A., H.A., A.A.S.A. and J.R.B.; formal analysis, M.A. and B.A.; investigation A.A.S.A. and H.A.; data curation, A.A.S.A. and H.A.; writing—original draft preparation, M.A. and A.M.; writing—review and editing, A.M., M.A., B.A., H.A., A.A.S.A. and J.R.B.; supervision, B.A. and J.R.B.; project administration, B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the manuscript.
Acknowledgments
The authors thank Welch Medical Library of the Johns Hopkins University School of Medicine, Baltimore, MD, USA, for providing access to publications.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CI | Confidence interval, the lower and upper limits of significance |
| TT | Triple therapy |
| H. pylori | Helicobacter pylori |
| MD | Mean difference |
| N/A | Not available |
| p | Probability |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses [42,43] |
| PZN | Polaprezinc |
| RCT | Randomized controlled trial |
| SD | Standard deviation |
| WOS | Web of Science |
Appendix A

Table A1.
PRISMA 2020 Checklist.
Table A1.
PRISMA 2020 Checklist.
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Page 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Page 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Page 2 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | page 2 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 3 Section 2.3 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Page 3 Section 2.2 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Page 2, 3, Table 1 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Page 3 Section 2.4 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Page 3 Section 2.4 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Page 3 Section 2.5 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Page 3 Section 2.5 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Page 3 Section 2.6 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Page 4 Section 2.7 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Page 4 Section 2.7 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Page 4 Section 2.7 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Page 4 Section 2.7 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Page 4 Section 2.7 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | Not applicable | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | Not applicable | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | Page 3 Section 2.6 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Page 4 Section 2.7 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 4, Section 3.1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Not applicable | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Page 5, Section 3.2 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Page 5, Section 3.3 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Pages 9, 10 Section 3.4 and 3.5 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | Page 5 Section 3.2 and 3.3 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Pages 9, 10 Section 3.4 and 3.5 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | Not applicable | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Not applicable | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Page 5, Section 3.3 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Page 9, 10 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Page 10, 11 |
| 23b | Discuss any limitations of the evidence included in the review. | Page 12 | |
| 23c | Discuss any limitations of the review processes used. | Page 12 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Page 12 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Page 2 Section 2.1 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Page 2 Section 2.1 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Page 2 Section 2.1 | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Page 12 |
| Competing interests | 26 | Declare any competing interests of review authors. | Page 12 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Page 12 |
Appendix B

Table A2.
Author Judgment for ROB Assessment.
Table A2.
Author Judgment for ROB Assessment.
| Study ID | Domain | Judgment |
|---|---|---|
| Isomoto et al. [26] 2005 | Allocation concealment (selection bias) | Unclear risk “no enough information’’ |
| Blinding of participants and personnel (performance bias) | High risk “The present study was open label trial” | |
| Blinding of outcome assessment (detection bias) | High Risk “The study was open-label” | |
| Selective reporting (reporting bias) | Unclear risk “no protocol was able to be retrieved” | |
| Kashimura et al. [27] 1999 | Random sequence generation (selection bias) | Unclear risk “did not mention the method of randomization” |
| Allocation concealment (selection bias) | Unclear risk “did not mention the method of allocation” | |
| Selective reporting (reporting bias) | Unclear risk “no protocol was able to be retrieved” | |
| Tan et al. [28] 2017 | Blinding of participants and personnel (performance bias) | High risk “This was an open-label clinical study.” |
| Blinding of outcome assessment (detection bias) | High risk “This was an open-label clinical study.” |
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong., V.W.S.; Wu., J.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Peng, Y.; Peng, L. Helicobacter pylori Infection—A risk factor for irritable bowel syndrome? An updated systematic review and meta-analysis. Medicina 2022, 58, 1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, H.; Graham, D.Y. An update on Helicobacter pylori as the cause of gastric cancer. Gastrointest. Tumors 2014, 1, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef]
- Graham, D.Y.; Yamaoka, Y. H. pylori and cagA: Relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter 1998, 3, 145–151. [Google Scholar] [CrossRef]
- Tsay, F.-W.; Hsu, P.-I. H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 65. [Google Scholar] [CrossRef]
- Fang, Y.; Fan, C.; Xie, H. Effect of Helicobacter pylori infection on the risk of acute coronary syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e18348. [Google Scholar] [CrossRef]
- Szwed, P.; Gąsecka, A.; Zawadka, M.; Eyileten, C.; Postuła, M.; Mazurek, T.; Szarpak, Ł.; Filipiak, K.J. Infections as novel risk factors of atherosclerotic cardiovascular diseases: Pathophysiological links and therapeutic implications. J. Clin. Med. 2021, 10, 2539. [Google Scholar] [CrossRef]
- Doheim, M.F.; Altaweel, A.A.; Elgendy, M.G.; Elshanbary, A.A.; Dibas, M.; Ali, A.; Dahy, T.M.; Sharaf, A.K.; Hassan, A.E. Association between Helicobacter pylori infection and stroke: A meta-analysis of 273, 135 patients. J. Neurol. 2021, 268, 3238–3248. [Google Scholar] [CrossRef]
- Doulberis, M.; Kotronis, G.; Gialamprinou, D.; Polyzos, S.A.; Papaefthymiou, A.; Katsinelos, P.; Kountouras, J. Alzheimer’s disease and gastrointestinal microbiota; impact of Helicobacter pylori infection involvement. Int. J. Neurosci. 2021, 131, 289–301. [Google Scholar] [CrossRef]
- Fu, P.; Gao, M.; Yung, K.K.L. Association of intestinal disorders with Parkinson’s disease and Alzheimer’s disease: A systematic review and meta-analysis. ACS Chem. Neurosci. 2020, 11, 395–405. [Google Scholar] [CrossRef]
- de Brito, B.B.; da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; de Melo, F.F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection-the Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Rokkas, T.; Gisbert, J.P.; Malfertheiner, P.; Niv, Y.; Gasbarrini, A.; Leja, M.; Megraud, F.; O’Morain, C.; Graham, D.Y. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: A network meta-analysis. Gastroenterology 2021, 161, 495–507.e4. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Bujanda, L.; Nyssen, O.P.; Vaira, D.; Saracino, I.M.; Fiorini, G.; Lerang, F.; Georgopoulos, S.; Tepes, B.; Heluwaert, F.; Gasbarrini, A.; et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013–2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics 2021, 10, 1058. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Andreev, D.N.; Maev, I.V.; Dicheva, D.T. Efficiency of the inclusion of rebamipide in the eradication therapy for Helicobacter pylori infection: Meta-analysis of randomized controlled studies. J. Clin. Med. 2019, 8, 1498. [Google Scholar] [CrossRef]
- Higuchi, K.; Watanabe, T.; Tanigawa, T.; Tominaga, K.; Fujiwara, Y.; Arakawa, T. Sofalcone, a gastroprotective drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori: A randomized controlled comparative trial with cimetidine, an H2-receptor antagonist. J. Gastroenterol. Hepatol. 2010, 25 (Suppl. 1), S155–S160. [Google Scholar] [CrossRef]
- Teng, G.; Liu, Y.; Wu, T.; Wang, W.; Wang, H.; Hu, F. Efficacy of sucralfate-combined quadruple therapy on gastric mucosal injury induced by Helicobacter pylori and its effect on gastrointestinal flora. BioMed Res. Int. 2020, 2020, 4936318. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 51051629, Polaprezinc. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/51051629 (accessed on 3 October 2022).
- Ibrahim, N.; El Said, H.; Choukair, A. Zinc carnosine-based modified bismuth quadruple therapy vs standard triple therapy for Helicobacter pylori eradication: A randomized controlled study. World J. Clin. Cases 2022, 10, 227–235. [Google Scholar] [CrossRef]
- Ishihara, R.; Iishi, H.; Sakai, N.; Yano, H.; Uedo, N.; Narahara, H.; Iseki, K.; Mikuni, T.; Ishiguro, S.; Tatsuta, M. Polaprezinc attenuates Helicobacter pylori-associated gastritis in Mongolian gerbils. Helicobacter 2002, 7, 384–389. [Google Scholar] [CrossRef]
- Isomoto, H.; Furusu, H.; Ohnita, K.; Wen, C.Y.; Inoue, K.; Kohno, S. Sofalcone, a mucoprotective agent, increases the cure rate of Helicobacter pylori infection when combined with rabeprazole, amoxicillin and clarithromycin. World J. Gastroenterol. 2005, 11, 1629–1633. [Google Scholar] [CrossRef]
- Kashimura, H.; Suzuki, K.; Hassan, M.; Ikezawa, K.; Sawahata, T.; Watanabe, T.; Nakahara, A.; Mutoh, H.; Tanaka, N. Polaprezinc, a mucosal protective agent, in combination with lansoprazole, amoxycillin and clarithromycin increases the cure rate of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1999, 13, 483–487. [Google Scholar] [CrossRef]
- Tan, B.; Luo, H.-Q.; Xu, H.; Lv, N.-H.; Shi, R.-H.; Luo, H.-S.; Li, J.-S.; Ren, J.-L.; Zou, Y.-Y.; Li, Y.-Q.; et al. Polaprezinc combined with clarithromycin-based triple therapy for Helicobacter pylori-associated gastritis: A prospective, multicenter, randomized clinical trial. PLoS ONE 2017, 12, e0175625. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, C. A review of zinc-L-carnosine and its positive effects on oral mucositis, taste disorders, and gastrointestinal disorders. Nutirents 2020, 12, 665. [Google Scholar] [CrossRef]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3782–3895. [Google Scholar] [CrossRef]
- Kawahara, M.; Tanaka, K.-i.; Kato-Negishi, M. Zinc, carnosine, and neurodegenerative diseases. Nutrients 2018, 10, 147. [Google Scholar] [CrossRef]
- Furihata, K.; Tsuchikawa, M.; Miwa, T.; Naito, Y.; Oba, K.; Sakagami, M. Efficacy and safety of polaprezinc (zinc compound) on zinc deficiency: A systematic review and dose-response meta-analysis of randomized clinical trials using individual patient data. Nutrients 2020, 12, 1128. [Google Scholar] [CrossRef] [PubMed]
- Handa, O.; Yoshida, N.; Tanaka, Y.; Ueda, M.; Ishikawa, T.; Takagi, T.; Matsumoto, N.; Naito, Y.; Yoshikawa, T. Inhibitory effect of polaprezinc on the inflammatory response to Helicobacter pylori. Can. J. Gastroenterol. 2002, 16, 785–789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuu-Matsuyama, M.; Shichijo, K.; Okaichi, K.; Nakayama, T.; Nakashima, M.; Uemura, T.; Niino, D.; Sekine, I. Protection by polaprezinc against radiation-induced apoptosis in rat jejunal crypt cells. J. Radiat. Res. 2008, 49, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, T.; Naito, Y.; Handa, O.; Mizushima, K.; Hayashi, N.I.; Qin, Y.; Harusato, A.; Hirata, I.; Kishimoto, E.; Okada, H.; et al. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J. Gastroenterol. 2010, 45, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Matsura, T.; Kai, M.; Kawasaki, H.; Yamada, K. Protection by polaprezinc, an anti-ulcer drug, against indomethacin-induced apoptosis in rat gastric mucosal cells. Jpn. J. Pharmacol. 2000, 84, 63–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Massimi, M.; Conti Devirgiliis, L.; Mengheri, E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [CrossRef]
- Mahmood, A.; FitzGerald, A.J.; Marchbank, T.; Ntatsaki, E.; Murray, D.; Ghosh, S.; Playford, R.J. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut 2007, 56, 168–175. [Google Scholar] [CrossRef]
- Kato, S.; Tanaka, A.; Ogawa, Y.; Kanatsu, K.; Seto, K.; Yoneda, T.; Takeuchi, K. Effect of polaprezinc on impaired healing of chronic gastric ulcers in adjuvant-induced arthritic rats: Role of insulin-like growth factors (IGF)-1. Med. Sci. Monit. 2001, 7, 20–25. [Google Scholar]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Chichester, UK, 2019; pp. 1–694. [Google Scholar]
- Covidence. Available online: http://www.covidence.org/ (accessed on 5 September 2022).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Cochrane Bias Methods Group; Cochrane Statistical Methods Group; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J.; GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ 2008, 336, 995–998. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef]
- RevMan. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 5 September 2022).
- Leja, M.; Grinberga-Derica, I.; Bilgilier, C.; Steininger, C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. 1), e12635. [Google Scholar] [CrossRef]
- Dunn, B.E.; Cohen, H.; Blaser, M.J. Helicobacter pylori. Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar] [CrossRef]
- Peek, R.M., Jr.; Crabtree, J.E. Helicobacter infection and gastric neoplasia. J. Pathol. 2006, 208, 233–248. [Google Scholar] [CrossRef]
- Matsukura, T.; Tanaka, H. Applicability of zinc complex of L-carnosine for medical use. Biochemistry 2000, 65, 817–823. [Google Scholar]
- Yakoob, J.; Abbas, Z.; Jafri, W.; Usman, M.W.; Awan, S. Zinc chloride inhibits Helicobacter pylori growth and reduces expression of interleukin-1beta by gastric epithelial cells. Br. J. Biomed. Sci. 2014, 71, 43–45. [Google Scholar] [CrossRef]
- Amin, M.; Iqbal, M.S.; Hughes, R.W.; Khan, S.A.; Reynolds, P.A.; Enne, V.I.; Rahman, S.-U.; Mirza, A.S. Mechanochemical synthesis and in vitro anti-Helicobacter pylori and uresase inhibitory activities of novel zinc(II)–famotidine complex. J. Enzyme Inhib. Med. Chem. 2010, 25, 383–390. [Google Scholar] [CrossRef]
- Fan, D.; Gong, Y.; Sun, L.; Zhang, Y.; Zhang, J. Comparative transcriptome analysis to investigate the mechanism of anti-Helicobacter pylori activity of zinc. Microb. Pathog. 2022, 168, 105611. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2003, 71, 635–700. [Google Scholar] [CrossRef]
- Sakae, K.; Yanagisawa, H. Oral treatment of pressure ulcers with polaprezinc (zinc L-carnosine complex): 8-week open-label trial. Biol. Trace Elem. Res. 2014, 158, 280–288. [Google Scholar] [CrossRef]
- Chinese Study Group on Helicobacter pylori; Liu, W.Z.; Xie, Y.; Cheng, H.; Lu, N.H.; Hu, F.L.; Zhang, W.D.; Zhou, L.Y.; Chen, Y.; Zeng, Z.R.; et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J. Dig. Dis. 2013, 14, 211–221. [Google Scholar] [CrossRef]
- Graham, D.Y. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: Effect of resistance, curation, and CYP2C19 genotype. Helicobacter 2016, 21, 85–90. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).