Nutritional Management of Patients with Fontan Circulation: A Potential for Improved Outcomes from Birth to Adulthood
Abstract
1. Introduction
2. Methodology
3. The Burden of Faltering Growth in Patients with Fontan Circulation
3.1. Epidemiology
3.2. Pathogenesis
4. Assessment of the Nutritional Status
5. Nutritional Management up to Fontan Surgery
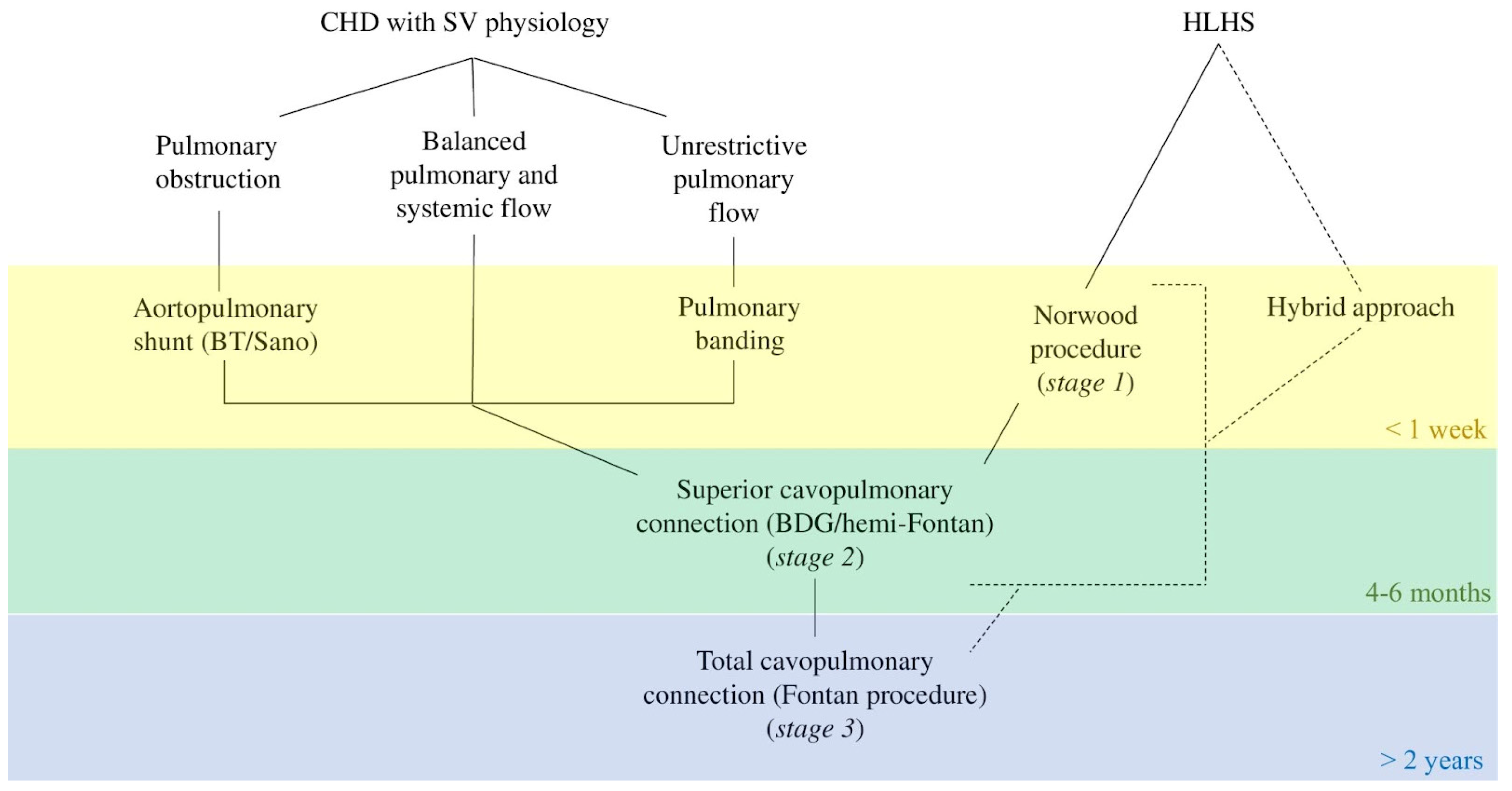
5.1. From Birth to Stage 1
5.2. From Interstage 1 to Stage 2
5.3. From Interstage 2 to Stage 3
5.4. General Approach up to Fontan Surgery
6. Nutritional Management after Fontan Surgery
6.1. Energy Requirements
6.2. Somatic Growth and Bone Mineralization
6.3. Altered Body Composition
6.4. Protein-Losing Enteropathy
7. Fontan Failure and Heart Transplant
8. Clinical Implications and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Driscoll, D.J.; Offord, K.P.; Feldt, R.H.; Schaff, H.V.; Puga, F.J.; Danielson, G.K. Five- to Fifteen-Year Follow-up after Fontan Operation. Circulation 1992, 85, 469–496. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, R.M.; Meliones, J.N.; McMillan, K.N.; Cooper, D.S.; Jacobs, J.P. Critical Heart Disease in Infants and Children. In Critical Heart Disease in Infants and Children; Elsevier: Amsterdam, The Netherlands, 2018; pp. 747–757.e2. ISBN 9781455707607. [Google Scholar]
- Sadineni, R.; Kumar, B.S.; Chander, N.; Boppana, D. Prenatal Sonographic Diagnosis of Hypoplastic Left Heart Syndrome. Int. J. Appl. Basic Med. Res. 2017, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Atz, A.M.; Travison, T.G.; Williams, I.A.; Pearson, G.D.; Laussen, P.C.; Mahle, W.T.; Cook, A.L.; Kirsh, J.A.; Sklansky, M.; Khaikin, S.; et al. Prenatal Diagnosis and Risk Factors for Preoperative Death in Neonates with Single Right Ventricle and Systemic Outflow Obstruction: Screening Data from the Pediatric Heart Network Single Ventricle Reconstruction Trial. J. Thorac. Cardiovasc. Surg. 2010, 140, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, C.L.; Clur, S.A.; Rijlaarsdam, M.E.B.; Bax, C.J.; Pajkrt, E.; Heymans, M.W.; Bekker, M.N.; Hruda, J.; De Groot, C.J.M.; Blom, N.A.; et al. Prenatal Detection of Congenital Heart Disease--Results of a National Screening Programme. BJOG 2016, 123, 400–407. [Google Scholar] [CrossRef]
- Poh, C.L.; Zannino, D.; Weintraub, R.G.; Winlaw, D.S.; Grigg, L.E.; Cordina, R.; Hornung, T.; Bullock, A.; Justo, R.N.; Gentles, T.L.; et al. Three Decades Later: The Fate of the Population of Patients Who Underwent the Atriopulmonary Fontan Procedure. Int. J. Cardiol. 2017, 231, 99–104. [Google Scholar] [CrossRef]
- Kreutzer, C.; Kreutzer, J.; Kreutzer, G.O. Reflections on Five Decades of the Fontan Kreutzer Procedure. Front. Pediatr. 2013, 1, 45. [Google Scholar] [CrossRef]
- Morris, S.A.; Ethen, M.K.; Penny, D.J.; Canfield, M.A.; Minard, C.G.; Fixler, D.E.; Nembhard, W.N. Prenatal Diagnosis, Birth Location, Surgical Center, and Neonatal Mortality in Infants with Hypoplastic Left Heart Syndrome. Circulation 2014, 129, 285–292. [Google Scholar] [CrossRef]
- Kverneland, L.S.; Kramer, P.; Ovroutski, S. Five Decades of the Fontan Operation: A Systematic Review of International Reports on Outcomes after Univentricular Palliation. Congenit. Heart Dis. 2018, 13, 181–193. [Google Scholar] [CrossRef]
- Hosein, R.B.M.; Clarke, A.J.B.; McGuirk, S.P.; Griselli, M.; Stumper, O.; De Giovanni, J.V.; Barron, D.J.; Brawn, W.J. Factors Influencing Early and Late Outcome Following the Fontan Procedure in the Current Era. The “Two Commandments”? Eur. J. Cardiothorac. Surg. 2007, 31, 344–353. [Google Scholar] [CrossRef]
- Brown, J.W.; Ruzmetov, M.; Deschner, B.W.; Rodefeld, M.D.; Turrentine, M.W. Lateral Tunnel Fontan in the Current Era: Is It Still a Good Option? Ann. Thorac. Surg. 2010, 89, 556–563. [Google Scholar] [CrossRef]
- Medium and Long-Term Outcomes of Fontan Operation. Available online: https://pubmed-ncbi-nlm-nih-gov.bibliopass.unito.it/21560840/ (accessed on 15 July 2022).
- Fontan, F.; Kirklin, J.W.; Fernandez, G.; Costa, F.; Naftel, D.C.; Tritto, F.; Blackstone, E.H. Outcome after a “Perfect” Fontan Operation. Circulation 1990, 81, 1520–1536. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Goldberg, D.; Rand, E.; Semeao, E.; Russo, P.; Dori, Y.; Dodds, K. End-Organ Consequences of the Fontan Operation: Liver Fibrosis, Protein-Losing Enteropathy and Plastic Bronchitis. Cardiol. Young 2013, 23, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, C.; Zak, V.; Williams, I.A.; Bellinger, D.C.; Gaynor, J.W.; Ghanayem, N.S.; Krawczeski, C.D.; Licht, D.J.; Mahony, L.; Newburger, J.W.; et al. Association of Impaired Linear Growth and Worse Neurodevelopmental Outcome in Infants with Single Ventricle Physiology: A Report from the Pediatric Heart Network Infant Single Ventricle Trial. J. Pediatr. 2013, 162, 250–256. [Google Scholar] [CrossRef]
- Anderson, J.B.; Beekman, R.H.; Border, W.L.; Kalkwarf, H.J.; Khoury, P.R.; Uzark, K.; Eghtesady, P.; Marino, B.S. Lower Weight-for-Age z Score Adversely Affects Hospital Length of Stay after the Bidirectional Glenn Procedure in 100 Infants with a Single Ventricle. J. Thorac. Cardiovasc. Surg. 2009, 138, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.J.; Radman, M.; Jacobs, M.L.; Sassano-Miguel, C.; Joffe, D.C.; Hill, K.D.; Chiswell, K.; Feng, L.; Jacobs, J.P.; Vener, D.F.; et al. Associations between Anthropometric Indices and Outcomes of Congenital Heart Operations in Infants and Young Children: An Analysis of Data from the Society of Thoracic Surgeons Database. Am. Heart J. 2020, 224, 85–97. [Google Scholar] [CrossRef]
- Hartman, D.M.; Medoff-Cooper, B. Transition to Home after Neonatal Surgery for Congenital Heart Disease. MCN Am. J. Matern. Child Nurs. 2012, 37, 95–100. [Google Scholar] [CrossRef]
- Kelleher, D.K.; Laussen, P.; Teixeira-Pinto, A.; Duggan, C. Growth and Correlates of Nutritional Status among Infants with Hypoplastic Left Heart Syndrome (HLHS) after Stage 1 Norwood Procedure. Nutrition 2006, 22, 237–244. [Google Scholar] [CrossRef]
- Menon, S.C.; McCandless, R.T.; MacK, G.K.; Lambert, L.M.; McFadden, M.; Williams, R.V.; Minich, L.L. Clinical Outcomes and Resource Use for Infants with Hypoplastic Left Heart Syndrome during Bidirectional Glenn: Summary from the Joint Council for Congenital Heart Disease National Pediatric Cardiology Quality Improvement Collaborative Registry. Pediatr. Cardiol. 2013, 34, 143–148. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Lu, M.; Sleeper, L.A.; Mahle, W.T.; Gaynor, J.W.; Williams, I.A.; Mussatto, K.A.; Ohye, R.G.; Graham, E.M.; Frank, D.U.; et al. Factors Associated with Neurodevelopment for Children with Single Ventricle Lesions. J. Pediatr. 2014, 165, 490–496. [Google Scholar] [CrossRef]
- Hehir, D.A.; Rudd, N.; Slicker, J.; Mussatto, K.A.; Simpson, P.; Li, S.H.; Frommelt, M.A.; Tweddell, J.S.; Ghanayem, N.S. Normal Interstage Growth after the Norwood Operation Associated with Interstage Home Monitoring. Pediatr. Cardiol. 2012, 33, 1315–1322. [Google Scholar] [CrossRef]
- Tume, L.N.; Valla, F.V.; Joosten, K.; Jotterand Chaparro, C.; Latten, L.; Marino, L.V.; Macleod, I.; Moullet, C.; Pathan, N.; Rooze, S.; et al. Nutritional Support for Children during Critical Illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) Metabolism, Endocrine and Nutrition Section Position Statement and Clinical Recommendations. Intensive Care Med. 2020, 46, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef] [PubMed]
- Burch, P.T.; Gerstenberger, E.; Ravishankar, C.; Hehir, D.A.; Davies, R.R.; Colan, S.D.; Sleeper, L.A.; Newburger, J.W.; Clabby, M.L.; William, I.A.S.; et al. Longitudinal Assessment of Growth in Hypoplastic Left Heart Syndrome: Results from the Single Ventricle Reconstruction Trial. J. Am. Heart Assoc. 2014, 3, 1445–1454. [Google Scholar] [CrossRef]
- Vogt, K.N.; Manlhiot, C.; Van Arsdell, G.; Russell, J.L.; Mital, S.; McCrindle, B.W. Somatic Growth in Children with Single Ventricle Physiology Impact of Physiologic State. J. Am. Coll. Cardiol. 2007, 50, 1876–1883. [Google Scholar] [CrossRef]
- Mehta, N.M.; Costello, J.M.; Bechard, L.J.; Johnson, V.M.; Zurakowski, D.; McGowan, F.X.; Laussen, P.C.; Duggan, C.P. Resting Energy Expenditure after Fontan Surgery in Children with Single-Ventricle Heart Defects. JPEN. J. Parenter. Enteral Nutr. 2012, 36, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Ensinger, H.; Takala, J. Metabolic Changes after Cardiac Surgery. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 149–155. [Google Scholar] [CrossRef]
- Dündar, B.; Akçoral, A.; Saylam, G.; Ünal, N.; Meşe, T.; Hüdaoǧlu, S.; Büyükgebiz, B.; Böber, E.; Büyükgebiz, A. Chronic Hypoxemia Leads to Reduced Serum IGF-I Levels in Cyanotic Congenital Heart Disease. J. Pediatr. Endocrinol. Metab. 2000, 13, 431–436. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, G.L.; Yang, L.L.; Sun, L.Q. Elevated Serum Levels of Ghrelin and TNF-α in Patients with Cyanotic and Acyanotic Congenital Heart Disease. World J. Pediatr. 2017, 13, 122–128. [Google Scholar] [CrossRef]
- Schuurmans, F.M.; Pulles-Heintzberger, C.F.M.; Gerver, W.J.M.; Kester, A.D.M.; Forget, P.P. Long-Term Growth of Children with Congenital Heart Disease: A Retrospective Study. Acta Paediatr. 1998, 87, 1250–1255. [Google Scholar] [CrossRef]
- Stenbøg, E.V.; Hjortdal, V.E.; Ravn, H.B.; Skjærbæk, C.; Sørensen, K.E.; Hansen, O.K. Improvement in Growth, and Levels of Insulin-like Growth Factor-I in the Serum, after Cavopulmonary Connections. Cardiol. Young 2000, 10, 440–446. [Google Scholar] [CrossRef]
- Ovroutski, S.; Ewert, P.; Alexi-Meskishvili, V.; Stiller, B.; Nürnberg, J.H.; Abdul-Khaliq, H.; Hetzer, R.; Lange, P.E. Comparison of Somatic Development and Status of Conduit after Extracardiac Fontan Operation in Young and Older Children. Eur. J. Cardiothorac. Surg. 2004, 26, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- François, K.; Bové, T.; Panzer, J.; De Groote, K.; Vandekerckhove, K.; De Wilde, H.; De Wolf, D. Univentricular Heart and Fontan Staging: Analysis of Factors Impacting on Body Growth. Eur. J. Cardiothorac. Surg. 2012, 41, e139–e145. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.D.; Silverman, A.H.; Noel, R.J.; Simpson, P.M.; Slicker, J.; Scott, A.E.; Bartz, P.J. Feeding Dysfunction in Children with Single Ventricle Following Staged Palliation. J. Pediatr. 2014, 164, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.; Silverman, A.; Noel, R.; Bartz, P.J. Feeding Dysfunction in Single Ventricle Patients with Feeding Disorder. Congenit. Heart Dis. 2014, 9, 26–29. [Google Scholar] [CrossRef]
- Shine, A.M.; Foyle, L.; Gentles, E.; Ward, F.; McMahon, C.J. Growth and Nutritional Intake of Infants with Univentricular Circulation. J. Pediatr. 2021, 237, 79–86. [Google Scholar] [CrossRef]
- Alsoufi, B.; McCracken, C.; Oster, M.; Shashidharan, S.; Kanter, K. Genetic and Extracardiac Anomalies Are Associated With Inferior Single Ventricle Palliation Outcomes. Ann. Thorac. Surg. 2018, 106, 1204–1212. [Google Scholar] [CrossRef]
- Pillo-Blocka, F.; Adatia, I.; Sharieff, W.; McCrindle, B.W.; Zlotkin, S. Rapid Advancement to More Concentrated Formula in Infants after Surgery for Congenital Heart Disease Reduces Duration of Hospital Stay: A Randomized Clinical Trial. J. Pediatr. 2004, 145, 761–766. [Google Scholar] [CrossRef]
- Shaw, V. Clinical Paediatric Dietetics: Fourth Edition. In Clinical Paediatric Dietetics, 4th ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 1–844. ISBN 9781118915349. [Google Scholar]
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F. Defining Pediatric Malnutrition: A Paradigm Shift toward Etiology-Related Definitions. JPEN J. Parenter. Enter. Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef]
- Sica, C.D.A.; Cesa, C.C.; Pellanda, L.C. Growth Curves in Down Syndrome with Congenital Heart Disease. Rev. Assoc. Med. Bras. 2016, 62, 414–420. [Google Scholar] [CrossRef][Green Version]
- Bertapelli, F.; Barros-Filho, A.D.A.; Antonio, M.Â.R.D.G.M.; Barbeta, C.J.D.O.; De Lemos-Marini, S.H.V.; Guerra-Junior, G. Growth Curves for Girls with Turner Syndrome. Biomed Res. Int. 2014, 2014, 687978. [Google Scholar] [CrossRef]
- WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: A Joint Statement by the World Health Organization and the United Nations Children’s Fund. Available online: https://pubmed-ncbi-nlm-nih-gov.bibliopass.unito.it/24809116/ (accessed on 15 July 2022).
- Rychik, J.; Goldberg, D.J.; Rand, E.; Mancilla, E.E.; Heimall, J.; Seivert, N.; Campbell, D.; O’Malley, S.; Dodds, K.M. A Path FORWARD: Development of a Comprehensive Multidisciplinary Clinic to Create Health and Wellness for the Child and Adolescent with a Fontan Circulation. Pediatr. Cardiol. 2022, 43, 1175–1192. [Google Scholar] [CrossRef] [PubMed]
- Human Energy Requirements. Available online: https://www.fao.org/3/y5686e/y5686e06.htm (accessed on 15 July 2022).
- Diao, J.; Chen, L.; Wei, J.; Shu, J.; Li, Y.; Li, J.; Zhang, S.; Wang, T.; Qin, J. Prevalence of Malnutrition in Children with Congenital Heart Disease: A Systematic Review and Meta-Analysis. J. Pediatr. 2022, 242, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe-Terilli, C.R.; Hartman, D.H.; Nagle, M.L.; Gallagher, P.R.; Ittenbach, R.F.; Burnham, N.B.; Gaynor, J.W.; Ravishankar, C. Enteral Feeding and Caloric Intake in Neonates after Cardiac Surgery. Am. J. Crit. Care 2009, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Mussatto, K.; Uhing, M.R.; Zimmerman, H.; Tweddell, J.; Ghanayem, N. Variability in the Preoperative Management of Infants with Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2008, 29, 515–520. [Google Scholar] [CrossRef]
- Iannucci, G.J.; Oster, M.E.; Mahle, W.T. Necrotising Enterocolitis in Infants with Congenital Heart Disease: The Role of Enteral Feeds. Cardiol. Young 2013, 23, 553–559. [Google Scholar] [CrossRef]
- Becker, K.C.; Hornik, C.P.; Cotten, C.M.; Clark, R.H.; Hill, K.D.; Smith, P.B.; Lenfestey, R.W. Necrotizing Enterocolitis in Infants with Ductal-Dependent Congenital Heart Disease. Am. J. Perinatol. 2015, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- O’Neal Maynord, P.; Johnson, M.; Xu, M.; Slaughter, J.C.; Killen, S.A.S. A Multi-Interventional Nutrition Program for Newborns with Congenital Heart Disease. J. Pediatr. 2021, 228, 66–73. [Google Scholar] [CrossRef]
- Martini, S.; Beghetti, I.; Annunziata, M.; Aceti, A.; Galletti, S.; Ragni, L.; Donti, A.; Corvaglia, L. Enteral Nutrition in Term Infants with Congenital Heart Disease: Knowledge Gaps and Future Directions to Improve Clinical Practice. Nutrients 2021, 13, 932. [Google Scholar] [CrossRef]
- Justice, L.; Buckley, J.R.; Floh, A.; Horsley, M.; Alten, J.; Anand, V.; Schwartz, S.M. Nutrition Considerations in the Pediatric Cardiac Intensive Care Unit Patient. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 333–343. [Google Scholar] [CrossRef]
- Mehta, N.M.; McAleer, D.; Hamilton, S.; Naples, E.; Leavitt, K.; Mitchell, P.; Duggan, C. Challenges to Optimal Enteral Nutrition in a Multidisciplinary Pediatric Intensive Care Unit. JPEN J. Parenter. Enter. Nutr. 2010, 34, 38–45. [Google Scholar] [CrossRef]
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafae, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic Left Heart Syndrome: Current Considerations and Expectations. J. Am. Coll. Cardiol. 2012, 59, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, H.E.; Wells, W.J.; Starnes, V.A.; Wetzel, R.C.; Moromisato, D.Y. Gastrointestinal Morbidity after Norwood Palliation for Hypoplastic Left Heart Syndrome. Ann. Thorac. Surg. 2006, 81, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Chen, S.; Dagincourt, N.; Bar-Cohen, Y.; Brothers, M.; Cain, N.; Colan, S.D.; Czosek, R.J.; Decker, J.A.; Gamboa, D.G.; et al. Development and Impact of Arrhythmias after the Norwood Procedure: A Report from the Pediatric Heart Network. J. Thorac. Cardiovasc. Surg. 2017, 153, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Kogon, B.; Jain, A.; Oster, M.; Woodall, K.; Kanter, K.; Kirshbom, P. Risk Factors Associated with Readmission after Pediatric Cardiothoracic Surgery. Ann. Thorac. Surg. 2012, 94, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Tume, L.; Carter, B.; Latten, L. A UK and Irish Survey of Enteral Nutrition Practices in Paediatric Intensive Care Units. Br. J. Nutr. 2013, 109, 1304–1322. [Google Scholar] [CrossRef]
- Fuller, S.; Nord, A.S.; Gerdes, M.; Wernovsky, G.; Jarvik, G.P.; Bernbaum, J.; Zackai, E.; Gaynor, J.W. Predictors of Impaired Neurodevelopmental Outcomes at One Year of Age after Infant Cardiac Surgery. Eur. J. Cardiothorac. Surg. 2009, 36, 40–48. [Google Scholar] [CrossRef]
- Nicholson, G.T.; Clabby, M.L.; Kanter, K.R.; Mahle, W.T. Caloric Intake during the Perioperative Period and Growth Failure in Infants with Congenital Heart Disease. Pediatr. Cardiol. 2013, 34, 316–321. [Google Scholar] [CrossRef]
- Ohye, R.G. Multi-Institutional Studies: Lessons Learned from the Pediatric Heart Network Single Ventricle Reconstruction Trial. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2010, 13, 76–78. [Google Scholar] [CrossRef]
- Ghanayem, N.S.; Tweddell, J.S.; Hoffman, G.M.; Mussatto, K.; Jaquiss, R.D.B. Optimal Timing of the Second Stage of Palliation for Hypoplastic Left Heart Syndrome Facilitated through Home Monitoring, and the Results of Early Cavopulmonary Anastomosis. Cardiol. Young 2006, 16 (Suppl. 1), 61–66. [Google Scholar] [CrossRef]
- Cohen, M.I.; Bush, D.M.; Ferry, R.J.; Spray, T.L.; Moshang, T.; Wernovsky, G.; Vetter, V.L. Somatic Growth Failure after the Fontan Operation. Cardiol. Young 2000, 10, 447–457. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Herridge, J.; Holtby, H.; Humpl, T.; Redington, A.N.; Van Arsdell, G.S. Energy Expenditure and Caloric and Protein Intake in Infants Following the Norwood Procedure. Pediatr. Crit. Care Med. 2008, 9, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Slicker, J.; Hehir, D.A.; Horsley, M.; Monczka, J.; Stern, K.W.; Roman, B.; Ocampo, E.C.; Flanagan, L.; Keenan, E.; Lambert, L.M.; et al. Nutrition Algorithms for Infants with Hypoplastic Left Heart Syndrome; Birth through the First Interstage Period. Congenit. Heart Dis. 2013, 8, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Furlong-Dillard, J.; Neary, A.; Marietta, J.; Jones, C.; Jeffers, G.; Gakenheimer, L.; Puchalski, M.; Eckauser, A.; Delgado-Corcoran, C. Evaluating the Impact of a Feeding Protocol in Neonates before and after Biventricular Cardiac Surgery. Pediatric Qual. Saf. 2018, 3, e080. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.M.; Fatusin, O.; Yenokyan, G.; Thompson, W.R.; Lefton-Greif, M.A. Feeding Methods for Infants with Single Ventricle Physiology Are Associated with Length of Stay during Stage 2 Surgery Hospitalization. Congenit. Heart Dis. 2019, 14, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Forchielli, M.L.; McColl, R.; Walker, W.A.; Lo, C. Children with Congenital Heart Disease: A Nutrition Challenge. Nutr. Rev. 1994, 52, 348–353. [Google Scholar] [CrossRef]
- Tokel, K.; Azak, E.; Ayabakan, C.; Varan, B.; Aşlamaci, S.A.; Mercan, S. Somatic Growth after Corrective Surgery for Congenital Heart Disease. Turk. J. Pediatr. 2010, 52, 58–67. [Google Scholar]
- Varan, B.; Tokel, K.; Yilmaz, G. Malnutrition and Growth Failure in Cyanotic and Acyanotic Congenital Heart Disease with and without Pulmonary Hypertension. Arch. Dis. Child. 1999, 81, 49–52. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Huang, R.; Sun, C.; Bao, N.; Xu, Z. Risk Factors of Malnutrition in Chinese Children with Congenital Heart Defect. BMC Pediatr. 2020, 20, 213. [Google Scholar] [CrossRef]
- Dalili, M.; Meraji, S.M.; Davari, P.; Moghaddam, M.Y.A.; Abkenar, H.B.; Vahidi, A.; Shahmohammadi, A. Growth Status of Iranian Children with Hemodynamically Important Congenital Heart Disease. Acta Med. Iran. 2011, 49, 103–108. [Google Scholar]
- Sandberg, C.; Rinnström, D.; Dellborg, M.; Thilén, U.; Sörensson, P.; Nielsen, N.E.; Christersson, C.; Wadell, K.; Johansson, B. Height, Weight and Body Mass Index in Adults with Congenital Heart Disease. Int. J. Cardiol. 2015, 187, 219–226. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Brubaker, P.H.; Morgan, T.M.; Kritchevsky, S.; Eggebeen, J.; Kitzman, D.W. Impaired Aerobic Capacity and Physical Functional Performance in Older Heart Failure Patients with Preserved Ejection Fraction: Role of Lean Body Mass. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Freud, L.R.; Webster, G.; Costello, J.M.; Tsao, S.; Rychlik, K.; Backer, C.L.; Deal, B.J. Growth and Obesity Among Older Single Ventricle Patients Presenting for Fontan Conversion. World J. Pediatr. Congenit. Heart Surg. 2015, 6, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.T.; Hong, B.; Patterson, L.; Petit, C.J.; Ham, J.N. High Overweight and Obesity in Fontan Patients: A 20-Year History. Pediatr. Cardiol. 2016, 37, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Zak, V.; Atz, A.M.; Printz, B.F.; Pinto, N.; Lambert, L.; Pemberton, V.; Li, J.S.; Margossian, R.; Dunbar-Masterson, C.; et al. Anthropometric Measures after Fontan Procedure: Implications for Suboptimal Functional Outcome. Am. Heart J. 2010, 160, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.M.; Goldberg, D.J.; Zemel, B.S.; Brodsky, J.L.; Dodds, K.; Hayden-Rush, C.; Whitehead, K.K.; Goldmuntz, E.; Rychik, J.; Leonard, M.B. Deficits in Bone Density and Structure in Children and Young Adults Following Fontan Palliation. Bone 2015, 77, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.S.; Dishmon, D.A.; Garg, N.; Weber, K.T. Secondary Hyperparathyroidism in Heart Failure. Am. J. Med. Sci. 2017, 354, 335–338. [Google Scholar] [CrossRef]
- Bendaly, E.A.; DiMeglio, L.A.; Fadel, W.F.; Hurwitz, R.A. Bone Density in Children with Single Ventricle Physiology. Pediatr. Cardiol. 2015, 36, 779–785. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Dodds, K.; Avitabile, C.M.; Glatz, A.C.; Brodsky, J.L.; Semeao, E.J.; Rand, E.B.; Mancilla, E.E.; Rychik, J. Children with Protein-Losing Enteropathy after the Fontan Operation Are at Risk for Abnormal Bone Mineral Density. Pediatr. Cardiol. 2012, 33, 1264–1268. [Google Scholar] [CrossRef]
- Witzel, C.; Sreeram, N.; Coburger, S.; Schickendantz, S.; Brockmeier, K.; Schoenau, E. Outcome of Muscle and Bone Development in Congenital Heart Disease. Eur. J. Pediatr. 2006, 165, 168–174. [Google Scholar] [CrossRef]
- Tran, D.; D’ambrosio, P.; Verrall, C.E.; Attard, C.; Briody, J.; D’souza, M.; Singh, M.F.; Ayer, J.; D’udekem, Y.; Twigg, S.; et al. Body Composition in Young Adults Living With a Fontan Circulation: The Myopenic Profile. J. Am. Heart Assoc. 2020, 9, e015639. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Petryk, A.; Mishra, P.E.; Polgreen, L.E.; Panoskaltsis-Mortari, A.; Brown, R.; Marino, B.S.; Gremmels, D.; Shepard, C.; Kelly, A.S.; et al. Early Characteristics of Bone Deficits in Children with Fontan Palliation. Cardiol. Young 2020, 30, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.G.; Godang, K.; Müller, L.S.O.; Almaas, R.; de Lange, C.; Brunvand, L.; Hansen, K.M.; Myhre, A.G.; Døhlen, G.; Thaulow, E.; et al. Progressive Loss of Bone Mass in Children with Fontan Circulation. Congenit. Heart Dis. 2019, 14, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Holler, F.; Hannes, T.; Germund, I.; Emmel, M.; Hoyer-Kuhn, H.; Khalil, M.; Sreeram, N.; Udink Ten Cate, F.E.A. Low Serum 25-Hydroxyvitamin D Levels and Secondary Hyperparathyroidism in Fontan Patients. Cardiol. Young 2016, 26, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Vaikunth, S.S.; Leonard, M.B.; Whitehead, K.K.; Goldberg, D.J.; Rychik, J.; Zemel, B.S.; Avitabile, C.M. Deficits in the Functional Muscle-Bone Unit in Youths with Fontan Physiology. J. Pediatr. 2021, 238, 202–207. [Google Scholar] [CrossRef]
- Cordina, R.; O’Meagher, S.; Gould, H.; Rae, C.; Kemp, G.; Pasco, J.A.; Celermajer, D.S. Skeletal Muscle Abnormalities and Exercise Capacity in Adults with a Fontan Circulation. Heart 2013, 99, 1530–1534. [Google Scholar] [CrossRef]
- Sandberg, C.; Johansson, K.; Christersson, C.; Hlebowicz, J.; Thilén, U.; Johansson, B. Sarcopenia Is Common in Adults with Complex Congenital Heart Disease. Int. J. Cardiol. 2019, 296, 57–62. [Google Scholar] [CrossRef]
- Powell, A.W.; Wittekind, S.G.; Alsaied, T.; Lubert, A.M.; Chin, C.; Veldtman, G.R.; Cordina, R.; Katz, D.A.; Mays, W.A.; Knecht, S.K.; et al. Body Composition and Exercise Performance in Youth With a Fontan Circulation: A Bio-Impedance Based Study. J. Am. Heart Assoc. 2020, 9, e018345. [Google Scholar] [CrossRef]
- Cordina, R.; D’Udekem, Y. Long-Lasting Benefits of Exercise for Those Living with a Fontan Circulation. Curr. Opin. Cardiol. 2019, 34, 79–86. [Google Scholar] [CrossRef]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Martinez, S.C.; Byku, M.; Novak, E.L.; Cedars, A.M.; Eghtesady, P.; Ludbrook, P.A.; Billadello, J.J. Increased Body Mass Index Is Associated with Congestive Heart Failure and Mortality in Adult Fontan Patients. Congenit. Heart Dis. 2016, 11, 71–79. [Google Scholar] [CrossRef]
- Byrne, R.D.; Weingarten, A.J.; Clark, D.E.; Healan, S.J.; Richardson, T.L.; Huang, S.; Menachem, J.N.; Frischhertz, B.P. Sizing Up Fontan Failure: Association with Increasing Weight in Adulthood. Pediatr. Cardiol. 2021, 42, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Al Balushi, A.; Mackie, A.S. Protein-Losing Enteropathy Following Fontan Palliation. Can. J. Cardiol. 2019, 35, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Copland, A.P.; Dibaise, J.K. Protein Losing Enteropathy: Diagnosis and Management—Mayo Clinic. Gastroenterol. Hepatol. 2017, 41, 22–37. [Google Scholar]
- John, A.S.; Johnson, J.A.; Khan, M.; Driscoll, D.J.; Warnes, C.A.; Cetta, F. Clinical Outcomes and Improved Survival in Patients with Protein-Losing Enteropathy after the Fontan Operation. J. Am. Coll. Cardiol. 2014, 64, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Meadows, J.; Jenkins, K. Protein-Losing Enteropathy: Integrating a New Disease Paradigm into Recommendations for Prevention and Treatment. Cardiol. Young 2011, 21, 363–377. [Google Scholar] [CrossRef]
- Allen, K.Y.; Downing, T.E.; Glatz, A.C.; Rogers, L.S.; Ravishankar, C.; Rychik, J.; Fuller, S.; Montenegro, L.M.; Steven, J.M.; Spray, T.L.; et al. Effect of Fontan-Associated Morbidities on Survival With Intact Fontan Circulation. Am. J. Cardiol. 2017, 119, 1866–1871. [Google Scholar] [CrossRef]
- Lin, W.-S.; Hwang, M.-S.; Chung, H.-T.; Chu, J.-J.; Lai, M.-W.; Yang, J.-S.; Huang, S.-C.; Huang, J.-L.; Su, W.-J. Protein-Losing Enteropathy after the Fontan Operation: Clinical Analysis of Nine Cases. Chang Gung Med. J. 2006, 29, 505–512. [Google Scholar]
- Itkin, M.; Piccoli, D.A.; Nadolski, G.; Rychik, J.; DeWitt, A.; Pinto, E.; Rome, J.; Dori, Y. Protein-Losing Enteropathy in Patients With Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2929–2937. [Google Scholar] [CrossRef]
- Rychik, J.; Gui-Yang, S. Relation of Mesenteric Vascular Resistance after Fontan Operation and Protein-Losing Enteropathy. Am. J. Cardiol. 2002, 90, 672–674. [Google Scholar] [CrossRef]
- Ostrow, A.M.; Freeze, H.; Rychik, J. Protein-Losing Enteropathy after Fontan Operation: Investigations into Possible Pathophysiologic Mechanisms. Ann. Thorac. Surg. 2006, 82, 695–700. [Google Scholar] [CrossRef]
- Bode, L.; Freeze, H.H. Applied Glycoproteomics--Approaches to Study Genetic-Environmental Collisions Causing Protein-Losing Enteropathy. Biochim. Biophys. Acta 2006, 1760, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.A.; Hooper, J.; Kemp, M.; Somerville, J. Gastro-Intestinal Protein Loss in Late Survivors of Fontan Surgery and Other Congenital Heart Disease. Eur. Heart J. 1998, 19, 514–520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujii, T.; Shimizu, T.; Takahashi, K.; Kishiro, M.; Ohkubo, M.; Akimoto, K.; Yamashiro, Y. Fecal Alpha1-Antitrypsin Concentrations as a Measure of Enteric Protein Loss after Modified Fontan Operations. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Bejiqi, R.; Retkoceri, R.; Zeka, N.; Bejiqi, H.; Vuqiterna, A.; Maloku, A. Treatment of Children with Protein—Losing Enteropathy after Fontan and Other Complex Congenital Heart Disease Procedures in Condition with Limited Human and Technical Resources. Mater. Sociomed. 2014, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Denolin, H.; Kuhn, H.; Krayenbuehl, H.; Loogen, F.; Reale, A. The Definition of Heart Failure. Eur. Heart J. 1983, 4, 445–448. [Google Scholar] [CrossRef]
- Tavazzi, L. Towards a More Precise Definition of Heart Failure Aetiology. Eur. Heart J. 2001, 22, 192–195. [Google Scholar] [CrossRef][Green Version]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, E234–E284. [Google Scholar] [CrossRef]
- Márquez-González, H.; Hernández-Vásquez, J.G.; Del Valle-Lom, M.; Yáñez-Gutiérrez, L.; Klünder-Klünder, M.; Almeida-Gutiérrez, E.; Koretzky, S.G. Failures of the Fontan System in Univentricular Hearts and Mortality Risk in Heart Transplantation: A Systematic Review and Meta-Analysis. Life 2021, 11, 1363. [Google Scholar] [CrossRef]
- Lezo, A.; Aidala, E.; Deorsola, L.; Cascarano, M.T.; Rizzo, A.; Iannandrea, S.; Peruzzi, L.; Runfola, F.; Pace Napoleone, C. Malnutrition and Chyle Leakage: A Life-Threatening Duo in Heart Transplantation Post-Fontan Procedure. Clin. Case Rep. 2020, 8, 2055–2059. [Google Scholar] [CrossRef]
- Lim, J.Y.J.; Wee, R.W.B.; Gandhi, M.; Lim, Y.P.; Tan, L.N.M.; Quek, S.C.; Aw, M.M.; Chen, C.K. The Associations Between Preoperative Anthropometry and Postoperative Outcomes in Infants Undergoing Congenital Heart Surgery. Front. Cardiovasc. Med. 2022, 9, 812680. [Google Scholar] [CrossRef]
- Revelly, J.P.; Tappy, L.; Berger, M.M.; Gersbach, P.; Cayeux, C.; Chioléro, R. Early Metabolic and Splanchnic Responses to Enteral Nutrition in Postoperative Cardiac Surgery Patients with Circulatory Compromise. Intensive Care Med. 2001, 27, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Barash, M.; Patel, J.J. Gut Luminal and Clinical Benefits of Early Enteral Nutrition in Shock. Curr. Surg. Rep. 2019, 7, 21. [Google Scholar] [CrossRef]
- Modir, R.; Hadhazy, E.; Teuteberg, J.; Hiesinger, W.; Tulu, Z.; Hill, C. Improving Nutrition Practices for Postoperative High-Risk Heart Transplant and Ventricular Assist Device Implant Patients in Circulatory Compromise: A Quality Improvement Pre- and Post-Protocol Intervention Outcome Study. Nutr. Clin. Pract. 2022, 37, 677–697. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Ohuchi, H.; Matsuyama, T.A.; Miyazaki, A.; Ishibashi-Ueda, H.; Yamada, O. Diverse Multi-Organ Histopathologic Changes in a Failed Fontan Patient. Pediatr. Int. 2016, 58, 1061–1065. [Google Scholar] [CrossRef]
- Khuong, J.N.; Wilson, T.G.; Grigg, L.E.; Bullock, A.; Celermajer, D.; Disney, P.; Wijesekera, V.A.; Hornung, T.; Zannino, D.; Iyengar, A.J.; et al. Fontan-Associated Nephropathy: Predictors and Outcomes. Int. J. Cardiol. 2020, 306, 73–77. [Google Scholar] [CrossRef]
- Balakumar, V.; Murugan, R.; Sileanu, F.E.; Palevsky, P.; Clermont, G.; Kellum, J.A. Both Positive and Negative Fluid Balance May Be Associated With Reduced Long-Term Survival in the Critically Ill. Crit. Care Med. 2017, 45, e749–e757. [Google Scholar] [CrossRef]
- Chalret Du Rieu, M.; Baulieux, J.; Rode, A.; Mabrut, J.Y. Management of Postoperative Chylothorax. J. Visc. Surg. 2011, 148, 392–399. [Google Scholar] [CrossRef]
- Van der Gaag, N.A.; Verhaar, A.C.; Haverkort, E.B.; Busch, O.R.C.; van Gulik, T.M.; Gouma, D.J. Chylous Ascites after Pancreaticoduodenectomy: Introduction of a Grading System. J. Am. Coll. Surg. 2008, 207, 751–757. [Google Scholar] [CrossRef]
- Roh, J.L.; Yoon, Y.H.; Park, C. Il Chyle Leakage in Patients Undergoing Thyroidectomy plus Central Neck Dissection for Differentiated Papillary Thyroid Carcinoma. Ann. Surg. Oncol. 2008, 15, 2576–2580. [Google Scholar] [CrossRef]
- Steven, B.R.; Carey, S. Nutritional Management in Patients with Chyle Leakage: A Systematic Review. Eur. J. Clin. Nutr. 2015, 69, 776–780. [Google Scholar] [CrossRef]
| Periodic nutritional assessment |
|
| Surgical phase |
|
| Chronic phase |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldini, L.; Librandi, K.; D’Eusebio, C.; Lezo, A. Nutritional Management of Patients with Fontan Circulation: A Potential for Improved Outcomes from Birth to Adulthood. Nutrients 2022, 14, 4055. https://doi.org/10.3390/nu14194055
Baldini L, Librandi K, D’Eusebio C, Lezo A. Nutritional Management of Patients with Fontan Circulation: A Potential for Improved Outcomes from Birth to Adulthood. Nutrients. 2022; 14(19):4055. https://doi.org/10.3390/nu14194055
Chicago/Turabian StyleBaldini, Letizia, Katia Librandi, Chiara D’Eusebio, and Antonella Lezo. 2022. "Nutritional Management of Patients with Fontan Circulation: A Potential for Improved Outcomes from Birth to Adulthood" Nutrients 14, no. 19: 4055. https://doi.org/10.3390/nu14194055
APA StyleBaldini, L., Librandi, K., D’Eusebio, C., & Lezo, A. (2022). Nutritional Management of Patients with Fontan Circulation: A Potential for Improved Outcomes from Birth to Adulthood. Nutrients, 14(19), 4055. https://doi.org/10.3390/nu14194055






