Relationship between Ideal Cardiovascular Health and Incident Proteinuria: A 5 Year Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
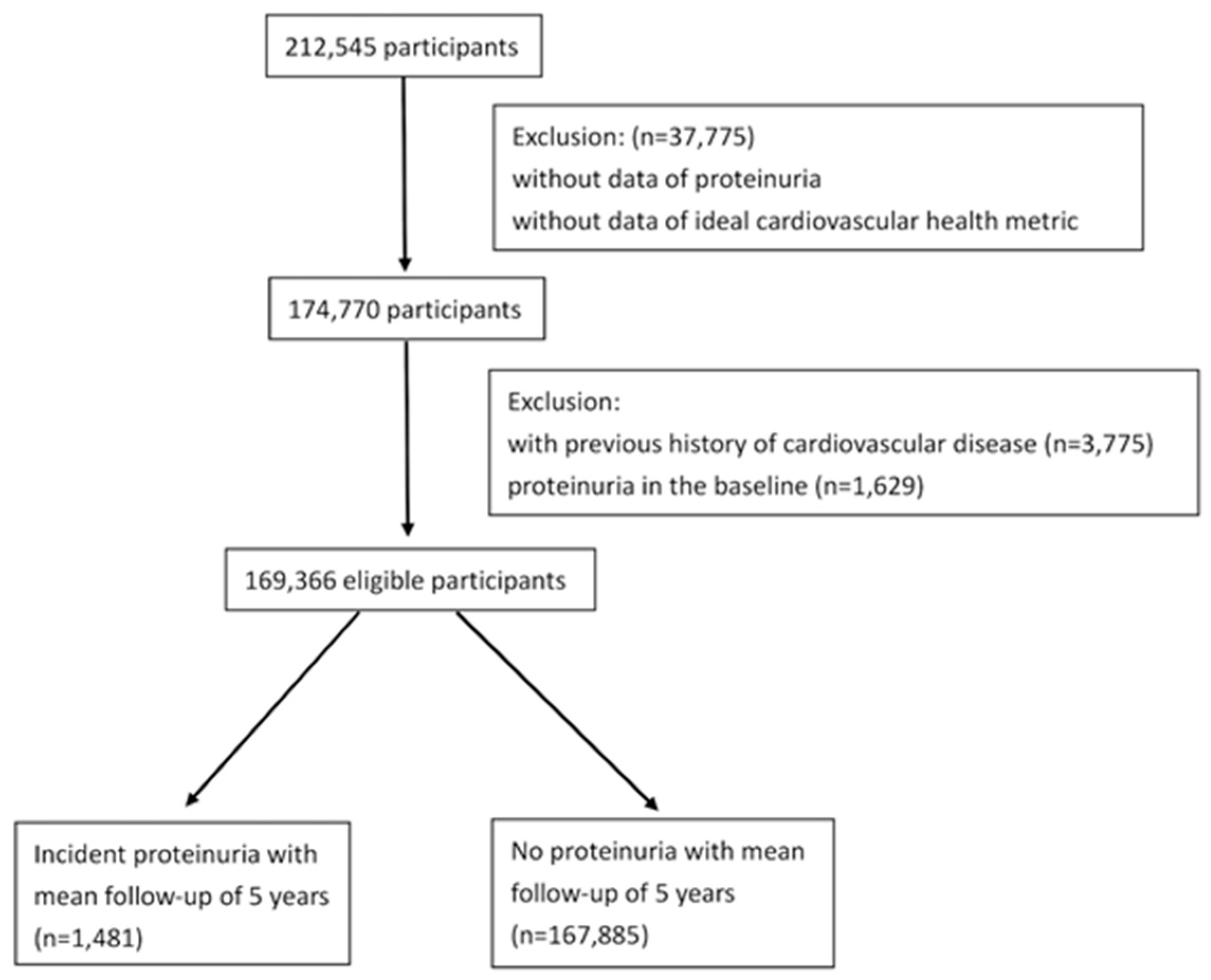
2.1. Study Population and Design
2.2. Sociodemographic and Clinical Variables
2.3. Proteinuria
2.4. Cardiovascular Health Metrics
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Proteinuria and Cardiovascular Health Metrics
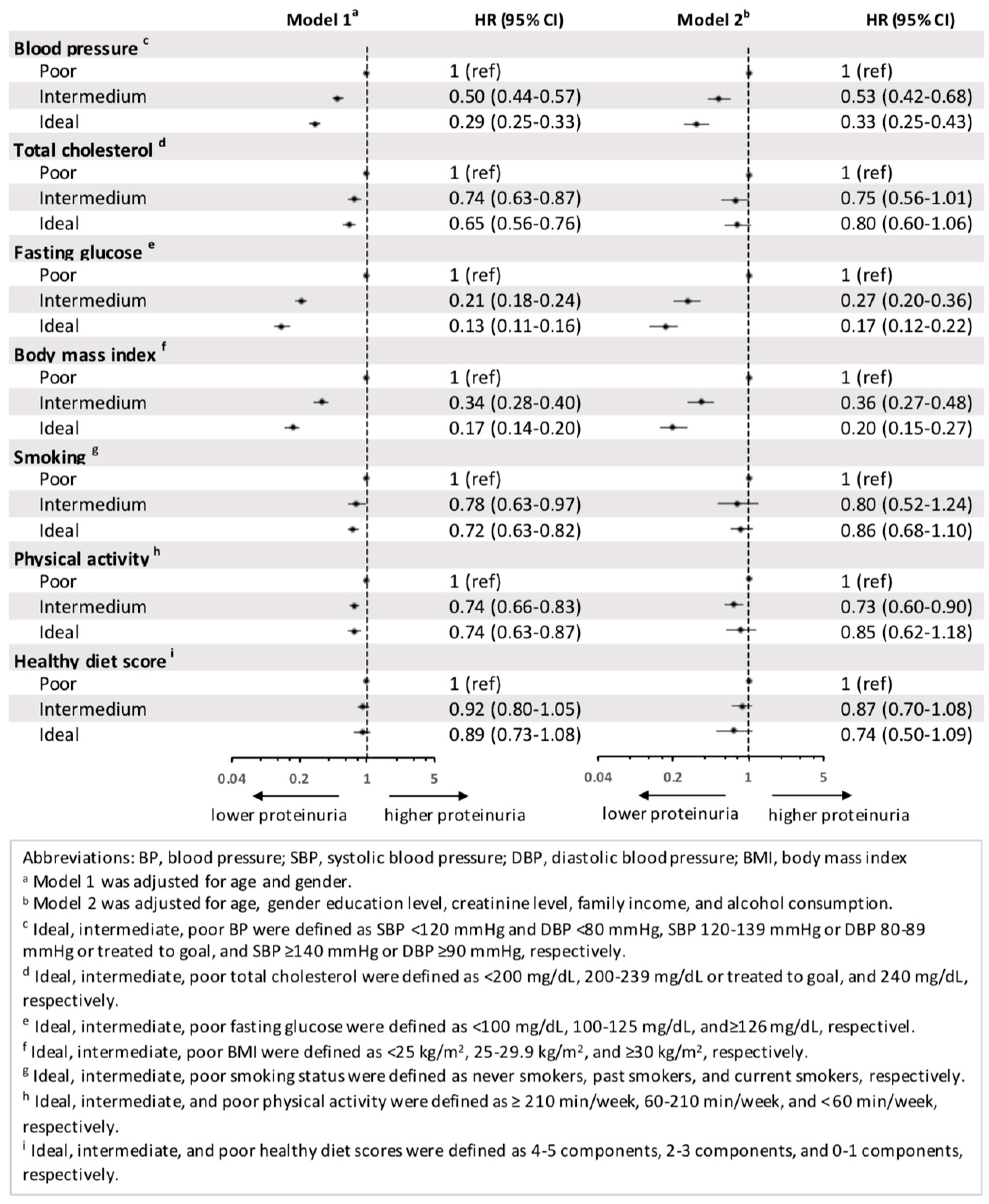
3.3. Proteinuria and Each Component of CVH Metrics
3.4. Proteinuria and CVH Healthy Diet Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Hemmelgarn, B.R.; Manns, B.J.; Lloyd, A.; James, M.T.; Klarenbach, S.; Quinn, R.R.; Wiebe, N.; Tonelli, M.; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010, 303, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Greene, T.; Tighiouart, H.; Gansevoort, R.T.; Coresh, J.; Simon, A.L.; Chan, T.M.; Hou, F.F.; Lewis, J.B.; Locatelli, F. Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019, 7, 128–139. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Tummalapalli, S.L.; Boulware, L.E.; Grams, M.E.; Ix, J.H.; Jha, V.; Kengne, A.-P.; Madero, M.; Mihaylova, B.; Tangri, N. The case for early identification and intervention of chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021, 99, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Hemmelgarn, B.; Lloyd, A.; James, M.T.; Manns, B.J.; Klarenbach, S.; Tonelli, M.; Alberta Kidney Disease Network. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin. J. Am. Soc. Nephrol. 2011, 6, 1418–1426. [Google Scholar] [CrossRef]
- Currie, G.; Delles, C. Proteinuria and its relation to cardiovascular disease. Int. J. Nephrol. Renov. Dis. 2013, 7, 13–24. [Google Scholar]
- Irie, F.; Iso, H.; Sairenchi, T.; Fukasawa, N.; Yamagishi, K.; Ikehara, S.; Kanashiki, M.; Saito, Y.; Ota, H.; Nose, T. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int. 2006, 69, 1264–1271. [Google Scholar] [CrossRef]
- Perkovic, V.; Verdon, C.; Ninomiya, T.; Barzi, F.; Cass, A.; Patel, A.; Jardine, M.; Gallagher, M.; Turnbull, F.; Chalmers, J. The relationship between proteinuria and coronary risk: A systematic review and meta-analysis. PLoS Med. 2008, 5, e207. [Google Scholar] [CrossRef]
- Xu, R.; Sun, S.; Huo, Y.; Yun, L.; Huang, S.; Li, G.; Yan, S. Effects of ACEIs versus ARBs on proteinuria or albuminuria in primary hypertension: A meta-analysis of randomized trials. Medicine 2015, 94, e1560. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Halimi, J.-M.; Giraudeau, B.; Cacès, E.; Nivet, H.; Lebranchu, Y.; Tichet, J. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int. 2000, 58, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Zhou, C.; He, P.; Nie, J.; Liang, M.; Liu, C.; Xu, F.; Liao, G.; Zhang, Y. Relationship of body mass index and waist circumference with risk of new-onset proteinuria in hypertensive patients. J. Clin. Endocrinol. Metab. 2020, 105, e511–e519. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.M.; Punithavathi, N.; Thurairatnam, D.; Zainal, H.; Beh, M.L.; Morad, Z.; Lee, S.Y.S.; Bavanandan, S.; Kok, L.S. Prevalence and risk factors for proteinuria: T he N ational K idney F oundation of M alaysia L ifecheck H ealth S creening programme. Nephrology 2013, 18, 569–575. [Google Scholar] [CrossRef]
- Ramirez, S.P.B.; McClellan, W.; Port, F.K.; Stephen, I.; Hsu, H. Risk factors for proteinuria in a large, multiracial, southeast Asian population. J. Am. Soc. Nephrol. 2002, 13, 1907–1917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tozawa, M.; Iseki, K.; Iseki, C.; Oshiro, S.; Ikemiya, Y.; Takishita, S. Influence of smoking and obesity on the development of proteinuria. Kidney Int. 2002, 62, 956–962. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Ford, E.S.; Greenlund, K.J.; Hong, Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012, 125, 987–995. [Google Scholar] [CrossRef]
- Joseph, J.J.; Echouffo-Tcheugui, J.B.; Carnethon, M.R.; Bertoni, A.G.; Shay, C.M.; Ahmed, H.M.; Blumenthal, R.S.; Cushman, M.; Golden, S.H. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis. Diabetologia 2016, 59, 1893–1903. [Google Scholar] [CrossRef]
- Muntner, P.; Judd, S.E.; Gao, L.; Gutiérrez, O.M.; Rizk, D.V.; McClellan, W.; Cushman, M.; Warnock, D.G. Cardiovascular risk factors in CKD associate with both ESRD and mortality. J. Am. Soc. Nephrol. 2013, 24, 1159–1165. [Google Scholar] [CrossRef]
- Wu, X.; Tsai, S.P.; Tsao, C.K.; Chiu, M.L.; Tsai, M.K.; Lu, P.J.; Lee, J.H.; Chen, C.H.; Wen, C.; Chang, S.S.; et al. Cohort Profile: The Taiwan MJ Cohort: Half a million Chinese with repeated health surveillance data. Int. J. Epidemiol. 2017, 46, 1744–1744g. [Google Scholar] [CrossRef]
- MJ Health Resource Center. Available online: http://www.mjhrf.org/main/page/resource/en/#resource08 (accessed on 4 August 2022).
- Cravedi, P.; Ruggenenti, P.; Remuzzi, G. Proteinuria should be used as a surrogate in CKD. Nat. Rev. Nephrol. 2012, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Huang, S. Progress in pathogenesis of proteinuria. Int. J. Nephrol. 2012, 2012, 314251. [Google Scholar] [CrossRef] [PubMed]
- Rashidbeygi, E.; Safabakhsh, M.; Mohammed, S.H.; Alizadeh, S. Metabolic syndrome and its components are related to a higher risk for albuminuria and proteinuria: Evidence from a meta-analysis on 10,603,067 subjects from 57 studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, M.; Kazama, J.; Narita, I.; Iseki, K.; Fujimoto, S.; Moriyama, T.; Yamagata, K.; Konta, T.; Tsuruya, K.; Asahi, K. Association between overall lifestyle changes and the incidence of proteinuria: A population-based, cohort study. Intern. Med. 2017, 56, 1475–1484. [Google Scholar] [CrossRef]
- Okada, R.; Tsushita, K.; Wakai, K.; Kato, K.; Wada, T.; Shinohara, Y. Healthy lifestyle reduces incidence of trace/positive proteinuria and rapid kidney function decline after 2 years: From the Japan Ningen Dock study. Nephrol. Dial. Transplant. 2021, 36, 1039–1048. [Google Scholar] [CrossRef]
- Bidani, A.K.; Griffin, K.A. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension 2004, 44, 595–601. [Google Scholar] [CrossRef]
- Upadhyay, A.; Earley, A.; Haynes, S.M.; Uhlig, K. Systematic review: Blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann. Intern. Med. 2011, 154, 541–548. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.-I.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Czajka, A.; Malik, A.N. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Redox Biol. 2016, 10, 100–107. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. Obesity and kidney disease: Hidden consequences of the epidemic. Braz. J. Nephrol. 2017, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wickman, C.; Kramer, H. Obesity and kidney disease: Potential mechanisms. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 33, pp. 14–22. [Google Scholar]
- Whaley-Connell, A.; Sowers, J.R. Obesity and kidney disease: From population to basic science and the search for new therapeutic targets. Kidney Int. 2017, 92, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Anderson, C.A.M.; Grams, M.E.; Bazzano, L.A.; Crews, D.C.; Chang, A.R.; Coresh, J.; Appel, L.J. Relationship of the American Heart Association’s Impact Goals (Life’s Simple 7) with risk of chronic kidney disease: Results from the Atherosclerosis Risk in Communities (ARIC) Cohort Study. J. Am. Heart Assoc. 2016, 5, e003192. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Yang, W.; Akkina, S.; Alper, A.; Anderson, A.H.; Appel, L.J.; He, J.; Raj, D.S.; Schelling, J.; Strauss, L. Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2014, 9, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative stress and cardiovascular risk: Obesity, diabetes, smoking, and pollution: Part 3 of a 3-part series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Maeda, I.; Hayashi, T.; Sato, K.K.; Koh, H.; Harita, N.; Nakamura, Y.; Endo, G.; Kambe, H.; Fukuda, K. Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin. J. Am. Soc. Nephrol. 2011, 6, 2462–2469. [Google Scholar] [CrossRef]
- Ito, K.; Maeda, T.; Tada, K.; Takahashi, K.; Yasuno, T.; Masutani, K.; Mukoubara, S.; Arima, H.; Nakashima, H. The role of cigarette smoking on new-onset of chronic kidney disease in a Japanese population without prior chronic kidney disease: Iki epidemiological study of atherosclerosis and chronic kidney disease (ISSA-CKD). Clin. Exp. Nephrol. 2020, 24, 919–926. [Google Scholar] [CrossRef]
- Xia, J.; Wang, L.; Ma, Z.; Zhong, L.; Wang, Y.; Gao, Y.; He, L.; Su, X. Cigarette smoking and chronic kidney disease in the general population: A systematic review and meta-analysis of prospective cohort studies. Nephrol. Dial. Transplant. 2017, 32, 475–487. [Google Scholar] [CrossRef]
- Robinson, E.S.; Fisher, N.D.; Forman, J.P.; Curhan, G.C. Physical activity and albuminuria. Am. J. Epidemiol. 2010, 171, 515–521. [Google Scholar] [CrossRef]
- Martens, R.J.H.; van der Berg, J.D.; Stehouwer, C.D.A.; Henry, R.M.A.; Bosma, H.; Dagnelie, P.C.; van Dongen, M.C.J.M.; Eussen, S.J.P.M.; Schram, M.T.; Sep, S.J.S. Amount and pattern of physical activity and sedentary behavior are associated with kidney function and kidney damage: The Maastricht Study. PLoS ONE 2018, 13, e0195306. [Google Scholar] [CrossRef]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical activity in the prevention and treatment of coronary artery disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Cavallo, P.; Zulli, E.; Villa, R.; Veneziano, R.; Costanzo, S.; Magnacca, S.; Di Castelnuovo, A.; Iacoviello, L.; Moli-Sani Study, I. Sodium Intake and Proteinuria/Albuminuria in the Population—Observational, Cross-Sectional Study. Nutrients 2021, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R. Urinary sodium and potassium excretion and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 1202–1212. [Google Scholar] [CrossRef]
- de Borst, M.H.; Navis, G. Sodium intake, RAAS-blockade and progressive renal disease. Pharmacol. Res. 2016, 107, 344–351. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; Nicola, L.D.; Conte, G. Dietary salt restriction in chronic kidney disease: A meta-analysis of randomized clinical trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef]
- Kwakernaak, A.J.; Krikken, J.A.; Binnenmars, S.H.; Visser, F.W.; Hemmelder, M.H.; Woittiez, A.-J.; Groen, H.; Laverman, G.D.; Navis, G. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: A randomised clinical trial. Lancet Diabetes Endocrinol. 2014, 2, 385–395. [Google Scholar] [CrossRef]
- Hu, E.A.; Steffen, L.M.; Grams, M.E.; Crews, D.C.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Dietary patterns and risk of incident chronic kidney disease: The Atherosclerosis Risk in Communities study. Am. J. Clin. Nutr. 2019, 110, 713–721. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller Iii, E.R.; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kaneko, H.; Okada, A.; Itoh, H.; Morita, K.; Fujiu, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H. Change in cardiovascular health metrics and risk for proteinuria development: Analysis of a nationwide population-based database. Am. J. Nephrol. 2022, 53, 240–248. [Google Scholar] [CrossRef]
| Ideal | Intermediate | Poor | |
|---|---|---|---|
| Smoking status | never smoking, | former smoking | current smoking |
| Healthy diet score a | intake of 4–5 components | intake of 2–3 components | intake of 0–1 components |
| Physical activity | exercise ≥ 210 min/week | exercise 60–210 min/week | exercise < 60 min/week |
| Blood pressure | SBP < 120 mmHg and DBP < 80 mmHg | SBP 120–139 mmHg or DBP 80–89 mmHg | SBP ≥ 140 mmHg or DBP ≥ 90 mmHg |
| Body mass index | <25 kg/m2 | 25–29.9 kg/m2 | ≥30 kg/m2 |
| Total cholesterol | <200 mg/dL | 200–239 mg/dL | ≥240 mg/dL |
| Fasting glucose | <100 mg/dL | 100–125 mg/dL | ≥126 mg/dL |
| Number of Ideal CVH Metrics | |||||||||
| Continuous Variables a | All (n = 169,366) | 0 (n = 2539) | 1 (n = 11,768) | 2 (n = 25,391) | 3 (n = 38,674) | 4 (n = 47,044) | 5 (n = 38,706) | 6–7 (n = 5244) | p-Value |
| Age, mean (SD), years | 39.3 (11.6) | 42.8 (10.2) | 43.8 (11.5) | 43.3 (12.1) | 41.1 (12.0) | 37.8 (11.0) | 35.2 (9.7) | 39.1 (11.4) | <0.01 |
| BMI, mean (SD), kg/m2 | 23.1 (3.5) | 28.0 (2.6) | 27.2 (3.1) | 25.5 (3.4) | 23.7 (3.2) | 22.0 (2.8) | 20.8 (2.3) | 21.2 (2.1) | <0.01 |
| Total cholesterol, mean (SD), mg/dL | 192.2 (35.1) | 231.9 (27.9) | 221.5 (32.9) | 209.6 (34.7) | 198.9 (34.3) | 187.2 (32.2) | 171.5 (24.0) | 171.2 (22.2) | <0.01 |
| Fasting sugar, mean (SD), mg/dL | 98.5 (18.6) | 116.0 (29.8) | 112.2 (29.7) | 106.4 (24.7) | 100.2 (18.3) | 94.9 (12.4) | 91.6 (8.0) | 91.5 (7.3) | <0.01 |
| Systolic pressure, mean (SD), mmHg | 117.9 (16.7) | 134.8 (12.5) | 133.0 (14.7) | 128.7 (15.6) | 122.5 (15.8) | 114.4 (14.6) | 107.0 (11.1) | 107.0 (10.4) | <0.01 |
| Diastolic pressure, mean (SD), mmHg | 71.1 (11.1) | 83.1 (10.0) | 81.0 (10.7) | 77.7 (10.6) | 73.7 (10.6) | 68.9 (9.8) | 64.6 (8.1) | 64.7 (7.9) | <0.01 |
| Creatinine, mean (SD), mg/dL | 0.96 (0.20) | 1.08 (0.16) | 1.05 (0.18) | 1.02 (0.19) | 0.99 (0.21) | 0.94 (0.19) | 0.88 (0.18) | 0.89 (0.18) | <0.01 |
| Categorical Variables b | All (n = 169,366) | 0 (n = 2539) | 1 (n = 11,768) | 2 (n = 25,391) | 3 (n = 38,674) | 4 (n = 47,044) | 5 (n = 38,706) | 6–7 (n = 5244) | p-Value |
| Sex | <0.01 | ||||||||
| Male (%) | 91,140 (53.8) | 2466 (97.1) | 9704 (82.5) | 18,835 (74.2) | 24,912 (64.4) | 22,661 (48.2) | 10,946 (28.3) | 1616 (30.8) | |
| Female (%) | 78,226 (46.2) | 73 (2.9) | 2064 (17.5) | 6556 (25.8) | 13,762 (35.6) | 24,383 (51.8) | 27,760 (71.7) | 3628 (69.2) | |
| Education | <0.01 | ||||||||
| Below high school (%) | 57,226 (34.3) | 1024 (40.7) | 5008 (43.2) | 10,414 (41.6) | 14,604 (38.3) | 14,811 (31.9) | 9604 (25.1) | 1761 (34.0) | |
| beyond high school (%) | 109,846 (65.7) | 1489 (59.3) | 6591 (56.8) | 14,591 (58.4) | 23,529 (61.7) | 31,621 (68.1) | 28,613 (74.9) | 3412 (66.0) | |
| Family income | <0.01 | ||||||||
| <1.2 million NTD (%) | 51,288 (76.1) | 938 (73.1) | 4122 (75.3) | 8062 (74.6) | 11,712 (75.6) | 14,043 (76.8) | 11,259 (77.5) | 1152 (74.1) | |
| >1.2 million NTD (%) | 16,142 (23.9) | 346 (26.9) | 1355 (24.7) | 2747 (25.4) | 3774 (24.4) | 4245 (23.2) | 3273 (22.5) | 402 (25.9) | |
| Alcohol consumption | <0.01 | ||||||||
| Non-drinker (%) | 134,006 (82.2) | 1388 (56.0) | 7559 (66.7) | 17,954 (73.6) | 29,605 (79.6) | 38,526 (85.2) | 34,425 (92.2) | 4549 (90.3) | |
| Former drinker (%) | 3976 (2.4) | 130 (5.2) | 504 (4.4) | 877 (3.6) | 988 (2.7) | 991 (2.2) | 402 (1.1) | 84 (1.7) | |
| 1–2 times/week (%) | 16,820 (10.3) | 562 (22.7) | 1976 (17.4) | 3606 (14.8) | 4410 (11.9) | 4042 (8.9) | 1916 (5.1) | 308 (6.1) | |
| 3–4 times/week (%) | 5501 (3.4) | 268 (10.8) | 843 (7.4) | 1324 (5.4) | 1451 (3.9) | 1129 (2.5) | 416 (1.1) | 70 (1.4) | |
| ≥5 times/week (%) | 2665 (1.6) | 130 (5.2) | 454 (4.0) | 634 (2.6) | 715 (1.9) | 528 (1.2) | 176 (0.5) | 28 (0.6) | |
| Blood pressure c | <0.01 | ||||||||
| Not ideal (%) | 74,827 (44.2) | 2539 (100.0) | 10,728 (91.2) | 19,804 (78.0) | 23,008 (59.5) | 15,894 (33.8) | 2620 (6.8) | 234 (4.5) | |
| Ideal (%) | 94,539 (55.8) | 0 | 1040 (8.8) | 5587 (22.0) | 15,666 (40.5) | 31,150 (66.2) | 36,086 (93.2) | 5010 (95.5) | |
| Total cholesterol d | <0.01 | ||||||||
| Not ideal (%) | 64,637 (38.2) | 2539 (100.0) | 9805 (83.3) | 16,423 (64.7) | 18,769 (48.5) | 14,694 (31.2) | 2227 (5.8) | 180 (3.4) | |
| Ideal (%) | 104,729 (61.8) | 0 | 1963 (16.7) | 8968 (35.3) | 19,905 (51.5) | 32,350 (68.8) | 36,479 (94.2) | 5064 (96.6) | |
| Fasting glucose e | <0.01 | ||||||||
| Not ideal (%) | 55,765 (32.9) | 2539 (100.0) | 9873 (83.9) | 16,543 (65.2) | 16,241 (42.0) | 9043 (19.2) | 1400 (3.6) | 126 (2.4) | |
| Ideal (%) | 113,601 (67.1) | 0 | 1895 (16.1) | 8848 (34.8) | 22,433 (58.0) | 38,001 (80.8) | 37,306 (96.4) | 5118 (97.6) | |
| Body mass index f | <0.01 | ||||||||
| Not ideal (%) | 45,136 (26.6) | 2539 (100.0) | 9908 (84.2) | 14,706 (57.9) | 11,914 (30.8) | 5315 (11.3) | 708 (1.8) | 46 (0.9) | |
| Ideal (%) | 124,230 (73.4) | 0 | 1860 (15.8) | 10,685 (42.1) | 26,760 (69.2) | 41,729 (88.7) | 37,998 (98.2) | 5198 (99.1) | |
| Smoking g | <0.01 | ||||||||
| Not ideal (%) | 45,765 (27.0) | 2539 (100.0) | 7266 (61.7) | 11,272 (44.4) | 13,180 (34.1) | 10,243 (21.8) | 1197 (3.1) | 68 (1.3) | |
| Ideal (%) | 123,601 (73.0) | 0 | 4502 (38.3) | 14,119 (55.6) | 25,494 (65.9) | 36,801 (78.2) | 37,509 (96.9) | 5176 (98.7) | |
| Physical activity h | <0.01 | ||||||||
| Not ideal (%) | 152,396 (90.0) | 2539 (100.0) | 11,456 (97.3) | 23,761 (93.6) | 35,266 (91.2) | 42,421 (90.2) | 34,482 (89.1) | 2471 (47.1) | |
| Ideal (%) | 16,970 (10.0) | 0 | 312 (2.7) | 1630 (6.4) | 3408 (8.8) | 4623 (9.8) | 4224 (10.9) | 2773 (52.9) | |
| Healthy diet score i | <0.01 | ||||||||
| Not ideal (%) | 155,000 (91.5) | 2539 (100) | 11,572 (98.3) | 24,446 (96.3) | 36,318 (93.9) | 43,522 (92.5) | 34,778 (89.9) | 1825 (34.8) | |
| Ideal (%) | 14,366 (8.5) | 0 | 196 (1.7) | 945 (3.7) | 2356 (6.1) | 3522 (7.5) | 3928 (10.1) | 3419 (65.2) | |
| Hazard Ratio (95% CI) | |||
|---|---|---|---|
| Unadjusted | Model 1 a | Model 2 b | |
| CVH status (Number of ideal CVH metrics) | |||
| Low CVH (0–2) | 1 (ref) | 1 (ref) | 1 (ref) |
| Moderate CVH (3–4) | 0.39 (0.35–0.44) | 0.45 (0.40–0.50) | 0.46 (0.37–0.57) |
| High CVH (5–7) | 0.20 (0.17–0.24) | 0.27 (0.23–0.32) | 0.41 (0.30–0.55) |
| Number of ideal CVH metrics | |||
| 0 | 1 (ref) | 1 (ref) | 1 (ref) |
| 1 | 0.64 (0.49–0.83) | 0.60 (0.46–0.78) | 0.73 (0.45–1.17) |
| 2 | 0.40 (0.31–0.51) | 0.38 (0.29–0.49) | 0.50 (0.31–0.80) |
| 3 | 0.26 (0.20–0.33) | 0.26 (0.20–0.34) | 0.34 (0.21–0.54) |
| 4 | 0.15 (0.11–0.19) | 0.16 (0.13–0.21) | 0.22 (0.13–0.36) |
| 5 | 0.10 (0.08–0.13) | 0.13 (0.09–0.17) | 0.25 (0.15–0.42) |
| 6–7 c | 0.10 (0.06–0.15) | 0.10 (0.06–0.16) | 0.12 (0.04–0.36) |
| Hazard Ratio (95% CI) | |||
|---|---|---|---|
| Unadjusted | Model 1 a | Model 2 b | |
| Fruits and vegetables (≥450 g/day) | 1.06 (0.95–1.18) | 0.98 (0.88–1.10) | 1.00 (0.82–1.22) |
| Fiber-rich whole grains (≥85 g/day) | 1.33 (1.05–1.69) | 1.04 (0.82–1.32) | 0.93 (0.54–1.62) |
| Sodium (<1500 mg/day) | 0.76 (0.63–0.91) | 0.68 (0.57–0.82) | 0.58 (0.43–0.79) |
| Fish (≥198 g/week) | 1.10 (0.99–1.23) | 1.02 (0.91–1.13) | 1.03 (0.84–1.26) |
| Sugar-sweetened beverages (≤1 L/week) | 1.24 (1.11–1.37) | 0.97 (0.87–1.08) | 0.88 (0.72–1.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.-M.; Chen, W.-L.; Kao, T.-W.; Wu, L.-W.; Yang, H.-F.; Peng, T.-C. Relationship between Ideal Cardiovascular Health and Incident Proteinuria: A 5 Year Retrospective Cohort Study. Nutrients 2022, 14, 4040. https://doi.org/10.3390/nu14194040
He Y-M, Chen W-L, Kao T-W, Wu L-W, Yang H-F, Peng T-C. Relationship between Ideal Cardiovascular Health and Incident Proteinuria: A 5 Year Retrospective Cohort Study. Nutrients. 2022; 14(19):4040. https://doi.org/10.3390/nu14194040
Chicago/Turabian StyleHe, Yu-Min, Wei-Liang Chen, Tung-Wei Kao, Li-Wei Wu, Hui-Fang Yang, and Tao-Chun Peng. 2022. "Relationship between Ideal Cardiovascular Health and Incident Proteinuria: A 5 Year Retrospective Cohort Study" Nutrients 14, no. 19: 4040. https://doi.org/10.3390/nu14194040
APA StyleHe, Y.-M., Chen, W.-L., Kao, T.-W., Wu, L.-W., Yang, H.-F., & Peng, T.-C. (2022). Relationship between Ideal Cardiovascular Health and Incident Proteinuria: A 5 Year Retrospective Cohort Study. Nutrients, 14(19), 4040. https://doi.org/10.3390/nu14194040






