Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges
Abstract
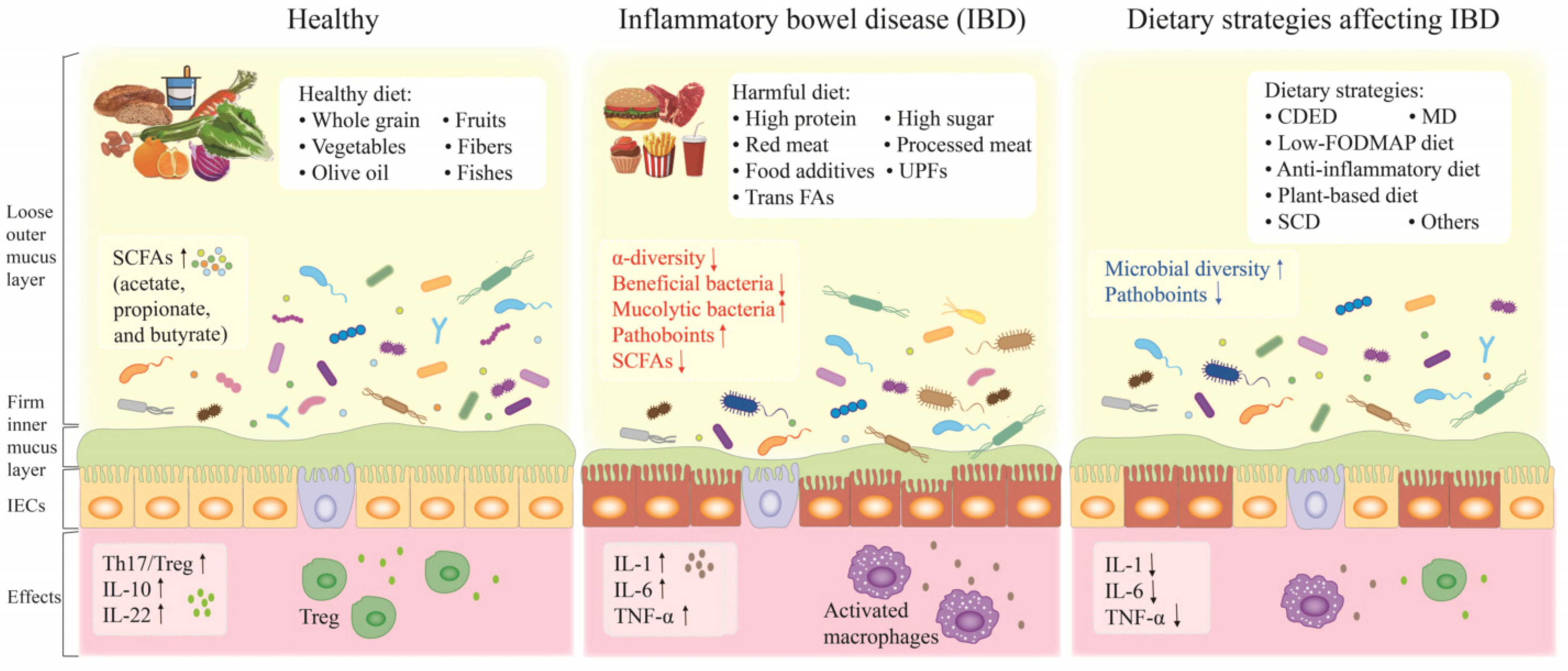
1. Introduction
2. Inflammatory Bowel Disease and Gut Microbiota
3. Westernized Diet, Gut Dysbiosis and IBD
3.1. High Protein
3.2. Heme
3.3. Fatty Acids
3.4. High Sugar
3.5. Food Additives
4. Dietary Strategies Alleviating IBD
4.1. Crohn’s Disease Exclusion Diet (CDED)
4.2. Mediterranean Diet (MD)
4.3. Low-FODMAP Diet
4.4. Anti-Inflammatory Diet
- (1)
- Restrict specific carbohydrates such as refined or processed complex carbohydrates and lactose.
- (2)
- Increase the consumption of prebiotics, probiotics and food rich in components that help restore the balance of intestinal flora.
- (3)
- Increase foods intake rich in omega-3 PUFAs while reducing total fat and saturated fatty acids intake.
- (4)
- Evaluate patient’s dietary pattern and monitor potential nutrient deficiencies. Modify food texture to improve nutrient absorption (e.g., homogenized, cooked, ground) [157].
4.5. Specific Carbohydrate Diet (SCD)
4.6. Plant-Based Diets
4.7. Other Dietary Patterns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Mack, D.R.; Guttmann, A.; Nguyen, G.C.; To, T.; Mojaverian, N.; Quach, P.; Manuel, D.G. Inflammatory bowel disease in immigrants to Canada and their children: A population-based cohort study. Am. J. Gastroenterol. 2015, 110, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental risk factors for inflammatory bowel diseases: An umbrella review of meta-analyses. Gastroenterology 2019, 157, 647–659.e644. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef]
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic review and meta-analysis: Association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 362–371. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. 2016, 1, 307–316. [Google Scholar] [CrossRef]
- Schreiner, P.; Martinho-Grueber, M.; Studerus, D.; Vavricka, S.R.; Tilg, H.; Biedermann, L.; on behalf of Swiss Ibdnet, an Official Working Group of the Swiss Society of Gastroenterology. Nutrition in Inflammatory Bowel Disease. Digestion 2020, 101 (Suppl. 1), 120–135. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Vasseur, P.; Dugelay, E.; Benamouzig, R.; Savoye, G.; Lan, A.; Srour, B.; Hercberg, S.; Touvier, M.; Hugot, J.P.; Julia, C.; et al. Dietary patterns, ultra-processed food, and the risk of inflammatory bowel diseases in the NutriNet-Sante cohort. Inflamm. Bowel Dis. 2021, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.L.; Devkota, S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Mol. Metab. 2016, 5, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Milagro, F.I.; Aranaz, P.; Martinez, J.A.; Riezu-Boj, J.I. Gut microbiota differences according to ultra-processed food consumption in a Spanish population. Nutrients 2021, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012, 30, 759–795. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Groen, R.N.; de Clercq, N.C.; Nieuwdorp, M.; Hoenders, H.J.R.; Groen, A.K. Gut microbiota, metabolism and psychopathology: A critical review and novel perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 283–293. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Yu, S.; Balasubramanian, I.; Laubitz, D.; Tong, K.; Bandyopadhyay, S.; Lin, X.; Flores, J.; Singh, R.; Liu, Y.; Macazana, C.; et al. Paneth cell-derived lysozyme defines the composition of mucolytic microbiota and the inflammatory tone of the intestine. Immunity 2020, 53, 398–416.e8. [Google Scholar] [CrossRef]
- Png, C.W.; Linden, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordevic, D.; Vitezova, M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J. Adv. Res. 2021, 27, 71–78. [Google Scholar] [CrossRef]
- Preda, C.M.; Manuc, T.; Chifulescu, A.; Istratescu, D.; Louis, E.; Baicus, C.; Sandra, I.; Diculescu, M.M.; Reenaers, C.; van Kemseke, C.; et al. Diet as an environmental trigger in inflammatory bowel disease: A retrospective comparative study in two European cohorts. Rev. Esp. Enferm. Dig. 2020, 112, 440–447. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, X.; Santos Rocha, C.; Rolston, M.; Garcia-Melchor, E.; Huynh, M.; Nguyen, M.; Law, T.; Haas, K.N.; Yamada, D.; et al. Short-term western diet intake promotes IL-23 mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J. Investig. Dermatol. 2021, 141, 1780–1791. [Google Scholar] [CrossRef]
- Agus, A.; Denizot, J.; Thevenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Cabral, D.J.; Wurster, J.I.; Korry, B.J.; Penumutchu, S.; Belenky, P. Consumption of a Western-style diet modulates the response of the murine gut microbiome to ciprofloxacin. mSystems 2020, 5, e00317-20. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Luo, Z.; Zhu, W. Temporal microbiota changes of high-protein diet intake in a rat model. Anaerobe 2017, 47, 218–225. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Song, S.; Zhang, M.; Zamaratskaia, G.; Xu, X.; Zhou, G.; Li, C. High-meat-protein high-fat diet induced dysbiosis of gut microbiota and tryptophan metabolism in Wistar rats. J. Agric. Food. Chem. 2020, 68, 6333–6346. [Google Scholar] [CrossRef]
- Li, D.P.; Cui, M.; Tan, F.; Liu, X.Y.; Yao, P. High red meat intake exacerbates dextran sulfate-induced colitis by altering gut microbiota in mice. Front. Nutr. 2021, 8, 646819. [Google Scholar] [CrossRef]
- Selmin, O.I.; Papoutsis, A.J.; Hazan, S.; Smith, C.; Greenfield, N.; Donovan, M.G.; Wren, S.N.; Doetschman, T.C.; Snider, J.M.; Snider, A.J.; et al. N-6 high fat diet induces gut microbiome dysbiosis and colonic inflammation. Int. J. Mol. Sci. 2021, 22, 6919. [Google Scholar] [CrossRef]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet rich in simple sugars promotes pro-inflammatory response via gut microbiota alteration and TLR4 signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.A.W.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C.; Peng, L.; Hooper, L.V.; Zaki, H. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci. Transl. Med. 2020, 12, eaay6218. [Google Scholar] [CrossRef]
- Montrose, D.C.; Nishiguchi, R.; Basu, S.; Staab, H.A.; Zhou, X.K.; Wang, H.; Meng, L.; Johncilla, M.; Cubillos-Ruiz, J.R.; Morales, D.K.; et al. Dietary fructose alters the composition, localization, and metabolism of gut microbiota in association with worsening colitis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 525–550. [Google Scholar] [CrossRef]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of food additives on gut homeostasis. Nutrients 2019, 11, 2334. [Google Scholar] [CrossRef]
- Zheng, J.; Hoffman, K.L.; Chen, J.S.; Shivappa, N.; Sood, A.; Browman, G.J.; Dirba, D.D.; Hanash, S.; Wei, P.; Hebert, J.R.; et al. Dietary inflammatory potential in relation to the gut microbiome: Results from a cross-sectional study. Br. J. Nutr. 2020, 124, 931–942. [Google Scholar] [CrossRef]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201.e125. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Investigators, I.B.D.i.E.S.; Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Nagel, G.; Linseisen, J.; Hallmans, G.; Palmqvist, R.; Sjodin, H.; Hagglund, G.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the etiology of ulcerative colitis: A nested case-control study within a European prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar] [CrossRef]
- Dong, C.; Chan, S.S.M.; Jantchou, P.; Racine, A.; Oldenburg, B.; Weiderpass, E.; Heath, A.K.; Tong, T.Y.N.; Tjønneland, A.; Kyrø, C.; et al. Meat intake is associated with a higher risk of ulcerative colitis in a large European prospective cohort studyø. J. Crohns Colitis 2022, 16, 1187–1196. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Qalqili, T.R.; Ajeen, R.; Rayyan, Y.M. Dietary patterns and the risk of inflammatory bowel disease: Findings from a case-control study. Nutrients 2021, 13, 1889. [Google Scholar] [CrossRef]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet rich in animal protein promotes pro-inflammatory macrophage response and exacerbates colitis in mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Kurata, K.; Kawahara, H.; Nishimura, K.; Jisaka, M.; Yokota, K.; Shimizu, H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem. Biophys. Res. Commun. 2019, 510, 649–655. [Google Scholar]
- Raffner Basson, A.; Gomez-Nguyen, A.; LaSalla, A.; Butto, L.; Kulpins, D.; Warner, A.; Di Martino, L.; Ponzani, G.; Osme, A.; Rodriguez-Palacios, A.; et al. Replacing animal protein with soy-pea protein in an “American Diet” controls murine Crohn disease-like ileitis regardless of Firmicutes: Bacteroidetes ratio. J. Nutr. 2021, 151, 579–590. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Yi, J.; Liu, Y.; Yu, Z.; Chen, S.; Liu, X. Increased mucin-degrading bacteria by high protein diet leads to thinner mucus layer and aggravates experimental colitis. J. Gastroenterol. Hepatol. 2021, 36, 2864–2874. [Google Scholar] [CrossRef]
- Rubin, K.H.; Rasmussen, N.F.; Petersen, I.; Kopp, T.I.; Stenager, E.; Magyari, M.; Hetland, M.L.; Bygum, A.; Glintborg, B.; Andersen, V. Intake of dietary fibre, red and processed meat and risk of late-onset Chronic Inflammatory Diseases: A prospective Danish study on the “diet, cancer and health” cohort. Int. J. Med. Sci. 2020, 17, 2487–2495. [Google Scholar] [CrossRef]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A diet low in red and processed meat does not reduce rate of Crohn’s disease flares. Gastroenterology 2019, 157, 128–136.e125. [Google Scholar] [CrossRef]
- Khalili, H.; de Silva, P.S.; Ananthakrishnan, A.N.; Lochhead, P.; Joshi, A.; Garber, J.J.; Richter, J.R.; Sauk, J.; Chan, A.T. Dietary iron and heme iron consumption, genetic susceptibility, and risk of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2017, 23, 1088–1095. [Google Scholar] [CrossRef]
- Constante, M.; Fragoso, G.; Calve, A.; Samba-Mondonga, M.; Santos, M.M. Dietary heme induces gut dysbiosis, aggravates colitis, and potentiates the development of adenomas in mice. Front. Microbiol. 2017, 8, 1809. [Google Scholar] [CrossRef]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The Imbalance between n-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A Comprehensive Review and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar]
- Tanaka, S.; Nemoto, Y.; Takei, Y.; Morikawa, R.; Oshima, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; Stutte, S.; et al. High-fat diet-derived free fatty acids impair the intestinal immune system and increase sensitivity to intestinal epithelial damage. Biochem. Biophys. Res. Commun. 2020, 522, 971–977. [Google Scholar] [CrossRef]
- Xie, M.; Yang, J.; Zhang, J.; Sherman, H.L.; Zhang, Z.; Minter, L.M.; Hammock, B.D.; Park, Y.; Zhang, G. Effects of linoleic acid-rich diet on plasma profiles of eicosanoids and development of colitis in Il-10−/− mice. J. Agric. Food. Chem. 2020, 68, 7641–7647. [Google Scholar] [CrossRef]
- Higashimura, Y.; Tanaka, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Niki, E.; Naito, Y. Trans-unsaturated fatty acid activates NLRP3 inflammasome in macrophages and exacerbates intestinal inflammation in mice. Biochem. Biophys. Res. Commun. 2020, 529, 243–250. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, W.; Tao, H.; Zhang, Y.; Liu, L.; Liu, Z.; Qiu, B.; Xu, T. Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur. J. Nutr. 2019, 58, 2625–2638. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Ge, Y.; Qiu, B.; Zhang, D.; Wang, X.; Liu, W.; Tao, H. Comparative transcriptome and microbiota analyses provide new insights into the adverse effects of industrial trans fatty acids on the small intestine of C57BL/6 mice. Eur. J. Nutr. 2021, 60, 975–987. [Google Scholar] [CrossRef]
- Opstelten, J.L.; de Vries, J.H.M.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J.M. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin. Nutr. 2019, 38, 1892–1898. [Google Scholar] [CrossRef]
- Fu, T.; Chen, H.; Chen, X.; Sun, Y.; Xie, Y.; Deng, M.; Hesketh, T.; Wang, X.; Chen, J. Sugar-sweetened beverages, artificially sweetened beverages and natural juices and risk of inflammatory bowel disease: A cohort study of 121,490 participants. Aliment. Pharmacol. Ther. 2022, 56, 1018–1029. [Google Scholar] [CrossRef]
- Wang, F.; Feng, J.; Gao, Q.; Ma, M.; Lin, X.; Liu, J.; Li, J.; Zhao, Q. Carbohydrate and protein intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. Clin. Nutr. 2017, 36, 1259–1265. [Google Scholar] [CrossRef]
- Khademi, Z.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Dietary intake of total carbohydrates, sugar and sugar-sweetened beverages, and risk of inflammatory bowel disease: A systematic review and meta-analysis of prospective cohort studies. Front. Nutr. 2021, 8, 707795. [Google Scholar] [CrossRef]
- Khalili, H.; Hakansson, N.; Chan, S.S.; Ludvigsson, J.F.; Olen, O.; Chan, A.T.; Hart, A.R.; Wolk, A. No association between consumption of sweetened beverages and risk of later-onset Crohn’s disease or ulcerative colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 123–129. [Google Scholar] [CrossRef]
- Nie, J.Y.; Zhao, Q. Beverage consumption and risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine 2017, 96, e9070. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, L.; He, J. Beverage intake and risk of Crohn disease: A meta-analysis of 16 epidemiological studies. Medicine 2019, 98, e15795. [Google Scholar] [CrossRef]
- Zeng, L.; Hu, S.; Chen, P.; Wei, W.; Tan, Y. Macronutrient intake and risk of Crohn’s disease: Systematic review and dose-response meta-analysis of epidemiological studies. Nutrients 2017, 9, 500. [Google Scholar] [CrossRef]
- Racine, A.; Carbonnel, F.; Chan, S.S.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.; Tjonneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary patterns and risk of inflammatory bowel disease in Europe: Results from the EPIC study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef]
- Li, J.M.; Yu, R.; Zhang, L.P.; Wen, S.Y.; Wang, S.J.; Zhang, X.Y.; Xu, Q.; Kong, L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Iossa, S.; Cigliano, L. Sweet but bitter: Focus on fructose impact on brain function in rodent models. Nutrients 2020, 13, 1. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Amamou, A.; Savoye, G.; Ghosh, S. Inflammatory bowel diseases and food additives: To add fuel on the flames! Nutrients 2019, 11, 1111. [Google Scholar] [CrossRef]
- Elmen, L.; Zlamal, J.E.; Scott, D.A.; Lee, R.B.; Chen, D.J.; Colas, A.R.; Rodionov, D.A.; Peterson, S.N. Dietary emulsifier sodium stearoyl lactylate alters gut microbiota in vitro and inhibits bacterial butyrate producers. Front. Microbiol. 2020, 11, 892. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environ. Toxicol. Pharmacol. 2020, 80, 103485. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Chang, H.; Wang, R.; You, Z.; Jiang, S.; Ma, C.; Huo, D.; Zhu, X.; Zhang, J. Potassium sorbate suppresses intestinal microbial activity and triggers immune regulation in zebrafish (Danio rerio). Food Funct. 2019, 10, 7164–7173. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Tang, Q.; Ma, J.; Liu, X.; Zhou, B.; Sun, Y.; Pang, X.; Guo, Z.; Xie, R.; Liu, T.; et al. Maternal emulsifier p80 intake induces gut dysbiosis in offspring and increases their susceptibility to colitis in adulthood. mSystems 2021, 6, e01337-20. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary therapy with the Crohn’s Disease Exclusion Diet is a successful strategy for induction of remission in children and adults failing biological therapy. J. Crohns Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 2019, 157, 440–450.e448. [Google Scholar] [CrossRef]
- Niseteo, T.; Sila, S.; Trivic, I.; Misak, Z.; Kolacek, S.; Hojsak, I. Modified Crohn’s disease exclusion diet is equally effective as exclusive enteral nutrition: Real-world data. Nutr. Clin. Pract. 2022, 37, 435–441. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary therapies induce rapid response and remission in pediatric patients with active Crohn’s disease. Clin. Gastroenterol. Hepatol. 2021, 19, 752–759. [Google Scholar] [CrossRef]
- Szczubelek, M.; Pomorska, K.; Korolczyk-Kowalczyk, M.; Lewandowski, K.; Kaniewska, M.; Rydzewska, G. Effectiveness of Crohn’s Disease Exclusion Diet for induction of remission in Crohn’s disease adult patients. Nutrients 2021, 13, 4112. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. 2022, 7, 49–59. [Google Scholar] [CrossRef]
- El Amrousy, D.; Elashry, H.; Salamah, A.; Maher, S.; Abd-Elsalam, S.M.; Hasan, S. Adherence to the Mediterranean Diet improved clinical scores and inflammatory markers in children with active inflammatory bowel disease: A randomized trial. J. Inflamm. Res. 2022, 15, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent prebiotic effect on gut microbiota with altered FODMAP intake in patients with Crohn’s disease: A randomised, controlled cross-over trial of well-defined diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef] [PubMed]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Valcheva, R.; Nickurak, C.; Park, H.; Mandal, R.; van Diepen, K.; Kroeker, K.I.; van Zanten, S.V.; Halloran, B.; Wishart, D.S.; et al. Anti-inflammatory diet prevents subclinical colonic inflammation and alters metabolomic profile of ulcerative colitis patients in clinical remission. Nutrients 2022, 14, 3294. [Google Scholar] [CrossRef]
- Suskind, D.L.; Wahbeh, G.; Gregory, N.; Vendettuoli, H.; Christie, D. Nutritional therapy in pediatric Crohn disease: The specific carbohydrate diet. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 87–91. [Google Scholar] [CrossRef]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef]
- Khandalavala, B.N.; Nirmalraj, M.C. Resolution of severe ulcerative colitis with the Specific Carbohydrate Diet. Case Rep. Gastroenterol. 2015, 9, 291–295. [Google Scholar] [CrossRef]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Wahbeh, G.; Cohen, S.A.; Damman, C.J.; Klein, J.; Braly, K.; Shaffer, M.; Lee, D. Patients perceive clinical benefit with the Specific Carbohydrate Diet for inflammatory bowel disease. Dig. Dis. Sci. 2016, 61, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Burgis, J.C.; Nguyen, K.; Park, K.T.; Cox, K. Response to strict and liberalized specific carbohydrate diet in pediatric Crohn’s disease. World J. Gastroenterol. 2016, 22, 2111–2117. [Google Scholar] [CrossRef]
- Wahbeh, G.T.; Ward, B.T.; Lee, D.Y.; Giefer, M.J.; Suskind, D.L. Lack of mucosal healing from modified Specific Carbohydrate Diet in pediatric patients with Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Cohen, S.A.; Brittnacher, M.J.; Wahbeh, G.; Lee, D.; Shaffer, M.L.; Braly, K.; Hayden, H.S.; Klein, J.; Gold, B.; et al. Clinical and fecal microbial changes with diet therapy in active inflammatory bowel disease. J. Clin. Gastroenterol. 2018, 52, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Lee, D.; Kim, Y.M.; Wahbeh, G.; Singh, N.; Braly, K.; Nuding, M.; Nicora, C.D.; Purvine, S.O.; Lipton, M.S.; et al. The Specific Carbohydrate Diet and diet modification as induction therapy for pediatric Crohn’s disease: A randomized diet controlled trial. Nutrients 2020, 12, 3749. [Google Scholar] [CrossRef]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A randomized trial comparing the Specific Carbohydrate Diet to a Mediterranean Diet in adults with Crohn’s disease. Gastroenterology 2021, 161, 837–852.e839. [Google Scholar] [CrossRef]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Ito, M.; Komatsu, M.; Sugawara, T. Induction with Infliximab and a Plant-Based Diet as First-Line (IPF) Therapy for Crohn Disease: A single-group trial. Perm. J. 2017, 21, 17-009. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Tsuji, T.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Obara, Y.; Komatsu, M.; Sugawara, T. Relapse prevention by Plant-Based Diet incorporated into induction therapy for ulcerative colitis: A single-group trial. Perm. J. 2019, 23, 18–220. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ishii, H.; Ohno, H.; Obara, Y.; Komatsu, M.; Tozawa, H. High remission rate with Infliximab and Plant-Based Diet as First-Line (IPF) therapy for severe ulcerative colitis: Single-group trial. Perm. J. 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scarallo, L.; Banci, E.; Pierattini, V.; Lionetti, P. Crohn’s disease exclusion diet in children with Crohn’s disease: A case series. Curr. Med. Res. Opin. 2021, 37, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Hakansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olen, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Cenni, S.; Serra, M.R.; Dolce, P.; Martinelli, M.; Staiano, A.; Miele, E. Effectiveness of Mediterranean Diet’s adherence in children with inflammatory bowel diseases. Nutrients 2020, 12, 3206. [Google Scholar] [CrossRef]
- Fiorindi, C.; Dinu, M.; Gavazzi, E.; Scaringi, S.; Ficari, F.; Nannoni, A.; Sofi, F.; Giudici, F. Adherence to Mediterranean diet in patients with inflammatory bowel disease. Clin. Nutr. ESPEN 2021, 46, 416–423. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, H.; Chen, S.; He, J.; Zhou, Y.; Nie, Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Illescas, O.; Rodriguez-Sosa, M.; Gariboldi, M. Mediterranean Diet to prevent the development of colon diseases: A meta-analysis of gut microbiota studies. Nutrients 2021, 13, 2234. [Google Scholar] [CrossRef] [PubMed]
- Farras, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the gut microbiota by olive oil phenolic compounds: Implications for lipid metabolism, immune system, and obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef] [PubMed]
- Balfego, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martinez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N.; et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Antoniussen, C.S.; Rasmussen, H.H.; Holst, M.; Lauridsen, C. Reducing disease activity of inflammatory bowel disease by consumption of plant-based foods and nutrients. Front. Nutr. 2021, 8, 733433. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.; Hayee, B.H.; Rodriguez-Mateos, A. (Poly)phenols in inflammatory bowel disease and irritable bowel syndrome: A review. Molecules 2021, 26, 1843. [Google Scholar] [CrossRef]
- Larussa, T.; Imeneo, M.; Luzza, F. Olive Tree biophenols in inflammatory bowel disease: When bitter is better. Int. J. Mol. Sci. 2019, 20, 1390. [Google Scholar] [CrossRef]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight adult subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.G.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols -induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory activity of extra virgin olive oil polyphenols: Which role in the prevention and treatment of immune-mediated inflammatory diseases? Endocr. Metab. Immune 2018, 18, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein decreases cyclooxygenase-2 and interleukin-17 expression and attenuates inflammatory damage in colonic samples from ulcerative colitis patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020, 586, 119515. [Google Scholar] [CrossRef]
- Mantzioris, E.; Muhlhausler, B.S.; Villani, A. Impact of the Mediterranean dietary pattern on n-3 fatty acid tissue levels-A systematic review. Prostaglandins Leukot. Essent. Fat. Acids 2022, 176, 102387. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Zhuang, P.; Liu, X.; Zhang, Y.; Zhang, P.; Jiao, J. Habitual fish oil supplementation and risk of incident inflammatory bowel diseases: A prospective population-based study. Front. Nutr. 2022, 9, 905162. [Google Scholar] [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2020, 59, 1–17. [Google Scholar] [CrossRef]
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coeffier, M.; Guerin, C.; Ghosh, S.; Savoye, G.; Marion-Letellier, R. Dietary n-3 PUFA may attenuate experimental colitis. Mediat. Inflamm. 2018, 2018, 8430614. [Google Scholar] [CrossRef]
- Scaioli, E.; Sartini, A.; Bellanova, M.; Campieri, M.; Festi, D.; Bazzoli, F.; Belluzzi, A. Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1268–1275.e1262. [Google Scholar] [CrossRef]
- Schwärzler, J.; Mayr, L.; Vich Vila, A.; Grabherr, F.; Niederreiter, L.; Philipp, M.; Grander, C.; Meyer, M.; Jukic, A.; Tröger, S.; et al. PUFA-induced metabolic enteritis as a fuel for Crohn’s disease. Gastroenterology 2022, 162, 1690–1704. [Google Scholar]
- Gibson, P.R. History of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Halpin, S.J.; Ford, A.C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.L.; Zhan, Y.A.; Dai, S.X. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin. Nutr. 2018, 37, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.L.; Pop, C.; Dumitrascu, D.L. Diet advice for Crohn’s disease: FODMAP and beyond. Nutrients 2020, 12, 3751. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Goulart, R.A.; Aranao, A.L.C.; de Oliveira, P.G.C. Inflammatory bowel diseases and fermentable oligosaccharides, disaccharides, monosaccharides, and polyols: An overview. J. Med. Food 2018, 21, 633–640. [Google Scholar] [CrossRef]
- Simoes, C.D.; Maganinho, M.; Sousa, A.S. FODMAPs, inflammatory bowel disease and gut microbiota: Updated overview on the current evidence. Eur. J. Nutr. 2022, 61, 1187–1198. [Google Scholar] [CrossRef]
- Vandeputte, D.; Joossens, M. Effects of low and high FODMAP diets on human gastrointestinal microbiota composition in adults with intestinal diseases: A systematic review. Microorganisms 2020, 8, 1638. [Google Scholar] [CrossRef]
- Gibson, P.R. Use of the low-FODMAP diet in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 40–42. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Nigdelis, M.P.; Papageorgiou, S.T.; Papamitsou, T.; Forbes, A.; Bogdanos, D.P. Low FODMAP Diet for functional gastrointestinal symptoms in quiescent inflammatory bowel disease: A systematic review of randomized controlled trials. Nutrients 2020, 12, 3648. [Google Scholar] [CrossRef]
- Tian, Z.; Zhuang, X.; Zhao, M.; Zhuo, S.; Li, X.; Ma, R.; Li, N.; Liu, C.; Zhu, Y.; Tang, C.; et al. Index-based dietary patterns and inflammatory bowel disease: A systematic review of observational studies. Adv. Nutr. 2021, 12, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.R.; de Roos, N.M.; Witteman, B.J.M. The association between inflammatory potential of diet and disease activity: Results from a cross-sectional study in patients with inflammatory bowel disease. BMC Gastroenterol. 2020, 20, 316. [Google Scholar] [CrossRef]
- Vagianos, K.; Shafer, L.A.; Witges, K.; Targownik, L.E.; Haviva, C.; Graff, L.A.; Sexton, K.A.; Lix, L.M.; Sargent, M.; Bernstein, C.N. Association between change in inflammatory aspects of diet and change in IBD-related inflammation and symptoms over 1 year: The Manitoba living with IBD Study. Inflamm. Bowel Dis. 2021, 27, 190–202. [Google Scholar] [CrossRef]
- Mirmiran, P.; Moslehi, N.; Morshedzadeh, N.; Shivappa, N.; Hebert, J.R.; Farsi, F.; Daryani, N.E. Does the inflammatory potential of diet affect disease activity in patients with inflammatory bowel disease? Nutr. J. 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and validation of an empirical dietary inflammatory index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr. J. 2014, 13, 5. [Google Scholar] [CrossRef]
- Urlep, D.; Benedik, E.; Brecelj, J.; Orel, R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Pediatr. 2019, 179, 431–438. [Google Scholar] [CrossRef]
- Campmans-Kuijpers, M.J.E.; Dijkstra, G. Food and food groups in Inflammatory Bowel Disease (IBD): The design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients 2021, 13, 1067. [Google Scholar] [CrossRef]
- Braly, K.; Williamson, N.; Shaffer, M.L.; Lee, D.; Wahbeh, G.; Klein, J.; Giefer, M.; Suskind, D.L. Nutritional adequacy of the Specific Carbohydrate Diet in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 533–538. [Google Scholar] [CrossRef]
- Morrison, A.; Braly, K.; Singh, N.; Suskind, D.L.; Lee, D. Differences in nutrient intake with homemade versus chef-prepared Specific Carbohydrate Diet therapy in inflammatory bowel disease: Insights into dietary research. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional update for physicians: Plant-based diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.S.J.; Christophersen, C.T.; Devine, A.; Lawrance, I.C. The role of a plant-based diet in the pathogenesis, etiology and management of the inflammatory bowel diseases. Expert. Rev. Gastroenterol. 2020, 14, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Amarapurkar, A.D.; Amarapurkar, D.N.; Rathi, P.; Sawant, P.; Patel, N.; Kamani, P.; Rawal, K.; Baijal, R.; Sonawane, A.; Narawane, N.; et al. Risk factors for inflammatory bowel disease: A prospective multi-center study. Indian J. Gastroenterol. 2018, 37, 189–195. [Google Scholar] [CrossRef]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology 2019, 157, 1279–1292.e11. [Google Scholar] [CrossRef]
- Schreiner, P.; Yilmaz, B.; Rossel, J.B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef]
- Weaver, K.N.; Herfarth, H. Gluten-Free Diet in IBD: Time for a recommendation? Mol. Nutr. Food. Res. 2021, 65, e1901274. [Google Scholar] [CrossRef]
- Menta, P.L.R.; Andrade, M.E.R.; Leocadio, P.C.L.; Fraga, J.R.; Dias, M.T.S.; Cara, D.C.; Cardoso, V.N.; Borges, L.F.; Capettini, L.S.A.; Aguilar, E.C.; et al. Wheat gluten intake increases the severity of experimental colitis and bacterial translocation by weakening of the proteins of the junctional complex. Br. J. Nutr. 2019, 121, 361–373. [Google Scholar] [CrossRef]
- Pickert, G.; Wirtz, S.; Matzner, J.; Ashfaq-Khan, M.; Heck, R.; Rosigkeit, S.; Thies, D.; Surabattula, R.; Ehmann, D.; Wehkamp, J.; et al. Wheat consumption aggravates colitis in mice via amylase trypsin inhibitor-mediated dysbiosis. Gastroenterology 2020, 159, 257–272.e217. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Sepulveda, R.; Hing, T.; Shah, N.D.; Choe, M.; Limsui, D.; Shah, S. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 147–151. [Google Scholar] [CrossRef]
- Lopes, E.W.; Lebwohl, B.; Burke, K.E.; Ivey, K.L.; Ananthakrishnan, A.N.; Lochhead, P.; Richter, J.M.; Ludvigsson, J.F.; Willett, W.C.; Chan, A.T.; et al. Dietary gluten intake is not associated with risk of inflammatory bowel disease in US adults without celiac disease. Clin. Gastroenterol. Hepatol. 2022, 20, 303–313.e306. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Q.; Liao, Q.; Li, M.; Zhang, P.; Santos, H.O.; Kord-Varkaneh, H.; Abshirini, M. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2020, 79–80, 110974. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [Google Scholar] [CrossRef]
- Song, S.; Bai, M.; Ling, Z.; Lin, Y.; Wang, S.; Chen, Y. Intermittent administration of a fasting-mimicking diet reduces intestinal inflammation and promotes repair to ameliorate inflammatory bowel disease in mice. J. Nutr. Biochem. 2021, 96, 108785. [Google Scholar] [CrossRef]
- Bagherniya, M.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. 2018, 47, 183–197. [Google Scholar] [CrossRef]
- Negm, M.; Bahaa, A.; Farrag, A.; Lithy, R.M.; Badary, H.A.; Essam, M.; Kamel, S.; Sakr, M.; Abd El Aaty, W.; Shamkh, M.; et al. Effect of Ramadan intermittent fasting on inflammatory markers, disease severity, depression, and quality of life in patients with inflammatory bowel diseases: A prospective cohort study. BMC. Gastroenterol. 2022, 22, 203. [Google Scholar] [CrossRef]
- Scott, P.M. Which diet is better—Low-fat or low-carb? JAAPA 2006, 19, 49. [Google Scholar] [CrossRef]
- Kong, C.; Yan, X.; Liu, Y.; Huang, L.; Zhu, Y.; He, J.; Gao, R.; Kalady, M.F.; Goel, A.; Qin, H.; et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct. Target. Ther. 2021, 6, 154. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Li, S.; Zhuge, A.; Wang, K.; Lv, L.; Bian, X.; Yang, L.; Xia, J.; Jiang, X.; Wu, W.; Wang, S.; et al. Ketogenic diet aggravates colitis, impairs intestinal barrier and alters gut microbiota and metabolism in DSS-induced mice. Food Funct. 2021, 12, 10210–10225. [Google Scholar] [CrossRef]
- Wang, J.; Lin, X.; Bloomgarden, Z.T.; Ning, G. The Jiangnan diet, a healthy diet pattern for Chinese. J. Diabetes 2020, 12, 365–371. [Google Scholar] [CrossRef]
| Diet | Characteristics | Changes of Gut Microbiota | References |
|---|---|---|---|
| Westernized diet | High-fat and high-sugar | Proteobacteria ↑ E. coli ↑ Bacteroides spp. ↑ Ruminococcus torques ↑ | [32,33] |
| High protein | E. coli ↑ Bifidobacterium ↓ Prevotella ↓ Ruminococcus bromii ↓ Roseburia ↓ Eubacterium rectale ↓ Faecalibacterium prausnitzii ↓ Lactobacillus ↑ XIII AD3011 group ↑ Desulfovibrio ↑ | [35,36,37] | |
| High red meat | Bacteroides ↑ Alistipes ↑ Lachnospiraceae_NK4A136_group ↓ Faecalibaculum ↓ Blautia ↓ Dubosiella ↓ | [38] | |
| High fat rich in n-6 PUFAs | Bacteroidetes ↑ Deferribacteraceae ↑ Firmicutes ↓ Clostridia ↓ Lachnospiraceae ↓ | [39] | |
| High sugar | E. coli ↑ Candida ↑ Akkermansia muciniphila ↑ Bacteroides fragilis ↑ Citrobacter Rodentium ↑ Bifidobacterium pseudolongum ↓ Lactobacillus johnsonii ↓ Verrucomicrobiaceae ↑ Porphyromonadaceae ↑ Anaeroplasmataceae ↓ Prevotellaceae ↓ Lachnospiraceae ↓ | [40,41,42,43] | |
| More food additives | α-diversity ↓ Bifidobacterium ↓ Lactobacillus ↓ Pathoboints ↑ Mucus-degrading bacteria ↑ | [44] | |
| Pro-inflammatory diet | More pro-inflammatory foods | Ruminococcus torques ↑ Eubacterium nodatum ↑ Acidaminococcus intestine ↑ Clostridium leptum ↑ Akkermansia muciniphila ↑ | [45] |
| Diet Regimen | First Author | Study Design | Population | Intervention (Duration) | Control Group | Key Findings | Changes of Gut Microbiota |
|---|---|---|---|---|---|---|---|
| Crohn’s disease exclusion diet (CDED) | Sigall-Boneh R (2014) [86] | R | 34 children and 13 adults with mild-to-moderate CD | PEN + CDED (12 weeks, n = 40) CDED (12 weeks, n = 7) | N/A | Clinical remission observed in 24/34 children and 9/13 adults at week 6 and maintained in 27/33 patients at week 12; Significant fall in clinical disease activity and C-reactive protein. | Not analyzed |
| Sigall-Boneh R (2017) [87] | R | 11 adults and ten children with loss of response to biologics in CD | PEN + CDED (12 weeks, n = 21) Paediatric pateints with severe flares: EEN (14 days); PEN + CDED (10 weeks) | N/A | Clinical remission obtained in 13/21 (62%); Decrease in CRP and Harvey Bradshaw Index and increase in albumin. | Not analyzed | |
| Levine A (2019) [88] | P | 74 children with mild to moderate CD, six withdrawed | PEN (50%) + CDED (6 weeks); PEN (25%) + CDED (6 weeks, n = 40) | EEN (6 weeks); PEN (25%) + free diet (6 weeks, n = 38) | Clinical remission observed in 28/37 received CDED plus PEN and 14/31 received EEN and then PEN; Sustained fall in serum level of C-reactive protein, fecal level of calprotectin. | Haemophilus ↓ Veillonella ↓ Bifidobacterium ↓ Prevotella ↓ Anaerostipes ↓ Oscillibacter ↑ Roseburia ↑ | |
| Niseteo T (2022) [89] | R | 61 children | EEN (1–2 weeks) CDED + PEN (n = 20) | EEN (n = 41) | Clinical remission observed in 15/20 received CDED + PEN and 27/41 received EEN; Higher weight gain in CDED + PEN group. | Not analyzed | |
| Sigall Boneh R (2021) [90] | P | 73 children with mild to moderate CD | CDED + PEN (6 weeks, n = 39) | EEN (6 weeks, n = 34) | Rapid response or remission observed in 32/39 received CDED + PEN and 29/34 received EEN at week 3. | Not analyzed | |
| Szczubełek M (2021) [91] | P | 32 adults | PEN (50%) + CDED (12 weeks) | N/A | Clinical remission observed in 76.7% patients at 6 weeks and 82.1% at 12 weeks. Significant fall in calprotectin level. | Not analyzed | |
| Yanai H (2022) [92] | P | 40 adults | CDED (24 weeks, n = 21) | CDED + PEN (24 weeks, n = 19) | Clinical remission observed in 12/21 received CDED alone and 13/19 received CDED plus PEN, endoscopic remission observed in 6/21 received CDED alone and 8/19 received CDED plus PEN. | Not analyzed | |
| Mediterranean diet | El Amrousy D (2022) [93] | P | 100 children and adolescents with mild/moderate active IBD | MD (12 weeks, n = 50) | Regular diet (12 weeks, n = 50) | Significant decrease in PCDAI, PUCAI and inflmmatory markers (CRP, calprotectin, TNF-α, IL-17, IL-12 and IL-13) | Not analyzed |
| Low-FODMAP diet | Halmos EP (2016) [94] | 9 patients with clinically quiescent CD | Low-FODMAP diet (21 days) received low or tipical FODMAP diet with ≥21 day washout | N/A | Symptoms relief in low-FODMAP diet, but no effect on calprotectin. | Ruminococcus torques ↑ Clostridium cluster XIVa ↓ Akkermansia muciniphila ↓ no difference in SCFA and total bacterial abundance | |
| Bonidi G (2019) [95] | P | 55 adults with IBD (38 CD/22 UC) | Low-FODMAP diet (6 weeks, n = 26) | Standard diet (6 weeks, n = 29) | Disease activity, median calprotectin decreased, and disease-specific quality of life significantly increased in Low-FODMAP diet group but not in the standard diet group. | Not analyzed | |
| Cox SR (2020) [96] | P | 52 patients | Low-FODMAP diet (4 weeks, n = 27) | Control diet (4 weeks, n = 25) | Adequate relief in gut symptoms received low-FODMAP diet (14/27, 52%) than the control diet (4/25, 16%); Greater reduction in irritable bowel syndrome severity scores and higher health-related quality of life scores received low-FODMAP diet | Bifidobacterium adolescentis ↓ Bifidobacterium longum ↓ Faecalibacterium prausnitzi ↓ | |
| Anti-inflammatory Diet (AID) | Keshteli AH (2022) [97] | P | 53 patients with UC | AID (6 months, n = 26) | Canada’s Food Guide (6 months, n = 27) | Higher subclinical response (FCP < 150 µg/g at the endpoint) in AID group(69.2 vs. 37.0%) | Bifidobacteriaceae ↑ Lachnospiraceae ↑ Ruminococcaceae ↑ |
| Specific Carbohydrate Diet (SCD) | Suskind DL (2014) [98] | R | 7 children with CD | SCD (5 to 30 months) | N/A | All patients’ PCDAI decreased to 0 after 3 months; Improvement to normalization of albumin, CRP, and hematocrit. | Not analyzed |
| Cohen SA (2014) [99] | P | 10 children with active CD (PCDAI ≥ 15) | SCD + prescribed medications (52 weeks) | N/A | Improvement of PCDAI in 9 patients who completed the initial 12-week trial; Continued improvement of PCDAI in 7 patients who maintained 52 weeks and mucocal healing in 2 patients. | Not analyzed | |
| Khandalavala BN (2015) [100] | Case series | 36 patients with CD 9 patients with UC 5 patients with indeterminate colitis | SCD or SCD + medications (mean time 35.4 months) SCD | N/A | Mean effectiveness of 91.3% in controlling acute flare symptoms; Mean effectiveness of 92.1% at maintaining remission. | Not analyzed | |
| Obih C (2016) [101] | R | 20 children with CD 6 children with UC | SCD (3 to 48 months) | N/A | Fall in PCDAI from 32.8 ± 13.2 to 20.8 ± 16.6 by 4 ± 2 wk, and to 8.8 ± 8.5 by 6 months; For in mean PUCAI from 28.3 ± 10.3 to 20.0 ± 17.3 at 4 ± 2 wk, to 18.3 ± 31.7 at 6 months. | Not analyzed | |
| Suskind DL (2016) [102] | Anonymous online survey | 417 patients with IBD (47% CD, 43% UC, 10% indeterminate colitis) | SCD (34.9 ± 16.4 years) | N/A | Clinical remission less than 2 weeks in 13% patients, 2 weeks to a month in 36% patients, 1–3 months in 36% patients, and greater than 3 months in 34% patients. | Not analyzed | |
| Burgis JC (2016) [103] | R | 11 pediatric patients with CD | SCD simple (diet alone, antibiotics or 5-ASA) for 7.7 ± 4.0 months (range 1–12) | SCD with immunomodulators (corticosteroids and/or stable thiopurine dosing) | Improvement in hematocrit, albumin and ESR in both groups; Weight and height gain in the majority of children. | Not analyzed | |
| Wahbeh GT (2017) [104] | R | 7 pediatric patients with CD | Modified SCD (mSCD, 26 months) | N/A | No active systoms before mSCD; Consistent normalization in CRP, albumin and hematocrit; Mild elevation in fecal calprotectin; No endoscopic mucosal healing in any patients. | Not analyzed | |
| Suskind DL (2018) [105] | P | 9 pediatric patients with CD and 3 pediatric patients with UC | SCD (12 weeks) | N/A | Decrease in CRP, PCDAI and PUCAI; No clear pattern of dysbiosis in all patients before the SCD; Correction of dysbiosis in most patients after dietary change. | Not analyzed | |
| Suskind DL (2020) [106] | P | 18 pediatric patients with mild/moderate CD | SCD (12 weeks, n-5) Modified SCD (mSCD 12 weeks, n = 6) | Whole foods diet (WF 12 weeks, n = 5) | Decrease in CRP, PCDIA, ESR in all groups; decrease in calprotectin in mSCD and WF groups; Changes of the microbiota composition showed largely patient specific; Increase in predicted metabolic mode of the organisms. | Blautia species ↑ Lachnospiraceae species ↑ Roseburia hominis ↑ Roseburia intestinalis ↑ Anaerobutyricum hallii ↑ Eubacterium eligens ↑ E. coli ↓ Faecalibacterium prausnitzii ↓ | |
| Lewis JD (2021) [107] | P | 194 patients with mild/moderate CD | SCD (12 weeks, n = 97) | MD (12 weeks, n = 97) | No difference in symptom remission, calprotectin and CRP. Greater ease to follow the MD | Not analyzed | |
| Plant-based diet | Chiba M (2010) [108] | prospective single-group | 22 adult CD patients with clinical remission | semi-vegetarian diet (n = 16, 2 years) | Omnivorous diet (n = 6, 2 years) | 100% in remission rate at 1 year and 92% at 2 years in semi-vegetarian diet group. | Not analyzed |
| Chiba M (2017) [109] | prospective single-group | 46 patients with CD (35 adults and 11 children) | A lacto-ovo-semivegetarian diet combined with infliximab (6 weeks, n = 46) | N/A | Decrease in CDAI score and CRP level; Mucosal healing achieved in 46% of cases. | Not analyzed | |
| Chiba M (2019) [110] | prospective single-group | 92 UC (51 initial episodes, 41 relapses) | A lacto-ovo-semivegetarian diet combined with medication | N/A | Cumulative relapse rate rates at 1 and 5 years follow up (Kaplan-Meier analysis) were 14% and 27% respectively for the initial episode of case, and 36% and 53% respectively for relapse cases. | Not analyzed | |
| Chiba M (2020) [111] | prospective single-group | 17 patients with severe UC | A lacto-ovo-semivegetarian diet combined with infliximab (4 years, n = 17) | N/A | 76% in remission rate and 6% in colectomy rate in the induction phase; Decrease in CRP and ESR at week 6; 25% in cumulative relapse and no colectomy at 1-year follow-up. | Not analyzed |
| Diet | Characteristics | Changes of Gut Microbiota | References |
|---|---|---|---|
| MD | MD adherence | Bifidobacteria ↑ Faecalibacterium prausnitzii ↑ Akkermansia ↑ E. coli ↓ Fusobacterium ↓ | [119,120,122] |
| Rich in Olive oil | Bacteroidetes ↑ Firmicutes/Bacteroidetes ratio ↓ Bifidobacteria ↓ Lactobacillus ↓ | [123] | |
| Rich in n-3 PUFAs | Bifidobacterium ↑ Roseburia ↑ Lactobacillus ↑ Firmicutes/Bacteroidetes ratio ↓ | [124,125] | |
| Plant-based diet | low in animal protein and fat and rich in dietary fiber and polyphenols | microbial diversity ↑ SCFAs ↑ Bifidobacterium ↑ Lactobacillus ↑ Faecalibacterium prausnitzii ↑ | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Wang, L.; Gu, Y.; Hou, H.; Liu, T.; Ding, Y.; Cao, H. Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges. Nutrients 2022, 14, 4003. https://doi.org/10.3390/nu14194003
Yan J, Wang L, Gu Y, Hou H, Liu T, Ding Y, Cao H. Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges. Nutrients. 2022; 14(19):4003. https://doi.org/10.3390/nu14194003
Chicago/Turabian StyleYan, Jing, Lei Wang, Yu Gu, Huiqin Hou, Tianyu Liu, Yiyun Ding, and Hailong Cao. 2022. "Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges" Nutrients 14, no. 19: 4003. https://doi.org/10.3390/nu14194003
APA StyleYan, J., Wang, L., Gu, Y., Hou, H., Liu, T., Ding, Y., & Cao, H. (2022). Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges. Nutrients, 14(19), 4003. https://doi.org/10.3390/nu14194003





