Abstract
Maternal hyperglycemia is associated with disrupted transplacental arachidonic acid (AA) supply and eicosanoid synthesis, which contribute to adverse pregnancy outcomes. Since placental inositol is lowered with increasing glycemia, and since myo-inositol appears a promising intervention for gestational diabetes, we hypothesized that myo-inositol might rectify glucose-induced perturbations in placental AA metabolism. Term placental explants (n = 19) from women who underwent a mid-gestation oral glucose-tolerance-test were cultured with 13C-AA for 48 h in media containing glucose (5, 10 or 17 mM) and myo-inositol (0.3 or 60 µM). Newly synthesized 13C-AA-lipids were quantified by liquid-chromatography-mass-spectrometry. Increasing maternal fasting glycemia was associated with decreased proportions of 13C-AA-phosphatidyl-ethanolamines (PE, PE-P), but increased proportions of 13C-AA-triacylglycerides (TGs) relative to total placental 13C-AA lipids. This suggests altered placental AA compartmentalization towards storage and away from pools utilized for eicosanoid production and fetal AA supply. Compared to controls (5 mM glucose), 10 mM glucose treatment decreased the amount of four 13C-AA-phospholipids and eleven 13C-AA-TGs, whilst 17 mM glucose increased 13C-AA-PC-40:8 and 13C-AA-LPC. Glucose-induced alterations in all 13C-AA lipids (except PE-P-38:4) were attenuated by concurrent 60 µM myo-inositol treatment. Myo-inositol therefore rectifies some glucose-induced effects, but further studies are required to determine if maternal myo-inositol supplementation could reduce AA-associated pregnancy complications.
1. Introduction
1.1. Arachidonic Acid in Pregnancy
Arachidonic acid (AA), an omega-6 long-chained polyunsaturated fatty acid, is vital for fetal-placental development and long-term offspring health [1]. AA and its derivatives are critical regulators of placental function and parturition, but both placenta and fetus have limited capacity to synthesize AA and rely on maternal supply [2,3]. Since AA is lowly abundant in the maternal circulation, well-regulated and preferential placental AA uptake, metabolism and fetal transfer is essential to meet fetal-placental demand [1,4]. However, transplacental AA transfer appears dysregulated in women with type 1, type 2 and gestational diabetes, resulting in reduced fetal-cord circulating AA lipids [5,6,7].
Diabetes and in vitro glucose treatment also disrupt the placental synthesis of AA-derived signalling molecules, increasing the synthesis of eicosanoids like thromboxane B2, HETES and LTB4, but decreasing the synthesis of 6-keto prostaglandin F1a [8,9,10]. Many of these molecules act as intracellular, paracrine and endocrine signals to regulate uteroplacental processes, inflammation and vascular function, so alterations impact fetal nutrient supply [11]. Indeed, placental and cord AA-phospholipids; a major source of AA for the synthesis of such molecules, are positively associated with birthweight [12,13]. Dysregulation of AA and eicosanoid metabolism in utero may also increase the risk of preterm birth, since the labor process is also driven by many AA-derived signaling molecules [14].
1.2. Myo-Inositol as a Potential Treatment for Disordered Placental Lipid Metabolism
Myo-inositol, the predominant inositol isomer in mammals, is an important regulatory polyol, synthesized endogenously in humans and abundant in dietary grains and fruits [15,16]. In pregnancy, inositols and inositol-containing derivatives play critical roles in regulating endocrine and paracrine signaling (including insulin signaling), placental physiology, nutrient transport, and lipid metabolism, which impact fetal growth and development [17]. However, inositol processing and action are dysregulated in disorders involving insulin resistance, including gestational diabetes (GDM), where placental inositol content is reduced [17]. Myo-inositol supplementation has shown inconsistency in the prevention of GDM and fetal macrosomia [18,19], but shows promise in reducing pre-term births [19,20], where dysregulated in utero AA metabolism may be implicated. We have also previously found that endogenous myo-inositol may specifically suppress glycemia-induced promotion of birthweight [21]. Myo-inositol also appears important for neural development. In humans low maternal circulatory myo-inositol is associated with spina bifida [22], whilst in mouse dams myo-inositol supplementation reduced the frequency of mouse neural tube defects [23]. Glucose-induced neural tube defects in rodent embryos were also prevented by myo-inositol through the rectification of AA-derived eicosanoid synthesis [24,25].
1.3. Placental AA Metabolism
Placental AA-metabolism regulates the availability of AA for use by the placenta, fetus and chorio-amniotic membranes, and is critical for healthy fetal development and parturition. Hence, glucose-induced alterations in placental AA-metabolism may play a role in the pathophysiology of hyperglycemia-related complications of pregnancy, including fetal macrosomia and preterm birth [14,26]. Thus, if inositol can attenuate the effects of glycemia on placental AA metabolism, adequate placental inositol may be protective against hyperglycemia-associated pregnancy complications.
Our study is designed to measure the production of 13C-AA-labeled lipids by placental explants from exogenous 13C-labeled-AA. We hypothesized that glucose induces perturbations in placental AA lipid metabolism that can be rectified by myo-inositol treatment. We thus aimed to characterize variations in in vitro placental AA-lipid processing capacity in relation to gestational glycemia and BMI, to describe changes induced directly by glucose in vitro and assess the effects of concurrent myo-inositol treatment, as well as seek supportive evidence in a separate cohort of snap-frozen placenta.
2. Methods and Materials
Placentas from nineteen pregnancies were collected with informed written consent from non-smoking mothers who delivered after 37 weeks’ gestation by elective Cesarean section at the National University Hospital, Singapore between 2018 and 2020 (Table 1). Women were tested for GDM at mid-gestation as part of universal screening by a three time-point 75 g oral glucose tolerance test (OGTT) using WHO 2013 criteria [27]. Placentas were collected from ten normoglycemic and nine GDM pregnancies and matched for first trimester BMI so that cases were balanced across a range of maternal BMIs in both the GDM and non-GDM populations (Table 1). Hence, neither fasting glycemia nor post-load glycemia (by OGTT) were significantly associated with BMI in our study population. All selected GDM pregnancies fulfilled only the criteria of high post-prandial glycemia (1 h ≥ 10.0 mmol/L and/or 2 h ≥ 8.5 mmol/L) and had normal fasting glycemia (<5.1 mmol/L) in order to limit heterogeneity within our GDM study group and reflect the predominant characteristics of GDM in our local Singapore population [28]. Cases of known pre-existing type 1 and type 2 diabetes mellitus were excluded, as were cases of possible pre-existing diabetes defined by antenatal OGTT results of 2 h glycemia ≥11.1 mmol/L. Only one of the GDM cases was treated with insulin, with the remainder diet-controlled.

Table 1.
Clinical characteristics of study population.
2.1. Placenta Collection and Placental Explant Culture
Placental explants were cultured as previously described [30]. Briefly, five pieces of villous placental tissue were biopsied from across the placenta then cut into small explants (~3 mm3). Explants were then cultured for 48 h in serum-free CMRL media containing 1.5% fatty acid free BSA and 24 µM 13C stable isotope labelled arachidonic acid (1,2,3,4,5–13C), alongside a range of glucose and myo-inositol treatments described in Table 2.

Table 2.
Placental explant culture conditions.
Three replicate wells each containing five explants and 1.8 mL media were conducted for each treatment. The control condition contained physiological amounts of glucose (5 mM) whilst 10 and 17 mM glucose treatments represent GDM-like conditions and a supraphysiological glucose concentration similar to those used in other placental trophoblast studies. Placental explants were treated with 60 µM myo-inositol to reflect the myo-inositol supplied to the placenta from mother and fetus when the mother is supplemented with myo-inositol. Un-esterified AA has previously been reported to be 12.7 (SD 2.1) µM in the maternal vein and 44.2 (5.2) µM in the placental intervillous space [31]. We therefore added 24 µM of 13C-AA in our experiments, which was high enough to enable quantification of a range of 13C-AA placental lipids. 13C-AA is metabolized identically to naturally occurring 12C-AA, but is not naturally present in placenta. Therefore, the abundance of 13C-AA-lipids in placental explants in vitro reflects the capacity of the placenta to take up AA and metabolize AA-lipids separately from the influence of maternal diet, and maternal and fetal lipid metabolism. Further experimental and material details can be found in Supplementary Methods S1. Lipids were extracted as described in Supplementary Methods S2.
2.2. LC-MS/MS Methodology
An LCMS method was developed based on the lipidomics methods of the Baker Institute [32]. A dMRM transition list was developed containing transitions for unlabeled lipids containing 12C20-AA (12C-AA-lipids) and the predicted transitions of stable isotope labeled lipids containing 13C5-12C15-AA (13C-AA-lipids). Only 13C-AA-lipids’ transitions which gave peaks that co-eluted with matching 12C-AA-lipids’ transitions, and which were only present in explants treated with 13C-AA were incorporated into the final method. Lipid extracts were injected into an Agilent 6490 triple quadrupole (QQQ) liquid chromatography mass spectrometry (LC-MS/MS) instrument and analyzed as described in Supplementary Methods S2 and S3.
2.3. Data and Statistical Analysis
LC-MS/MS data was analyzed as described in Supplementary Methods S4 and the amount of each lipid expressed as pmol/mg dry placenta. We also calculated the relative 13C-AA lipid amount in each lipid class for each placenta (i.e., ∑ amount 13C-AA lipid in lipid class/Σ amount 13C-AA lipid in total) to explore the compartmentalization of newly introduced 13C-AA into different lipid pools.
Linear regression was then run for each lipid under control conditions (outcome: log2 amount or log2 relative amount of lipid) with each variable of interest (predictor: maternal BMI, fasting glycemia or post-load 2 h glycemia). Where indicated, multiple linear regression was then performed with mutual adjustments for these variables. The Benjamini-Hochberg method was used to correct for multiple testing to minimize false discovery and statistical significance was set at a two-sided alpha level of p < 0.05.
We then considered the effects of glucose and myo-inositol treatment, calculating the log2 fold-change in lipid amount for each placenta between each treatment condition (Table 2) and control (5 mM glucose, 0.3 µM myoinositol). A log2 fold-change in lipid amount > 0 indicates an increase in 13C-AA lipids compared to the control, whilst values < 0 indicate a decrease. Statistical difference between treatment and control for each lipid was tested using a one sample t-test comparing the treatment log2 fold-change to the reference control (i.e., 0). The Benjamini-Hochberg method was used to correct for multiple testing (for multiple lipids and multiple treatments) and statistical significance was set at a two-sided alpha level of p < 0.05. Alterations in relative 13C-AA lipid amount in each lipid class were also calculated using the same methodology.
2.4. Analysis of Data from Snap Frozen Placenta
We also analyzed an existing dataset from a separate cohort of 50 snap frozen placental biopsies, where endogenous placental inositol content was quantified by an enzymatic assay [33,34], and lipidomics data were acquired using a triple quadrupole LC-MS/MS methodology (Supplementary Methods S5). We were specifically interested in comparing endogenous placental AA lipid data to our in vitro work. Therefore, data was only extracted for lipids that were also analyzed as part of our placental explant work.
Linear regression was then run for each lipid with each variable of interest (predictor: maternal BMI, fasting glycemia or post-load 2 h glycemia). In order to determine to what extent variations in endogenous 12C-AA-lipids may depend on variations in placental inositol content, these models were then re-rerun with the inclusion of inositol content in the model. The Benjamini-Hochberg method was used to correct for multiple testing and statistical significance was set at a two-sided alpha level of p < 0.05. Further information on this data set and its analysis can be found in Supplementary Methods S5.
3. Results
3.1. Placental Incorporation of Exogenous 13C-AA into 13C-AA Labeled Lipids
Placental explants incubated with stable-isotope 13C-AA for 48 h produced stable-isotope labeled 13C-AA-lipids of which seventeen could be reliably quantified using our LCMS method. Quantifiable 13C-AA lipids included five phospholipids [phosphatidyl-ethanolamines (PE-38:4 [18:0_20:4]) phosphatidylethanolamine plasmalogen (PE-P 36:4 [16:0_20:4], PE-P 38:4 [18:0_20:4]), phosphatidyl-choline (PC 40:8 [20:4_20:4]; phosphatidyl inositol PI 38:4 [18:0_20:4])], one lyso-phospholipid [lyso-phosphatidyl-choline (LPC 20:4)] and eleven triacylglycerides (TGs) (Figure 1). While the bulk of endogenous 12C-AA lipids were PEs (32.8%) and PE-Ps (35.4%), for freshly produced 13C-AA lipids most were TGs (75.1%), with less found in PEs (2.8%), PE-Ps (6.3%) and PIs (5.9%) (Figure 1). This indicates that TGs made up a much greater proportion of newly synthesized AA lipids, while PE, PE-P and PI phospholipids were produced more slowly consistent with previous findings for other fatty acids (Palmitic acid: PA, Oleic acid: OA and Docosahexaenoic acid: DHA) [30,35,36].
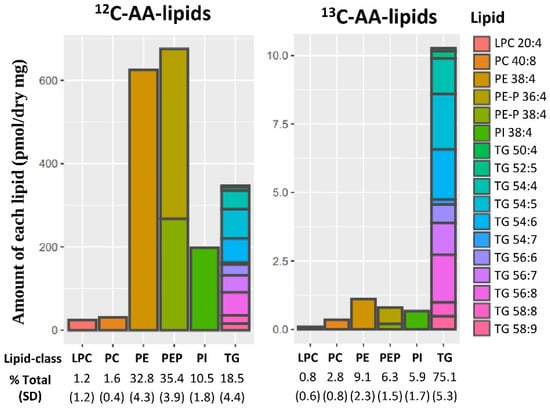
Figure 1.
Placental lipids containing endogenous 12C-AA or newly synthesized stable isotope labeled 13C-AA in placental explants from 19 placenta incubated for 48 h with 24 µM 13C-AA under physiological glucose conditions (5 mM). Amount of each lipid (pmol/mg dry placenta) with percentages showing relative amount of each lipid class compared to total 12C-AA or 13C-AA lipids respectively. Brackets show standard deviation (SD). Colors represent individual lipids. AA: arachidonic acid, LPC: lyso-phosphatidylcholine, PC: phosphatidyl-choline, PE: phosphatidyl-ethanolamine, PE-P: PE-plasmalogen, PI: Phosphatidylinositol, TG: triacylglycerols.
3.2. Association of Maternal Glycemia and BMI with Placental 13C-AA Incorporation under Culture with Physiological Glucose (5 mM)
The absolute amount of newly synthesized 13C-AA lipids in placental explants was not associated with prior in vivo exposure to the maternal metabolic factors of BMI or glycemia or GDM status for any 13C-AA lipid (Figure 2A). However, the proportions of 13C-AA lipids in different lipid classes relative to total 13C-AA lipids demonstrated distinct patterns of associations with maternal metabolic factors. Increasing maternal fasting glycemia across a normal gestational range (4.1–4.7 mM) was associated with a lower proportion of 13C-AA phosphatidyl-ethanolamines (both PE and PE-P) with a similar trend for PI, but positively associated with the proportion of 13C-AA TGs (Figure 2B). These findings indicate a shift in AA lipid metabolism away from PE, PE-P and PI and towards TG with increasing fasting glycemia. Adjustment for maternal BMI did not materially change the result (Figure 2). No associations were observed between AA-lipid class proportions with either maternal BMI or 2 h glycemia.
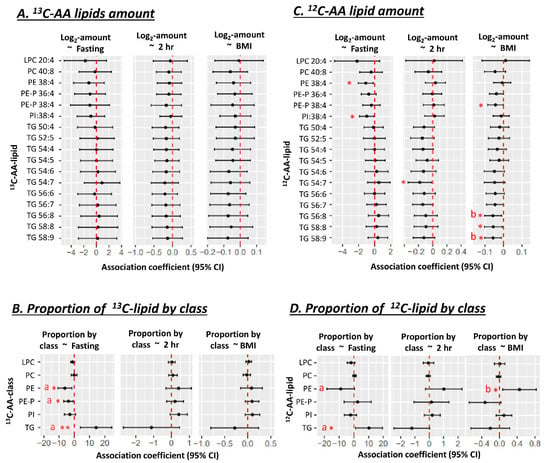
Figure 2.
Associations of maternal glycemia and BMI with 13C or 12C AA-lipids in placental explants (n = 19) after 48 h of culture in a physiological glucose concentration (5 mM). (A,B) Associations with lipid amount (pmol/dry mg placenta). (C,D) Associations with the proportions of 13C or 12C AA-lipids relative to total quantified 13C or 12C AA-lipids. Proportion of 13C-lipid in each class = 100 × Sum amount of 13C-lipid in lipid class/total quantified 13C-lipid in all classes. Results for 13C-AA-lipids and corresponding 12C-AA lipids are from the same placental explants. Linear regression was run with lipid amount or proportion as the outcome and glycemia (in mmol/L) or BMI (in kg/m2) as the predictor. Forest plots show association coefficients and 95% confidence intervals. * shows significant p < 0.05. ** shows significance at p < 0.01. “a” shows significant p < 0.05 for glycemia after adjusting for maternal BMI. “b” shows significance with BMI p < 0.05 after adjusting for maternal glycemia. The Benjamini-Hochberg (BH) method was used to correct for multiple testing. Abbreviations—AA: Arachidonic acid, LPC: lyso-phosphatidylcholine, LPE: lyso-phosphatidylethanolamine, PC: phosphatidylcholine, PE: phosphatidylethanolamine, PE-P: phosphatidylethanolamine-plasmalogen, TG: triacylglycerol.
Within the same placental explants, the absolute amounts of unlabeled endogenous 12C-AA-lipids (matching our previously discussed 13C-AA-lipids), demonstrated associations with maternal glycemia and BMI. Amounts of unlabeled 12C-AA PE 38:4 and PI 38:4 were negatively associated with fasting glycemia, with similar trends for 12C-AA PE-P. Meanwhile, amounts of 12C-AA TG 54:7 was negatively associated with 2 h glycemia, and 12C-AA TG 58:9, TG 58:8, TG 56:8 and PE-P 38:4 were negatively associated with maternal BMI (Figure 2C). Although amounts of newly synthesized 13C-AA lipids showed hints of corresponding directional trends, significant associations were only observed for 12C-AA lipids. Quantified 12C-AA lipids were synthesized both during pregnancy and under explant culture, whilst quantified 13C-AA lipids were only synthesized during culture. Differences could thus be due to the relatively short period of culture (48 h) and hence a limited ability to generate large amounts of slowly synthesized 13C-AA phospholipids. Alternatively, differences could be due to other in vivo factors such as maternal lipid supply that would affect 12C-AA-lipids but not in vitro 13C-AA-lipid metabolism.
When the proportion of 12C-AA lipids (relative to total 12C-AA lipids matching quantifiable 13C-AA-lipids) was analyzed (Figure 2D), as for 13C-AA-lipids, increasing maternal fasting glycemia was positively associated with the proportion of 12C-AA TGs. However, maternal fasting glycemia was only negatively associated with the proportion of PE, after adjusting for maternal BMI. BMI itself was positively associated with the proportion of 12C-AA PE even after adjusting for fasting glycemia.
3.3. Effect of In Vitro Glucose Treatment on Placental 13C-AA Lipid Metabolism
Next, we investigated if glucose could directly impact placental 13C-AA-lipid metabolism during in vitro explant culture, by measuring the amounts of each AA-lipid after 48 h, which reflects the net effects of lipid synthesis and catabolism. Compared to controls (5 mM glucose), placental explants treated with 10 mM glucose (representing GDM-like conditions, Figure 3A), showed decreases in the amount of all eleven TGs and all PE-P and PI, while 13C-AA-PC and 13C-AA-LPC were unchanged. The mean difference compared with control ± SD, averaged for each lipid class, was as follows 13C-AA-PE (12.3% ± 5), 13C-AA-PE-P (25% ± 11), 13C-AA-PI (29% ± 13) and 13C-AA-TG (28% ± 13). Meanwhile, placental explants treated with supraphysiological 17 mM glucose, showed increases in amounts of only 13C-AA-LPC (by 84% ± 62) and 13C-AA-PC (33% ± 15) compared with controls. In vitro glucose treatment thus directly alters placental AA metabolism, but the direction of effect and the lipids influenced depend on the concentration of glucose. In vitro glucose treatment had generally similar effects on the amount of unlabeled 12C-AA lipids to those seen with labeled 13C-AA lipids (Supplementary Figure S1A). These results suggest that increases in glucose concentration can impact both newly synthesized 13C-AA lipids and existing endogenous placental 12C-AA lipids over the relatively short time span of 48 h.
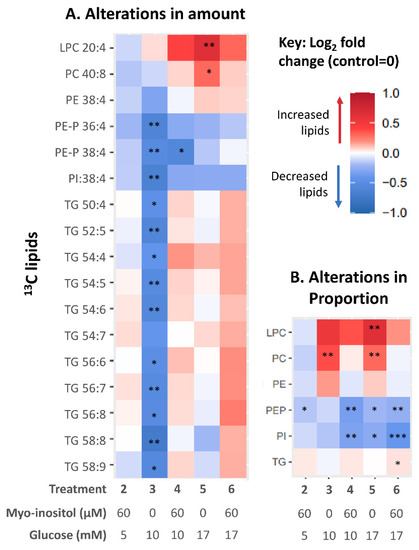
Figure 3.
Heat map illustrating alterations in 13C-AA lipids in placental explants in response to glucose and myo-inositol treatment. Color indicates the relative change (Log2-fold change) in AA lipid in placental explants treated with myoinositol (60 µM) and/or glucose (10 or 17 mM) compared with controls from the same placenta treated with no additional glucose (5 mM) or myo-inositol (0.3 µM). (A) Alterations in amount compared to control; (B) Alterations in the proportion of each AA lipid class compared to control. Asterisks indicate significant differences by one sample t test compared to control (test value = 0) after Benjamini-Hochberg correction for multiple comparisons * p < 0.05, ** p < 0.01, *** p < 0.001.
Among the 13C-AA-lipids, the proportion of 13C-AA PC was increased by in vitro 10 mM glucose treatment (Figure 3B) compared with controls. In vitro 17 mM glucose treatment increased the proportion of both 13C-AA PC and LPC while decreasing the proportion of PE-P and PI. Thus, in vitro glucose treatment alone could not explain the association between maternal glycemia and altered AA-lipid compartmentalization, and chronic exposure to other in vivo factors associated with glycemia are likely involved in programming this response.
3.4. Effect of In Vitro Myo-Inositol or Combined Glucose and Myo-Inositol Treatment on Placental 13C-AA Lipids
Myo-inositol has been suggested as an intervention to lower gestational glycemia, to prevent GDM and lower the risk of adverse effects in pregnancy and the offspring [37]. We therefore investigated the effects of in vitro myo-inositol treatment on placental 13C-AA metabolism and whether myo-inositol could attenuate the changes caused by glucose treatment. Treatment with myo-inositol alone (60 µM, at physiological 5 mM glucose) did not change the amount of 13C-AA-lipids after 48 h of culture compared with controls (no additional myo-inositol, 5 mM glucose) (Figure 3A). Furthermore, unlike treatment with glucose, treatment with combined glucose-myo-inositol (10 mM–60 µM or 17 mM–60 µM) also did not change the amount of most 13C-AA-lipids compared with controls (Figure 3A). Myo-inositol thus suppressed glucose-induced increases and glucose-induced decreases in placental 13C-AA-lipids. The exception was 13C-AA PE-P-38:4 which was decreased relative to controls with both glucose alone (10 mM) and glucose-myo-inositol (10 mM–60 µM). Overall, myo-inositol appeared better able to attenuate glucose-induced decreases in TG than phospholipids (PE-P, PE and PI). Similar effects were also observed with 12C-AA lipids (Supplementary Figure S1). Thus myo-inositol, by suppressing some glucose-induced alterations in AA lipid metabolism, whilst allowing others, may be an important regulatory factor in how glucose affects placental AA lipid metabolism.
When considering the proportions of 13C-AA-lipids, compared with controls, treatment with myo-inositol alone significantly reduced the proportion of 13C-AA-PE-P relative to total 13C-AA lipids suggesting that myo-inositol itself may decrease compartmentalization into PE-P (Figure 3B). Meanwhile, the glucose-induced increase in proportions of 13C-AA PC and LPC were attenuated with combined glucose/myo-inositol treatment, while the proportions of 13C-AA PE-P and PI were further reduced. Thus, while myo-inositol attenuated most glucose-induced changes, decreases in the amount and proportion of 13C-AA PE-P and the proportion of 13C-AA PI remained largely unresolved, suggesting particular challenges in suppressing these specific glucose-induced alterations. Such findings may relate to why these particular endogenous phospholipids appear decreased with increasing fasting glycemia (Figure 2).
3.5. Association of Prior Maternal Fasting Glycemia Exposure In Vivo with Alterations in Placental AA Lipids Induced by Glucose and Myoinositol Treatment In Vitro
Our previous work suggested that the maternal metabolic environment in utero programs the response of the placenta to in vitro treatments [30,38]. We therefore tested for associations between maternal BMI, fasting glycemia and 2 h glycemia, with the subsequent alterations in 13C-AA lipids in response to glucose or myo-inositol or combined treatments in vitro.
Prior exposure to increasing fasting glycemia influenced how 13C-AA phospholipids were altered by in vitro glucose treatment compared with controls, with significant associations between fasting glycemia and response to 10 mM glucose observed for PC 40:8, PE 38:4, PE-P 36:4, PE-P 38:4 and PI 38:4 (Figure 4-column 3 and Supplementary Figure S2). Even though in vitro glucose (10 mM) generally induced a decline in most AA-phospholipids (dots below y = 0), the higher the fasting glycemia, the more blunted the glucose-induced decrease (reduced downward distance between the dots and y = 0 (Figure 4, column 3). Indeed, placenta previously exposed to higher than average fasting glycemia even showed increases in AA-LPC and PC with 10 mM glucose treatment; echoing the overall increase in AA-LPC and PC with 17 mM glucose shown in Figure 3. Glucose-induced alterations in 13C-AA TGs, in contrast, appeared less influenced by fasting glycemia with significant associations only observed for TG 56:6 (Supplementary Figure S2). Similar trends were observed with 17 mM glucose-induced alterations, but significant associations were only observed for PE 38:4 (Supplementary Figure S2) suggesting that very high glucose treatment may override prior glycemia-induced programming. No associations were found between glucose-induced alterations in 13C-AA lipids and prior exposure to increasing 2 h glycemia. Overall, these findings suggest that increasing levels of fasting glycemia in prior in vivo exposure may program the placenta to blunt its response to glucose.
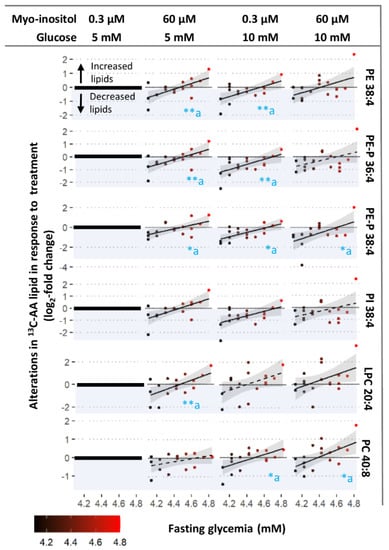
Figure 4.
Associations between maternal fasting glycemia and degree of alterations in 13C-AA lipid in response to treatment with glucose (10 mM), myo-inositol (60 µM) and combined glucose and myo-inositol. Alterations in 13C-AA-lipids expressed in log2-fold change relative to amount of 13C-AA lipid in control explants (0.3 µM myo-inositol and 5 mM glucose) from the same placenta (zero on y-axis). Positive log2-fold change values indicate an increase in 13C-AA lipids compared to the control, whilst negative values (blue shading) indicate a decrease. Linear regression was run with log2-fold change in lipids as the outcome and fasting glycemia as the exposure variable. Asterisks indicate significant associations * p < 0.05, ** p < 0.01, “a” indicates that the association remains after adjusting for maternal BMI. The Benjamini-Hochberg (BH) method was used to correct for multiple testing. Solid lines show significant associations while dashed lines show non-significant trends, shaded areas show 95% confidence intervals.
Meanwhile, associations between myo-inositol-induced alterations in 13C-AA lipids and prior exposure to increasing fasting glycemia were significant for most 13C-AA phospholipids (LPC 20:4, PE 38:4, PE-P 36:4, PE-P 38:4, PI 38:4), but were only significant for one TG (TG 50:4, Supplementary Figure S2). With prior exposure to lower maternal fasting glycemia, myo-inositol treatment generally decreased newly synthesized 13C-AA phospholipids (dots below y =0) compared with controls (0.3 µM myo-inositol, 5 mM glucose). In contrast with prior exposure to higher maternal fasting glycemia myo-inositol treatment generally increased 13C-AA phospholipids (dots above y =0) (Figure 4, column 2). The association crossed the x axis around a fasting glycemia level of 4.4–4.5 mM, which is the median concentration in the general obstetric population [39]. Since endogenous phospholipids were lower with increasing fasting glycemia exposure (Figure 2C), this suggests that myo-inositol could act to bring AA-phospholipids towards physiological means, thus moderating AA placental metabolism.
The influences of prior fasting glycemia on 13C-AA phospholipid alterations with combined glucose-myo-inositol treatment (10 mM/ 60 µM, or 17 mM/60 µM; Figure 5B) were similar to those of myo-inositol (60 µM with 5 mM physiological glucose). Prior exposure to increasing 2 h glycemia or increasing BMI did not influence myo-inositol-induced alterations. Findings were also similar for alterations in unlabeled 12C-AA lipids (Supplementary Figure S3).
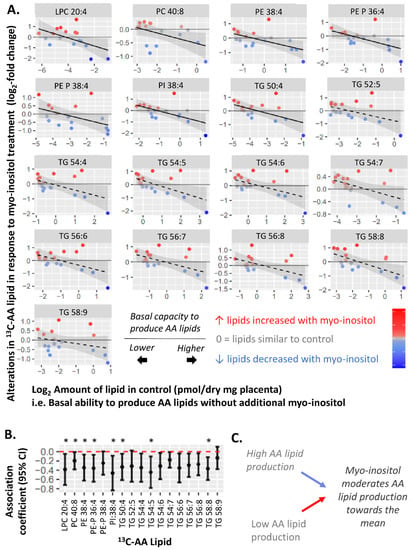
Figure 5.
Negative associations between amount of each 13C-AA lipid in the control and alterations in 13C-AA lipid (log2-fold change) in response to myo-inositol treatment. Myo-inositol-induced alterations represent the relative amount of 13C-AA lipid in placental explants treated with myo-inositol (60 µM myo-inositol, 5 mM glucose) compared to control explants (0.3 µM myo-inositol, 5 mM glucose) from the same placenta. Positive log2-fold-change values indicate an increase in 13C-AA lipids compared to the control, whilst negative values indicate a decrease. Linear regression was run with myo-inositol response as the outcome and fasting glycemia as the exposure variable. The Benjamini-Hochberg (BH) method was used to correct for multiple testing. (A) Solid lines show significant associations while dashed lines show non-significant trends, shaded areas show 95% confidence intervals. (B) Forest plot showing association coefficients and 95% confidence intervals. Asterisks indicate significant associations * p < 0.05. (C) Overall, these findings suggest that myo-inositol may moderate variations in basal placental AA metabolism, shifting them towards a physiological mean.
To explore the moderating effect of myo-inositol, we further assessed whether myo-inositol could indeed moderate the basal placental AA-lipid metabolism represented by the amount of 13C-AA-lipid in the control (0.3 µM myo-inositol, 5 mM glucose). We found that in vitro myo-inositol treatment (60 µM) generally increased 13C-AA lipids in explants from placenta which basally produced less 13C-AA-lipid, but decreased 13C-AA lipids in placenta that basally produced more 13C-AA-lipid (Figure 5). These findings were most prominent for phospholipids as reflected by the significant negative associations observed between the amount of 13C-AA-lipid in the control and the myo-inositol-induced alterations in 13C-AA LPC 20:4, PE 38:4, PE-P 36:4, PE-P 38:4, PI 38:4 and TG 50:4, with similar trends for remaining AA-lipids. Findings were similar for alterations in unlabeled 12C-AA lipids (Supplementary Figure S4).
Overall, these findings suggest that myo-inositol may moderate variations in basal placental AA metabolism, shifting them towards a physiological mean. All of these may constitute adaptive responses in placental AA metabolism to cope with prevailing levels of maternal glycemia or other factors influencing placental AA metabolism.
3.6. Supporting Evidence from a Separate Cohort: Endogenous 12C-AA Lipids in Snap-Frozen Placental Biopsies
We sought to replicate some of our findings using an existing ex vivo dataset from a separate cohort of 50 placental biopsies (snap frozen within 30 min of cesarean delivery) with measurements of endogenous placental inositol content (quantified by an enzymatic assay) and lipidomic data (Supplementary Methods S5). Data on 12C-AA-lipids matching those in our current study was extracted to determine whether endogenous inositol may influence the relationship between maternal glycemia, and the absolute and proportional (relative to total 12C-AA lipids of interest) amounts of endogenous 12C-AA-lipids.
Maternal glycemia was positively associated with the amount of several placental 12C-AA TGs (with fasting glycemia: TG 54:6, TG 54:7, TG 58:8; with 2 h glycemia: TG 54:5, TG 54:7, TG 56:6, TG 56:7 and TG 58:8) (Figure 6A). Both fasting and 2 h glycemia also positively associated with the proportion of endogenous 12C-AA-TGs, but negatively associated with the proportion of 12C-AA-PE-P and PI (Figure 6B), similar to patterns for 13C-AA lipids observed in placental explants following 48 h culture under control conditions (Figure 2). Since 13C-AA lipids represent only the lipids that were synthesized during the 48 h culture in the absence of on-going maternal-fetal influences, these similar variations in 12C-AA in ex vivo biopsies are likely also due to variations specifically in placental processing and metabolism rather than in the supply of AA from maternal sources or utilization by the fetus.
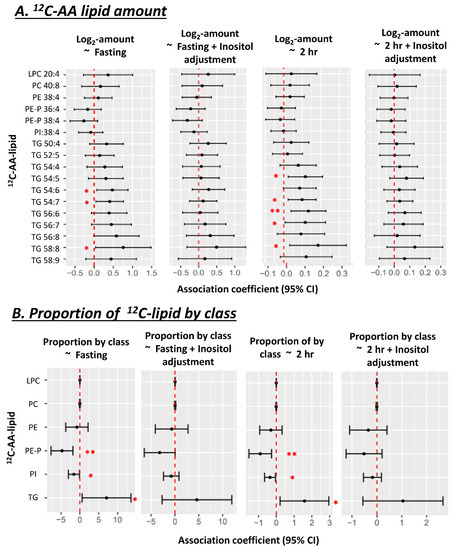
Figure 6.
Associations between amount (A) and proportion (B) of 12C-AA lipids with maternal glycemia, before and after adjusting for total placental inositol content, in uncultured snap-frozen placental biopsies (n = 50). 12C-AA-lipids were extracted from this existing dataset to match 13C-AA lipids measured in our placental explant study. Proportion of 12C-AA-lipids in each class = 100 × Sum amount of 12C-lipid in lipid class/total quantified 12C-lipid in all classes. Linear regression was run with lipid proportion as the outcome and fasting glycemia as the predictor. Forest plots show association coefficient and 95% confidence intervals for each lipid class. Asterisks indicate significant associations * p < 0.05, ** p < 0.01. The Benjamini-Hochberg (BH) method was used to correct for multiple testing.
As increasing glycemia is known to associate with reduced endogenous inositol in placenta [33], we explored the consequences of neutralizing such an influence by statistically adjusting for placental inositol. Adjusting for endogenous placental inositol content in the snap frozen placenta dataset, attenuated associations between maternal glycemia (fasting and 2 h) and the amount and proportion of AA lipids (Figure 6B). Consistent with our in vitro data, this suggests that variations in in vivo endogenous placental myo-inositol may regulate the relationship between maternal glycemia and endogenous placental AA-lipids. No associations were seen between placental AA-lipids and maternal BMI.
4. Discussion
Our findings suggest increasing maternal fasting glycemia programs the placenta to decrease the compartmentalization of AA into placental phospholipid pools, but increase compartmentalization into TGs. Meanwhile in vitro glucose treatment directly decreases the abundance of most freshly synthesized placental AA lipids, with most of these effects attenuated by myo-inositol treatment. Myo-inositol treatment alone also moderated basal placental AA-lipid processing towards physiological means. Overall, these observations suggest that myo-inositol promotes adaptive responses to overcome the effects of glucose on placental AA lipid metabolism. This notion is consistent with analysis of endogenous AA lipid data from a separate cohort, where endogenous myo-inositol also appears to modulate glycemia-induced placental AA-lipid alterations in vivo.
4.1. Maternal Glycemia and Placental AA-Lipid Compartmentalization and Bioavailability
The compartmentalization of AA into different lipid pools (Figure 7) is key to its bioavailability, its utilization and to placental function and signaling [40,41,42]. Placental AA-phospholipids are thought to be the source of fetal AA supply, and the source of AA for eicosanoid synthesis for paracrine and endocrine signaling [43,44,45]. Meanwhile, placental AA-TGs act as AA storage pools [44,46]. Thus, our finding of a glycemia associated shift in placental compartmentalization away from PEs, PE-Ps and PI and thus away from AA pools utilized for fetal supply and eicosanoid synthesis, could potentially impact placental function and fetal development.
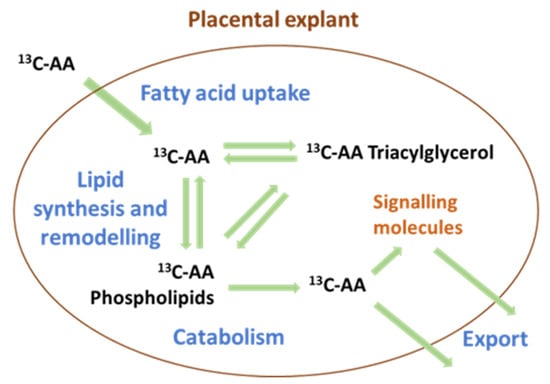
Figure 7.
The compartmentalization of arachidonic acid (AA) into different lipid pools regulates both the uptake and export of AA. AA-triacylglycerols often act as storage, while AA-phospholipids are the primary source of bioactive signaling molecules such as eicosanoids and of free AA for export to the fetus.
Our findings are consistent with a study [47] of perfused placentas from women with pre-existing diabetes showing increased radiolabeled placental 14C-AA TG and decreased 14C-AA phospholipids alongside decreased export of un-esterified 14C-AA to the fetus compared with non-diabetic placentas. Altered compartmentalization could thus potentially explain the reduction in fetal circulating non-esterified AA in maternal diabetes [5,6,7]. We next investigated whether such associations could be partly explained by a direct effect of high glucose on placental AA-metabolism.
4.2. Glucose-Induced Changes in Placental AA-Lipids In Vitro
There was a general decrease in all classes of freshly synthesized 13C-AA-containing lipids with 10 mM in vitro glucose treatment suggesting impact on an upstream process such as decreased placental AA uptake and/or activation. One possibility is AA-CoA synthesis, which prevents re-export into the maternal circulation and which is an essential first step for all lipid synthesis. This would be consistent with studies in isolated human trophoblasts from GDM pregnancies demonstrating less radiolabeled 14C-AA accumulation and decreased ACSL1 (acyl-CoA synthetase) mRNA expression compared to non-GDM controls [48].
However, with 17 mM glucose, most lipids were left unaltered compared to controls, whilst 13C-AA-PC and 13C-AA-LPC were actually increased. This lack of change with very high glucose mirrors published findings where 20 mM glucose was found to have little impact on overall 14C-AA uptake by primary cytotrophoblasts [48]. One possible explanation for increased PC and LPC may be increases in the activity of phosphocholine cytidylyltransferase or phosphatidylethanolamine N-methyltransferase, which catalyze the rate limiting steps in PC synthesis, and which are known to be upregulated by hyperglycemia or glucose treatment in a range of tissue types [49,50,51]. However, alterations in these genes would not explain why other lipids are decreased by 10 mM but not 17 mM glucose.
Decreases in the proportion of PE and PE-P both in association with increasing fasting glycemia and by in vitro 10 mM glucose treatment could also suggest that glucose may specifically increase catabolism of PE-P and PI, but not LPC and PC. This could be mediated by phospholipase PLA2G2A, which is abundant in the placenta [52,53,54], is known to be increased in GDM [55,56] and which in non-placental tissue has particular specificity for AA-PE over AA-PC [57,58].
4.3. Placental Programming by Prior Maternal Glycemia
Our findings suggest that prior exposure to increasing fasting glycemia blunts the effects of in vitro glucose treatment on placental AA-phospholipid metabolism, potentially representing an adaptive response to protect the placenta and fetus from high glucose. For example, decreased placental lipid synthesis or increased PE and PE-P catabolism could make more un-esterified AA available for fetal transfer even in a low AA environment. However, any alterations in placental lipid metabolism could have both positive and negative effects. For example, glucose-induced increases in placental AA-LPC could increase net export of AA to the fetus in the AA-LPC form to compensate for decreased supply of AA in the classical un-esterified form. This would be consistent with reports of increased transfer of an unidentified AA-containing phospholipid species, from perfused diabetic placenta into the fetal effluent compared to non-diabetic controls [47]. However, a possible trade-off of this form of compensation could be an increased risk of macrosomia since many LPCs are known to promote fetal growth [59,60,61], which would further exacerbate the direct effects of increased transplacental glucose and fetal hyperinsulinemia.
4.4. Myo-Inositol as a Moderator of Placental AA Metabolism
Prior exposure to increasing maternal fasting glycemia across the normal range also influenced the effects of in vitro myo-inositol on AA-lipid metabolism, with myo-inositol bringing basal AA-lipid amounts towards a physiological mean. Thus, myo-inositol likely plays a regulatory rather than direct role; influencing metabolic pathways that are programmed by glucose and other factors in order to moderate placental lipids. Prior exposure to higher maternal glycemia may also alter the effects of myo-inositol treatment by reducing inositol uptake and synthesis [33].
Lipid metabolism involves many pathways that use inositol-containing signaling molecules. For example, the phosphorylation of phosphatidyl inositol diphosphate (PIP2) into phosphatidyl inositol triphosphate (PIP3) activates protein kinase B, an enzyme which upregulates proteins important for fatty acid uptake [62]. Meanwhile the cleavage of PIP2 activates protein kinase C [63,64] which inhibits ACSL4 (an enzyme important for AA uptake and lipid synthesis [65,66]) and stimulates PLA2 (which is important for AA-lipid catabolism [63,67,68]). Both PIP2-related regulatory pathways are impaired in the placenta and in other tissues with hyperglycemia [64,69,70,71]. If such impairment is due to a deficiency in inositol-containing signaling molecules, increasing myo-inositol may attenuate many of these changes.
Myo-inositol appeared more able to attenuate glucose-induced decreases in AA-TG than AA-phospholipids. Differential regulation of lipids by myo-inositol has also previously been reported in the livers of myo-inositol deficient rats where AA-TGs were decreased but phospholipids remained unchanged [72]. However, myo-inositol in our study also appeared less able to attenuate glucose-induced decreases in PE-P 36:4 and PI 38:4 than other phospholipids and did not attenuate PE-P 38:4. These findings suggest the existence of glucose-induced processes targeting PE-P and PI metabolism which either do not involve inositol (nor amenable to regulation by inositol), or where inositol might have a similar effect to glucose. For example, inositol may also increase AA-phospholipid catabolism since lecithin:cholesterol acyltransferase (LCAT), a phospholipase with a preference for AA-phospholipids [73,74], is known to be decreased in the plasma of myo-inositol deficient rats [75,76]. The resistance of PE-P and PI to modulation could also contribute to explaining why maternal fasting glycemia is associated with decreased compartmentalization of AA into these phospholipids since these may be decreased by glucose even when myo-inositol supply is adequate.
There is also the possibility of bidirectional interplay between inositol signaling and AA bioavailability. Previous in vitro experiments reported that AA can stimulate the hydrolysis of placental phosphatidyl-inositol-phosphates to release inositol phosphates and human placental lactogen (hPL), a hormone important for maternal adaption and fetal development [25,77]. Many inositol containing signaling molecules also contain AA, so their abundance will be influenced by AA availability. Indeed, our experiments show that glucose-induced decreases in 13C-AA lipids include decreased 13C-AA phosphatidyl inositol (PI). Thus, the dysregulation of inositol metabolites and inositol signaling in hyperglycemia should be seen as a consequence not only of inadequate inositol but also dysregulated AA metabolism [17,33].
4.5. Clinical Implications
AA released from different phospholipids are directed towards synthesis of particular eicosanoids, with prostaglandins more likely to be synthesized from AA released from PEs than other phospholipids [40,78,79,80]. It is thus plausible that glycemia-induced alterations in AA metabolism underlie pathological changes in eicosanoid production in maternal diabetes and explain why glucose decreases some eicosanoids while increasing the synthesis of others [8,9,10], which may have implications for fetal growth disorders and preterm labor.
It is not clear yet which glucose-induced alterations in placental AA metabolism are involved in hyperglycemia-related pathophysiology. If myo-inositol can suppress pathological glucose-induced changes in AA metabolism during pregnancy, while sparing or enabling desired/beneficial adaptive changes, myo-inositol supplementation could be used to minimize glucose-induced disruptions in placental AA metabolism and potentially reduce hyperglycemia-related pregnancy complications.
5. Strengths and Limitations
This work isolated placental AA metabolism itself for study, minimizing the influence of maternal supply (reflective of diet, maternal tissue metabolism and placental blood flow) and fetal utilization. Our work is thus able to demonstrate the impact of maternal glycemia associated programming specifically within placental tissue and the direct effects of in vitro glucose and myo-inositol treatment. However, this work can only be done on cultured term placenta following delivery and therefore does not necessarily represent the in vivo situation earlier in pregnancy. Explant based stable isotope experiments cannot be run on large numbers of placenta, limiting the statistical power to explore concurrently the effects of multiple other factors such as parity, ethnicity and socio-demographics. Our experiments were also limited to Indian and Chinese women in Singapore and results may differ in other populations. GDM in Singapore is generally characterized by an elevated post-prandial glycemia in the OGTT, rather than by high fasting glycemia, with limited numbers of the latter group suitable for recruitment. Thus, our sample only included women with fasting glycemia within clinically-defined normal ranges yet we were able to elicit fasting glycemia-induced variations in placental AA-metabolism consistent with notions that maternal glycemia demonstrates a continuum of effect across the glycemic range [26]. However, whether our AA results continue to follow a similar linear trajectory with fasting glycemia above the levels studied is unclear. Most GDM cases (8 out of 9) were diet controlled and it is unknown whether results would be altered by insulin or metformin treatment in pregnancy.
Larger cohort studies will be needed to determine if inositol supplementation pre-pregnancy and during gestation could change placental AA metabolism to impact maternal and fetal outcomes such as preterm birth. Placental perfusion studies may be useful in determining specifically how inositol may influence glucose-induced changes in transplacental nutrient transport and placental signaling.
6. Conclusions
Prior exposure to different levels of fasting glycemia was associated with variations in how the placenta metabolizes AA, while in vitro evidence suggested that glucose directly alters some placental AA metabolic pathways. Since well-regulated placental AA-lipid metabolism is critical for maternal and fetal health, glucose-associated alterations in placental AA metabolism may negatively impact maternal-placental-fetal signaling, fetal growth and development, and parturition.
Myo-inositol moderated AA metabolism towards the mean and attenuated most glucose-induced alterations in AA metabolism. If myo-inositol is also able to moderate placental AA-lipid metabolism during pregnancy, including the effects induced by maternal hyperglycemia, then optimizing placental inositol content may physiologically play an important regulatory role for healthy fetal development and parturition. Thus, in conditions where placental inositol is decreased such as gestational diabetes, maternal inositol supplementation may be useful. Further studies are required to understand the role of inositol in maternal-placental-fetal physiology and whether maternal inositol supplementation is beneficial in pregnancy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14193988/s1, Supplementary Methods S1: Placental explant culture; Supplementary Methods S2: Lipid extraction; Supplementary Methods S3: LCMS Methodology; Supplementary Methods S4: Data analysis and statistics; Supplementary Methods S5: Placental biopsy cohort; Figure S1. Heat map illustrating alterations in 12C-AA lipids in placental explants in response to glucose and myo-inositol treatment; Figure S2. Forest plots showing positive associations of fasting glycemia with alterations in 13C lipids (log2-fold change) induced by glucose, myo-inositol and combination glucose myo-inositol. Figure S3. Forest plots showing positive associations of fasting glycemia with alterations in 12C lipids (log2-fold change) induced by glucose, myo-inositol and combination glucose myo-inositol. Figure S4. Negative associations between amount of each 12C-AA lipid in the control and alterations in 12C-AA lipid (log2-fold change) in response to myo-inositol treatment.
Author Contributions
Conceptualization, O.C.W., R.M.L., M.R.W. and S.-Y.C.; Data curation, O.C.W.; Formal analysis, O.C.W. and S.-Y.C.; Funding acquisition, A.C.-G., K.M.G., R.M.L., M.R.W. and S.-Y.C.; Investigation, O.C.W., R.A.P., P.S., H.E.J.Y., N.S., A.C.-G. and S.-Y.C.; Methodology, O.C.W., V.K.B.C.-H., R.A.P., P.S., H.E.J.Y., N.S., A.C.-G. and S.-Y.C.; Project administration, V.K.B.C.-H., R.A.P., P.S., A.C.-G., A.K.B., K.M.G. and S.-Y.C.; Resources, V.K.B.C.-H., R.A.P., P.S., H.E.J.Y., N.S., S.N.P., A.K.B. and S.-Y.C.; Software, O.C.W.; Supervision, A.C.-G., A.K.B., K.M.G., R.M.L., M.R.W. and S.-Y.C.; Writing—original draft, O.C.W., V.K.B.C.-H. and S.-Y.C.; Writing—review and editing, O.C.W., V.K.B.C.-H., R.A.P., P.S., H.E.J.Y., N.S., S.N.P., A.C.-G., A.K.B., K.M.G., R.M.L., M.R.W. and S.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by a Clinician Scientist Award awarded to SYC from the Singapore National Medical Research Council (NMRC/CSA-INV/0010/2016; MOH-CSAINV19nov-0002), by the National University of Singapore, National University Health System Singapore and the Singapore Institute for Clinical Sciences A*STAR. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus+ Programme ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP). The Singapore Lipidomics Incubator receives funding from the Life Sciences Institute, the National University of Singapore Yong Loo Lin School of Medicine and the National Research Foundation (grant number NRFI2015-05).
Institutional Review Board Statement
Ethical approval was obtained from the National Healthcare Group Domain Specific Review Board (2016/00183).
Informed Consent Statement
Informed written consent was obtained from all subjects involved in the study.
Data Availability Statement
Supporting data can be found in the Supplementary. Further information can be supplied upon request.
Acknowledgments
The authors would like to thank Samantha Grace Loon Magadia, Celes Maria Catherine Dado, and Chen Zhenzhi in coordinating the recruitment of the women involved in this study, the staff of the National University Hospital who kindly assisted with placental collection, and the women who generously donating their placenta for research.
Conflicts of Interest
S.Y.-C. and K.M.-G. are part of an academic consortium that has received research funding from Abbott Nutrition, Nestlé S.A., Danone and BenevolentAI Bio Ltd. for work unrelated to this manuscript. S.Y.-C. and K.M.-G. are co-inventors on patent filings by Nestlé S.A. which covers the use of inositol in human health applications but which do not draw on the work in this manuscript. Godfrey has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. Shiao-Yng Chan has received reimbursement and honoraria into her research funds from Société Des Produits Nestlé S.A. for speaking at a conference. The other authors have no financial or personal conflict of interest to declare.
Abbreviations
AA: arachidonic acid, BMI: body mass index, BSA: bovine serum albumin, CMRL: Connaught Medical Research Laboratories, DG: diacylglycerols, DHA: docosahexaenoic acid, dMRM: dynamic Multiple Reaction Monitoring, GDM: gestational diabetes mellitus, IS: Internal standard, LC-MS/MS: tandem liquid chromatography mass spectrometry, LC-PUFA: long-chain polyunsaturated fatty acid, LPC: lyso-phosphatidylcholine, LPE: lyso-phosphatidylethanolamine, PC: phosphatidylcholine, PE: phosphatidylethanolamine, PE-P: phosphatidylethanolamine plasmalogen, PI: phosphatidyl-inositols, PUFA: polyunsaturated fatty acids, TG: triacylglycerol.
References
- Bitsanis, D.; Crawford, M.A.; Moodley, T.; Holmsen, H.; Ghebremeskel, K.; Djahanbakhch, O. Arachidonic Acid Predominates in the Membrane Phosphoglycerides of the Early and Term Human Placenta. J. Nutr. 2005, 135, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Winkler, L.; Möller, U.; Schubert, H.; Goetze, E. Synthesis of arachidonic acid in the human placenta in vitro. Neonatology 1979, 35, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Chambaz, J.; Ravel, D.; Manier, M.-C.; Pepin, D.; Mulliez, N.; Bereziat, G. Essential fatty acids interconversion in the human fetal liver. Neonatology 1985, 47, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M. Placental delivery of arachidonic and docosahexaenoic acids: Implications for the lipid nutrition of preterm infants. Am. J. Clin. Nutr. 2000, 71, 275S–284S. [Google Scholar] [CrossRef] [PubMed]
- Ghebremeskel, K.; Thomas, B.; Lowy, C.; Min, Y.; Crawford, M.A. Type 1 diabetes compromises plasma arachidonic and docosahexaenoic acids in newborn babies. Lipids 2004, 39, 335–342. [Google Scholar] [CrossRef]
- Min, Y.; Lowy, C.; Ghebremeskel, K.; Thomas, B.; Offley-Shore, B.; Crawford, M. Unfavorable effect of type 1 and type 2 diabetes on maternal and fetal essential fatty acid status: A potential marker of fetal insulin resistance. Am. J. Clin. Nutr. 2005, 82, 1162–1168. [Google Scholar] [CrossRef]
- Thomas, B.; Ghebremeskel, K.; Lowy, C.; Offley-Shore, B.; Crawford, M.A. Plasma fatty acids of neonates born to mothers with and without gestational diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 335–341. [Google Scholar] [CrossRef]
- Kuhn, D.C.; Botti, J.J.; Cherouny, P.H.; Demers, L.M. Eicosanoid production and transfer in the placenta of the diabetic pregnancy. Prostaglandins 1990, 40, 205–215. [Google Scholar] [CrossRef]
- Jawerbaum, A.; Catafau, J.R.; González, E.T.; Novaro, V.; Gómez, G.; Gelpí, E.; Gimeno, M.A.F. Eicosanoid production by placental and amnion tissues from control and non-insulin-dependent diabetic rats. Influence of oxytocin in the incubating medium. Prostaglandins Leukot. Essent. Fat. Acids 1997, 56, 425–429. [Google Scholar] [CrossRef]
- White, V.; Jawerbaum, A.; Sinner, D.; Pustovrh, C.; Capobianco, E.; González, E. Oxidative stress and altered prostanoid production in the placenta of streptozotocin-induced diabetic rats. Reprod. Fertil. Dev. 2002, 14, 117–123. [Google Scholar] [CrossRef]
- Pearson, T.; Warren, A.Y.; Barrett, D.A.; Khan, R.N. Detection of EETs and HETE-generating cytochrome P-450 enzymes and the effects of their metabolites on myometrial and vascular function. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E647–E656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uhl, O.; Demmelmair, H.; Segura, M.T.; Florido, J.; Rueda, R.; Campoy, C.; Koletzko, B. Effects of obesity and gestational diabetes mellitus on placental phospholipids. Diabetes Res. Clin. Pract. 2015, 109, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Bitsanis, D.; Ghebremeskel, K.; Moodley, T.; Crawford, M.A.; Djahanbakhch, O. Gestational diabetes mellitus enhances arachidonic and docosahexaenoic acids in placental phospholipids. Lipids 2006, 41, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Godhamgaonkar, A.A.; Wadhwani, N.S.; Joshi, S.R. Exploring the role of LC-PUFA metabolism in pregnancy complications. Prostaglandins Leukot. Essent. Fat. Acids 2020, 163, 102203. [Google Scholar] [CrossRef]
- Clements, R., Jr. The metabolism of myo-inositol by the human kidney. J. Lab. Clin. Med. 1979, 93, 210–219. [Google Scholar]
- Holub, B.J. Metabolism and function of myo-inositol and inositol phospholipids. Annu. Rev. Nutr. 1986, 6, 563–597. [Google Scholar] [CrossRef]
- Watkins, O.C.; Yong, H.E.J.; Sharma, N.; Chan, S.Y. A review of the role of inositols in conditions of insulin dysregulation and in uncomplicated and pathological pregnancy. Crit. Rev. Food Sci. Nutr. 2022, 62, 1626–1673. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wong, M.M.H.; Pang, S.S.H.; Lo, K.K.H. Dietary supplementation for gestational diabetes prevention and management: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2021, 303, 1381–1391. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barton, S.J.; El-Heis, S.; Kenealy, T.; Nield, H.; Baker, P.N.; Chong, Y.S.; Cutfield, W.; Chan, S.-Y. Myo-Inositol, Probiotics, and Micronutrient Supplementation From Preconception for Glycemia in Pregnancy: NiPPeR International Multicenter Double-Blind Randomized Controlled Trial. Diabetes Care 2021, 44, 1091–1099. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, Y.; Li, Z.; Sun, L.; Guo, W. The efficacy of myo-inositol supplementation to prevent gestational diabetes onset: A meta-analysis of randomized controlled trials. J. Matern-Fetal Neonatal Med. 2019, 32, 2249–2255. [Google Scholar] [CrossRef]
- Chu, A.H.Y.; Tint, M.T.; Chang, H.F.; Wong, G.; Yuan, W.L.; Tull, D.; Nijagal, B.; Narayana, V.K.; Meikle, P.J.; Chang, K.T.E.; et al. High placental inositol content associated with suppressed pro-adipogenic effects of maternal glycaemia in offspring: The GUSTO cohort. Int. J. Obes. 2021, 45, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Groenen, P.M.; Merkus, H.M.; Sweep, F.C.; Wevers, R.A.; Janssen, F.S.; Steegers-Theunissen, R.P. Kinetics of myo-inositol loading in women of reproductive age. Ann. Clin. Biochem. 2003, 40, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cogram, P.; Tesh, S.; Tesh, J.; Wade, A.; Allan, G.; Greene, N.D.; Copp, A.J. D-chiro-inositol is more effective than myo-inositol in preventing folate-resistant mouse neural tube defects. Hum. Reprod. 2002, 17, 2451–2458. [Google Scholar] [CrossRef]
- Baker, L.; Piddington, R.; Goldman, A.; Egler, J.; Moehring, J. Myo-inositol and prostaglandins reverse the glucose inhibition of neural tube fusion in cultured mouse embryos. Diabetologia 1990, 33, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, P.; Wu, Y.Q.; Handwerger, S. Melittin stimulates phosphoinositide hydrolysis and placental lactogen release: Arachidonic acid as a link between phospholipase A2 and phospholipase C signal-transduction pathways. Life Sci. 1991, 48, 2089–2095. [Google Scholar] [CrossRef]
- Group, H.S.C.R. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with maternal body mass index. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 575–584. [Google Scholar]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36, S67. [Google Scholar] [CrossRef]
- Chen, L.-W.; Soh, S.E.; Tint, M.-T.; Loy, S.L.; Yap, F.; Tan, K.H.; Lee, Y.S.; Shek, L.P.-C.; Godfrey, K.M.; Gluckman, P.D. Combined analysis of gestational diabetes and maternal weight status from pre-pregnancy through post-delivery in future development of type 2 diabetes. Sci. Rep. 2021, 11, 5021. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157. [Google Scholar] [CrossRef]
- Watkins, O.C.; Selvam, P.; Pillai, R.A.; Cracknell-Hazra, V.; Yong, H.E.J.; Sharma, N.; Cazenave-Gassiot, A.; Bendt, A.K.; Godfrey, K.M.; Lewis, R.M.; et al. Placental 13C-DHA metabolism and relationship with maternal BMI, glycemia and birthweight. Mol. Med. 2021, 27, 84. [Google Scholar] [CrossRef]
- Benassayag, C.; Mignot, T.; Haourigui, M.; Civel, C.; Hassid, J.; Carbonne, B.; Nunez, E.; Ferre, F. High polyunsaturated fatty acid, thromboxane A2, and alpha-fetoprotein concentrations at the human feto-maternal interface. J. Lipid Res. 1997, 38, 276–286. [Google Scholar] [CrossRef]
- Weir, J.M.; Wong, G.; Barlow, C.K.; Greeve, M.A.; Kowalczyk, A.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Jowett, J.B.; Shaw, J. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013, 54, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.A.; Islam, M.O.; Selvam, P.; Sharma, N.; Chu, A.H.; Watkins, O.C.; Godfrey, K.M.; Lewis, R.M.; Chan, S.Y. Placental Inositol Reduced in Gestational Diabetes as Glucose alters Inositol Transporters and IMPA1 enzyme expression. J. Clin. Endocrinol. Metab. 2021, 106, e875–e890. [Google Scholar] [CrossRef]
- Islam, M.O.; Selvam, P.; Pillai, R.A.; Watkins, O.C.; Chan, S.Y. An enzymatic assay for quantification of inositol in human term placental tissue. Anal. Biochem. 2019, 586, 113409. [Google Scholar] [CrossRef]
- Watkins, O.C.; Islam, M.O.; Selvam, P.; Pillai, R.A.; Cazenave-Gassiot, A.; Bendt, A.K.; Karnani, N.; Godfrey, K.M.; Lewis, R.M.; Wenk, M.R.; et al. Metabolism of 13C-Labeled Fatty Acids in Term Human Placental Explants by Liquid Chromatography–Mass Spectrometry. Endocrinology 2019, 160, 1394–1408. [Google Scholar] [CrossRef] [PubMed]
- Watkins, O.C.; Yong, H.E.J.; Mah, T.K.; Cracknell-Hazra, V.K.B.; Pillai, R.A.; Selvam, P.; Sharma, N.; Cazenave-Gassiot, A.; Bendt, A.K.; Godfrey, K.M.; et al. Sex-Dependent Regulation of Placental Oleic Acid and Palmitic Acid Metabolism by Maternal Glycemia and Associations with Birthweight. Int. J. Mol. Sci. 2022, 23, 8685. [Google Scholar] [CrossRef]
- Russo, M.; Forte, G.; Montanino Oliva, M.; Laganà, A.S.; Unfer, V. Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth. Int. J. Mol. Sci. 2021, 22, 8433. [Google Scholar] [CrossRef] [PubMed]
- Watkins, O.C.; Islam, M.O.; Selvam, P.; Pillai, R.A.; Cazenave-Gassiot, A.; Bendt, A.K.; Karnani, N.; Godfrey, K.M.; Lewis, R.M.; Wenk, M.R.; et al. Myo-inositol alters 13C-labeled fatty acid metabolism in human placental explants. J. Endocrinol. 2019, 243, 73–84. [Google Scholar] [CrossRef]
- Tint, M.-T.; Sadananthan, S.A.; Soh, S.-E.; Aris, I.M.; Michael, N.; Tan, K.H.; Shek, L.P.; Yap, F.; Gluckman, P.D.; Chong, Y.-S. Maternal glycemia during pregnancy and offspring abdominal adiposity measured by MRI in the neonatal period and preschool years: The Growing Up in Singapore Towards healthy Outcomes (GUSTO) prospective mother–offspring birth cohort study. Am. J. Clin. Nutr. 2020, 112, 39–47. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Chilton, F.H. Mobilization of different arachidonate pools and their roles in the generation of leukotrienes and free arachidonic acid during immunologic activation of mast cells. J. Immunol. 1993, 150, 563–570. [Google Scholar]
- Tessner, T.; Greene, D.G.; Wykle, R. Selective deacylation of arachidonate-containing ethanolamine-linked phosphoglycerides in stimulated human neutrophils. J. Biol. Chem. 1990, 265, 21032–21038. [Google Scholar] [CrossRef]
- Waku, K. Origins and fates of fatty acyl-CoA esters. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1992, 1124, 101–111. [Google Scholar] [CrossRef]
- Johnson, M.M.; Vaughn, B.; Triggiani, M.; Swan, D.D.; Fonteh, A.N.; Chilton, F.H. Role of arachidonyl triglycerides within lipid bodies in eicosanoid formation by human polymorphonuclear cells. Am. J. Respir. Cell Mol. Biol. 1999, 21, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, M.; Marone, G. Differential roles for triglyceride and phospholipid pools of arachidonic acid in human lung macrophages. J. Immunol. 1994, 152, 1394–1403. [Google Scholar] [PubMed]
- Balsinde, J.; Winstead, M.V.; Dennis, E.A. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002, 531, 2–6. [Google Scholar] [CrossRef]
- Triggiani, M.; Giannattasio, G.; Granata, F.; Loffredo, S.; Rossi, F.W.; Salzano, S.; Marone, G. Remodeling of arachidonic acid in inflammatory cells of the human lung. In Arachidonate Remodeling and Inflammation; Springer: Berlin/Heidelberg, Germany, 2004; pp. 115–130. [Google Scholar]
- Kuhn, D.C.; Crawford, M.A.; Stuart, M.J.; Botti, J.J.; Demers, L.M. Alterations in transfer and lipid distribution of arachidonic acid in placentas of diabetic pregnancies. Diabetes 1990, 39, 914–918. [Google Scholar] [CrossRef]
- Araújo, J.R.; Correia-Branco, A.; Ramalho, C.; Keating, E.; Martel, F. Gestational diabetes mellitus decreases placental uptake of long-chain polyunsaturated fatty acids: Involvement of long-chain acyl-CoA synthetase. J. Nutr. Biochem. 2013, 24, 1741–1750. [Google Scholar] [CrossRef]
- Setton-Avruj, C.P.; Speziale, E.H.S.; Sterin-Speziale, N.B. High Glucose Concentrations Stimulate Renal Papillary Phosphatidylcholine Biosynthesis. Nephron Exp. Nephrol. 2001, 9, 301–308. [Google Scholar] [CrossRef]
- Tashiro, S.-I.; Sudou, K.; Imoh, A.Y.A.; Koide, M.; Akazawa, Y. Phosphatidylethanolamine Methyltransferase Activity in Developing, Demyelinating, and Diabetic Mouse Brain. Tohoku J. Exp. Med. 1983, 141, 485–490. [Google Scholar] [CrossRef]
- Hartz, C.S.; Nieman, K.M.; Jacobs, R.L.; Vance, D.E.; Schalinske, K.L. Hepatic phosphatidylethanolamine N-methyltransferase expression is increased in diabetic rats. J. Nutr. 2006, 136, 3005–3009. [Google Scholar] [CrossRef][Green Version]
- Freed, K.; Moses, E.; Brennecke, S.; Rice, G. Differential expression of type II, IV and cytosolic PLA2 messenger RNA in human intrauterine tissues at term. Mol. Hum. Reprod. 1997, 3, 493–499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farrugia, W.; Rice, G.; Wong, M.; Scott, K.; Brennecke, S. Release of Type II phospholipase A2 immunoreactivity and phospholipase A2 enzymatic activity from human placenta. J. Endocrinol. 1997, 153, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.; Wong, M.; Farrugia, W.; Scott, K. Contribution of Type II phospholipase A2 to in vitro phospholipase A2 enzymatic activity in human term placenta. J. Endocrinol. 1998, 157, 25–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schliefsteiner, C.; Hirschmugl, B.; Kopp, S.; Curcic, S.; Bernhart, E.M.; Marsche, G.; Lang, U.; Desoye, G.; Wadsack, C. Maternal Gestational Diabetes Mellitus increases placental and foetal lipoprotein-associated Phospholipase A2 which might exert protective functions against oxidative stress. Sci. Rep. 2017, 7, 12628. [Google Scholar] [CrossRef]
- Diez, E.; Chilton, F.; Stroup, G.; Mayer, R.; Winkler, J.; Fonteh, A. Fatty acid and phospholipid selectivity of different phospholipase A2 enzymes studied by using a mammalian membrane as substrate. Biochem. J. 1994, 301, 721–726. [Google Scholar] [CrossRef]
- Ishizaki, J.; Suzuki, N.; Higashino, K.; Yokota, Y.; Ono, T.; Kawamoto, K.; Fujii, N.; Arita, H.; Hanasaki, K. Cloning and characterization of novel mouse and human secretory phospholipase A(2)s. J. Biol. Chem. 1999, 274, 24973–24979. [Google Scholar] [CrossRef]
- Suzuki, N.; Ishizaki, J.; Yokota, Y.; Higashino, K.; Ono, T.; Ikeda, M.; Fujii, N.; Kawamoto, K.; Hanasaki, K. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A(2)s. J. Biol. Chem. 2000, 275, 5785–5793. [Google Scholar] [CrossRef]
- Ferchaud-Roucher, V.; Kramer, A.; Silva, E.; Pantham, P.; Weintraub, S.T.; Jansson, T.; Powell, T.L. A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 394–402. [Google Scholar] [CrossRef]
- Hellmuth, C.; Uhl, O.; Standl, M.; Demmelmair, H.; Heinrich, J.; Koletzko, B.; Thiering, E. Cord blood metabolome is highly associated with birth weight, but less predictive for later weight development. Obes. Facts 2017, 10, 85–100. [Google Scholar] [CrossRef]
- Foreman-van Drongelen, M.M.; van Houwelingen, A.C.; Kester, A.D.; Hasaart, T.H.; Blanco, C.E.; Hornstra, G. Long-chain polyunsaturated fatty acids in preterm infants: Status at birth and its influence on postnatal levels. J. Pediatrics 1995, 126, 611–618. [Google Scholar] [CrossRef]
- Ruiz-Palacios, M.; Prieto-Sánchez, M.T.; Ruiz-Alcaraz, A.J.; Blanco-Carnero, J.E.; Sanchez-Campillo, M.; Parrilla, J.J.; Larqué, E. Insulin Treatment May Alter Fatty Acid Carriers in Placentas from Gestational Diabetes Subjects. Int. J. Mol. Sci. 2017, 18, 1203. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, D.B.; Patterson, R.L. PKC and PLA2: Probing the complexities of the calcium network. Cell Calcium 2009, 45, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Beeson, M.; Sajan, M.P.; Dizon, M.; Grebenev, D.; Gomez-Daspet, J.; Miura, A.; Kanoh, Y.; Powe, J.; Bandyopadhyay, G.; Standaert, M.L. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3, 4, 5-(PO4) 3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: Amelioration by rosiglitazone and exercise. Diabetes 2003, 52, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Saraceno, G.E.; Capani, F.; Castilla, R. Long-chain acyl-CoA synthetase 4 is regulated by phosphorylation. Biochem. Biophys. Res. Commun. 2013, 430, 272–277. [Google Scholar] [CrossRef]
- Radaelli, T.; Lepercq, J.; Varastehpour, A.; Basu, S.; Catalano, P.M.; Hauguel-De Mouzon, S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am. J. Obstet. Gynecol. 2009, 201, 209.e1–209.e10. [Google Scholar] [CrossRef]
- Wentzel, P.; Welsh, N.; Eriksson, U.J. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered prostaglandin E2 levels in rat embryos exposed to a diabetic environment. Diabetes 1999, 48, 813–820. [Google Scholar] [CrossRef]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Unique enzyme specificity of three human phospholipases A2 toward phospholipids containing sn-2 omega-3 and omega-6 fatty acids. FASEB J. 2019, 33, 489–493. [Google Scholar] [CrossRef]
- Colomiere, M.; Permezel, M.; Riley, C.; Desoye, G.; Lappas, M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur. J. Endocrinol. 2009, 160, 567–578. [Google Scholar] [CrossRef]
- Varma, S.; Lal, B.K.; Zheng, R.; Breslin, J.W.; Saito, S.; Pappas, P.J.; Hobson, R.W.; Durán, W.N. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H1744–H1751. [Google Scholar] [CrossRef]
- Xu, K.-P.; Li, Y.; Ljubimov, A.V.; Fu-Shin, X.Y. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 2009, 58, 1077–1085. [Google Scholar] [CrossRef]
- Hayashi, E.; Maeda, T.; Tomita, T. The effect of myo-inositol deficiency on lipid metabolism in rats: I. The alteration of lipid metabolism in myo-inositol deficient rats. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1974, 360, 134–145. [Google Scholar] [CrossRef]
- Adam, O. Immediate and long range effects of the uptake of increased amounts of arachidonic acid. Clin. Investig. 1992, 70, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, P.V.; Horvath, P.; Achar, S.B. Regulation of the activity and fatty acid specificity of lecithin-cholesterol acyltransferase by sphingomyelin and its metabolites, ceramide and ceramide phosphate. Biochemistry 2006, 45, 5029–5038. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.E.; Wells, W.W. Characterization of the lactation-dependent fatty liver in myo-inositol deficient rats. J. Nutr. 1977, 107, 1871–1883. [Google Scholar] [CrossRef]
- Wells, I.C.; Hogan, J. Effects of dietary deficiencies of lipotropic factors on plasma cholesterol esterification and tissue cholesterol in rats. J. Nutr. 1968, 95, 55–62. [Google Scholar] [CrossRef]
- Petit, A.; Guillon, G.; Tence, M.; Jard, S.; Gallo-Payet, N.; Bellabarba, D.; Lehoux, J.G.; Belisle, S. Angiotensin II stimulates both inositol phosphate production and human placental lactogen release from human trophoblastic cells. J. Clin. Endocrinol. Metab. 1989, 69, 280–286. [Google Scholar] [CrossRef]
- Deykin, D.; Jakubowski, J.; Brown, M. Ionophore-induced metabolism of phospholipids and arachidonic acid (AA) in porcine aortic endothelial cells (PAEC): Release of AA from alkenyl-linked phosphatidylethanolamine (PE). Thromb. Haemost. 1987, 58, 1826. [Google Scholar]
- Chilton, F.H. Potential phospholipid source (s) of arachidonate used for the synthesis of leukotrienes by the human neutrophil. Biochem. J. 1989, 258, 327–333. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Pérez-Chacón, G.; Meana, C.; Balgoma, D.; Pol, A.; del Pozo, M.A.; Balboa, M.A.; Balsinde, J. Altered arachidonate distribution in macrophages from caveolin-1 null mice leading to reduced eicosanoid synthesis. J. Biol. Chem. 2011, 286, 35299–35307. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).