The Obesity-Related Dietary Pattern Is Associated with Higher Risk of Sleep Disorders: A Cross-Sectional Study from NHANES
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Study Population
2.2. Definition of Outcome
2.3. Dietary Assessment
2.4. Covariates
2.5. Statistical Analysis
2.5.1. Extraction of the Obesity-Related Dietary Patterns
2.5.2. Descriptive Analysis and Modeling
3. Results
3.1. Study Population
3.2. Obesity-Related Dietary Patterns
3.3. Dietary Patterns and Sleep Disorders
3.4. Dietary Patterns and Sleep Duration
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorpy, M.J. Classification of Sleep Disorders. Neurotherapeutics 2012, 9, 687–701. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; Colten, H.R., Altevogt, B.M., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2006; ISBN 978-0-309-10111-0. [Google Scholar]
- Anothaisintawee, T.; Reutrakul, S.; Van Cauter, E.; Thakkinstian, A. Sleep Disturbances Compared to Traditional Risk Factors for Diabetes Development: Systematic Review and Meta-Analysis. Sleep Med. Rev. 2016, 30, 11–24. [Google Scholar] [CrossRef]
- McDermott, M.; Brown, D.L.; Chervin, R.D. Sleep Disorders and the Risk of Stroke. Expert Rev. Neurother. 2018, 18, 523–531. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Yang, C.; Wang, L. The Role of Sleep Disorders in Cardiovascular Diseases: Culprit or Accomplice? Life Sci. 2021, 283, 119851. [Google Scholar] [CrossRef]
- Huyett, P.; Siegel, N.; Bhattacharyya, N. Prevalence of Sleep Disorders and Association with Mortality: Results from the NHANES 2009–2010. Laryngoscope 2021, 131, 686–689. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D. Global, Regional, and National Consumption Levels of Dietary Fats and Oils in 1990 and 2010: A Systematic Analysis Including 266 Country-Specific Nutrition Surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef]
- Deng, M.-G.; Nie, J.-Q.; Li, Y.-Y.; Yu, X.; Zhang, Z.-J. Higher HEI-2015 Scores Are Associated with Lower Risk of Sleep Disorder: Results from a Nationally Representative Survey of United States Adults. Nutrients 2022, 14, 873. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Wood, A.C.; Redline, S.; Reid, M.; Johnson, D.A.; Maras, J.E.; Jacobs, D.R.; Shea, S.; Crawford, A.; St-Onge, M.-P. Mediterranean Diet Pattern and Sleep Duration and Insomnia Symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy158. [Google Scholar] [CrossRef]
- Rostami, H.; Khayyatzadeh, S.S.; Tavakoli, H.; Bagherniya, M.; Mirmousavi, S.J.; Farahmand, S.K.; Tayefi, M.; Ferns, G.A.; Ghayour-Mobarhan, M. The Relationship between Adherence to a Dietary Approach to Stop Hypertension (DASH) Dietary Pattern and Insomnia. BMC Psychiatry 2019, 19, 234. [Google Scholar] [CrossRef]
- Gaona-Pineda, E.B.; Martinez-Tapia, B.; Rodríguez-Ramírez, S.; Guerrero-Zúñiga, S.; Perez-Padilla, R.; Shamah-Levy, T. Dietary Patterns and Sleep Disorders in Mexican Adults from a National Health and Nutrition Survey. J. Nutr. Sci. 2021, 10, e34. [Google Scholar] [CrossRef]
- Kurotani, K.; Kochi, T.; Nanri, A.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Akter, S.; Ito, R.; Pham, N.M.; Kabe, I.; et al. Dietary Patterns and Sleep Symptoms in Japanese Workers: The Furukawa Nutrition and Health Study. Sleep Med. 2015, 16, 298–304. [Google Scholar] [CrossRef]
- Yu, C.; Shi, Z.; Lv, J.; Guo, Y.; Bian, Z.; Du, H.; Chen, Y.; Tao, R.; Huang, Y.; Chen, J.; et al. Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank. Nutrients 2017, 9, 232. [Google Scholar] [CrossRef]
- Gębski, J.; Jezewska-Zychowicz, M.; Guzek, D.; Świątkowska, M.; Stangierska, D.; Plichta, M. The Associations between Dietary Patterns and Short Sleep Duration in Polish Adults (LifeStyle Study). Int. J. Environ. Res. Public. Health 2018, 15, 2497. [Google Scholar] [CrossRef]
- Mondin, T.C.; Stuart, A.L.; Williams, L.J.; Jacka, F.N.; Pasco, J.A.; Ruusunen, A. Diet Quality, Dietary Patterns and Short Sleep Duration: A Cross-Sectional Population-Based Study. Eur. J. Nutr. 2019, 58, 641–651. [Google Scholar] [CrossRef]
- Jansen, E.C.; Stern, D.; Monge, A.; O’Brien, L.M.; Lajous, M.; Peterson, K.E.; López-Ridaura, R. Healthier Dietary Patterns Are Associated with Better Sleep Quality among Midlife Mexican Women. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2020, 16, 1321–1330. [Google Scholar] [CrossRef]
- Lisan, Q.; Tafflet, M.; Thomas, F.; Boutouyrie, P.; Guibout, C.; Haba-Rubio, J.; Climie, R.; Périer, M.C.; Van Sloten, T.; Pannier, B.; et al. Body Silhouette Trajectories Over the Lifespan and Insomnia Symptoms: The Paris Prospective Study 3. Sci. Rep. 2019, 9, 1581. [Google Scholar] [CrossRef]
- Lallukka, T.; Haario, P.; Lahelma, E.; Rahkonen, O. Associations of Relative Weight with Subsequent Changes over Time in Insomnia Symptoms: A Follow-up Study among Middle-Aged Women and Men. Sleep Med. 2012, 13, 1271–1279. [Google Scholar] [CrossRef]
- Batool-Anwar, S.; Li, Y.; De Vito, K.; Malhotra, A.; Winkelman, J.; Gao, X. Lifestyle Factors and Risk of Restless Legs Syndrome: Prospective Cohort Study. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016, 12, 187–194. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Gao, T.; Zhong, F.; Sun, Y.; Cai, J.; Ma, A. The Association between Obesity and Restless Legs Syndrome: A Systemic Review and Meta-Analysis of Observational Studies. J. Affect. Disord. 2018, 235, 384–391. [Google Scholar] [CrossRef]
- Hargens, T.A.; Kaleth, A.S.; Edwards, E.S.; Butner, K.L. Association between Sleep Disorders, Obesity, and Exercise: A Review. Nat. Sci. Sleep 2013, 5, 27–35. [Google Scholar] [CrossRef]
- Fung, T.T.; Pan, A.; Hou, T.; Chiuve, S.E.; Tobias, D.K.; Mozaffarian, D.; Willett, W.C.; Hu, F.B. Long-Term Change in Diet Quality Is Associated with Body Weight Change in Men and Women. J. Nutr. 2015, 145, 1850–1856. [Google Scholar] [CrossRef]
- Arabshahi, S.; Ibiebele, T.I.; Hughes, M.C.B.; Lahmann, P.H.; Williams, G.M.; van der Pols, J.C. Dietary Patterns and Weight Change: 15-Year Longitudinal Study in Australian Adults. Eur. J. Nutr. 2017, 56, 1455–1465. [Google Scholar] [CrossRef]
- Lotfi, K.; Saneei, P.; Hajhashemy, Z.; Esmaillzadeh, A. Adherence to the Mediterranean Diet, Five-Year Weight Change, and Risk of Overweight and Obesity: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. Bethesda Md. 2022, 13, 152–166. [Google Scholar] [CrossRef]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a New Statistical Method to Derive Dietary Patterns in Nutritional Epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- Pinto, A.; Santos, A.C.; Lopes, C.; Oliveira, A. Dietary Patterns at 7 Year-Old and Their Association with Cardiometabolic Health at 10 Year-Old. Clin. Nutr. Edinb. Scotl. 2020, 39, 1195–1202. [Google Scholar] [CrossRef]
- Amir, A.; Masterson, R.M.; Halim, A.; Nava, A. Restless Leg Syndrome: Pathophysiology, Diagnostic Criteria, and Treatment. Pain Med. Malden Mass 2022, 23, 1032–1035. [Google Scholar] [CrossRef]
- Mannarino, M.R.; Di Filippo, F.; Pirro, M. Obstructive Sleep Apnea Syndrome. Eur. J. Intern. Med. 2012, 23, 586–593. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2 2013, 161, 1–24. [Google Scholar]
- NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 30 March 2022).
- Food Surveys Research Group: USDA ARS. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/ (accessed on 30 March 2022).
- Kirkpatrick, S.I.; Dodd, K.W.; Reedy, J.; Krebs-Smith, S.M. Income and Race/Ethnicity Are Associated with Adherence to Food-Based Dietary Guidance among US Adults and Children. J. Acad. Nutr. Diet. 2012, 112, 624–635.e6. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report; US Department of Health and Human Services: Washington, DC, USA, 2018.
- NHANES. Tutorials. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx (accessed on 13 June 2022).
- Naja, F.; Itani, L.; Hwalla, N.; Sibai, A.M.; Kharroubi, S.A. Identification of Dietary Patterns Associated with Elevated Blood Pressure among Lebanese Men: A Comparison of Principal Component Analysis with Reduced Rank Regression and Partial Least Square Methods. PLoS ONE 2019, 14, e0220942. [Google Scholar] [CrossRef]
- Jessri, M.; Wolfinger, R.D.; Lou, W.Y.; L’Abbé, M.R. Identification of Dietary Patterns Associated with Obesity in a Nationally Representative Survey of Canadian Adults: Application of a Priori, Hybrid, and Simplified Dietary Pattern Techniques. Am. J. Clin. Nutr. 2017, 105, 669–684. [Google Scholar] [CrossRef]
- Pinto, A.; Severo, M.; Oliveira, A. Use of a Hybrid Method to Derive Dietary Patterns in 7 Years Olds with Explanatory Ability of Body Mass Index at Age 10. Eur. J. Clin. Nutr. 2021, 75, 1598–1606. [Google Scholar] [CrossRef]
- Jessri, M.; Lou, W.Y.; L’Abbé, M.R. Evaluation of Different Methods to Handle Misreporting in Obesity Research: Evidence from the Canadian National Nutrition Survey. Br. J. Nutr. 2016, 115, 147–159. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar]
- Garriguet, D. Under-Reporting of Energy Intake in the Canadian Community Health Survey. Health Rep. 2008, 19, 37–45. [Google Scholar]
- St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef]
- Wells, A.S.; Read, N.W.; Uvnas-Moberg, K.; Alster, P. Influences of Fat and Carbohydrate on Postprandial Sleepiness, Mood, and Hormones. Physiol. Behav. 1997, 61, 679–686. [Google Scholar] [CrossRef]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-Glycemic-Index Carbohydrate Meals Shorten Sleep Onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Hale, L.; St-Onge, M.-P.; Choi, L.; LeBlanc, E.S.; Malaspina, D.; Opler, M.G.; Shadyab, A.H.; Shikany, J.M.; Snetselaar, L.; et al. High Glycemic Index and Glycemic Load Diets as Risk Factors for Insomnia: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2020, 111, 429–439. [Google Scholar] [CrossRef]
- Zhao, M.; Tuo, H.; Wang, S.; Zhao, L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediators Inflamm. 2020, 2020, 3142874. [Google Scholar] [CrossRef]
- Lennerz, B.; Lennerz, J.K. Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Boeing, H.; Fritsche, A.; Schleicher, E.; Joost, H.-G.; Schulze, M.B.; Steffen, A.; Pischon, T. Consumption of Red Meat and Whole-Grain Bread in Relation to Biomarkers of Obesity, Inflammation, Glucose Metabolism and Oxidative Stress. Eur. J. Nutr. 2013, 52, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Escalante, M.L.; Coop-Gamas, F.; Cervantes-Rodríguez, M.; Méndez-Iturbide, D.; Aranda-González, I.I. The Effect of Diet on Oxidative Stress and Metabolic Diseases-Clinically Controlled Trials. J. Food Biochem. 2020, 44, e13191. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Schwingshackl, L.; Agogo, G.O.; Sonestedt, E.; Boeing, H.; Knüppel, S. Associations of Food Groups and Cardiometabolic and Inflammatory Biomarkers: Does the Meal Matter? Br. J. Nutr. 2019, 122, 707–716. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative Stress and Inflammation Biomarker Expression in Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef]
- Dowsett, J.; Didriksen, M.; von Stemann, J.H.; Larsen, M.H.; Thørner, L.W.; Sørensen, E.; Erikstrup, C.; Pedersen, O.B.; Hansen, M.B.; Eugen-Olsen, J.; et al. Chronic Inflammation Markers and Cytokine-Specific Autoantibodies in Danish Blood Donors with Restless Legs Syndrome. Sci. Rep. 2022, 12, 1672. [Google Scholar] [CrossRef]
- Tan, X.; Alén, M.; Wang, K.; Tenhunen, J.; Wiklund, P.; Partinen, M.; Cheng, S. Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial. Nutrients 2016, 8, 751. [Google Scholar] [CrossRef]
- Paradis, A.-M.; Godin, G.; Pérusse, L.; Vohl, M.-C. Associations between Dietary Patterns and Obesity Phenotypes. Int. J. Obes. 2009, 33, 1419–1426. [Google Scholar] [CrossRef]
- Schulze, M.B.; Fung, T.T.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dietary Patterns and Changes in Body Weight in Women. Obesity 2006, 14, 1444–1453. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Wang, Z.; Huang, F.; Zhang, X.; Du, W.; Su, C.; Ouyang, Y.; Li, L.; Bai, J.; et al. Trajectories of Dietary Patterns and Their Associations with Overweight/Obesity among Chinese Adults: China Health and Nutrition Survey 1991–2018. Nutrients 2021, 13, 2835. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/2020-advisory-committee-report (accessed on 15 July 2022).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017, 288, 1–8. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, N.; Khayyatzadeh, S.S.; Banazadeh, V.; Bagherniya, M.; Tayefi, M.; Eslami, S.; Ferns, G.A.; Ghayour-Mobarhan, M. Adherence to a Dietary Approach to Stop Hypertension (DASH)-Style in Relation to Daytime Sleepiness. Nat. Sci. Sleep 2020, 12, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Subar, A.F.; Thompson, F.E.; Troiano, R.; Schatzkin, A.; Kipnis, V. Psychosocial Predictors of Energy Underreporting in a Large Doubly Labeled Water Study. Am. J. Clin. Nutr. 2004, 79, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.; Taren, D.L.; Teixeira, P.J.; Thomson, C.A.; Lohman, T.G.; Going, S.B.; Houtkooper, L.B. The Psychosocial and Behavioral Characteristics Related to Energy Misreporting. Nutr. Rev. 2006, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Risso, T.T.; Poyares, D.; Rizzi, C.F.; Pulz, C.; Guilleminault, C.; Tufik, S.; de Paola, A.A.V.; Cintra, F. The Impact of Sleep Duration in Obstructive Sleep Apnea Patients. Sleep Breath. 2013, 17, 837–843. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s Updated Sleep Duration Recommendations: Final Report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An Overview of USDA’s Dietary Intake Data System. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
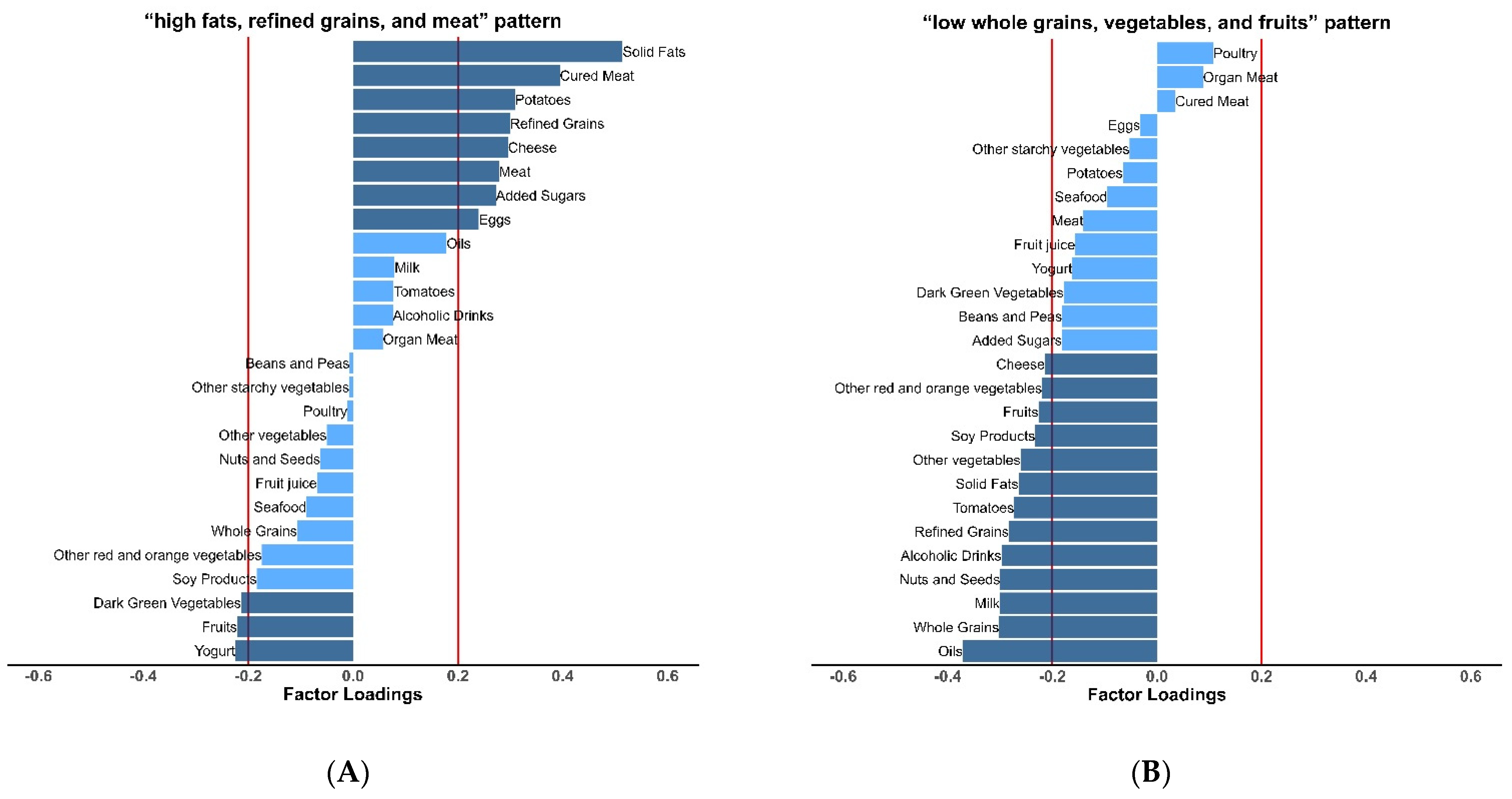
| Total | Sleep Disorders | Without Sleep Disorders | p Value 4 | |
|---|---|---|---|---|
| n | 19,892 | 1671 | 18,221 | |
| Age, % | <0.001 | |||
| 20–39 | 6824 (37.5) | 335 (21.6) | 6489 (39.0) | |
| 40–59 | 6651 (38.1) | 711 (50.1) | 5940 (37.0) | |
| 60+ | 6417 (24.4) | 625 (28.3) | 5792 (24.0) | |
| Age (year)2 | 46.8 (16.7) | 51.0 (14.5) | 46.4 (16.8) | <0.001 |
| Female, % | 10,432 (52.5) | 814 (48.4) | 9618 (52.9) | 0.014 |
| Race, % | <0.001 | |||
| MA | 2987 (8.1) | 160 (5.0) | 2827 (8.4) | |
| OH | 1647 (4.7) | 154 (4.8) | 1493 (4.7) | |
| NHW | 9533 (69.8) | 918 (74.7) | 8615 (69.4) | |
| NHB | 4117 (10.9) | 350 (10.9) | 3767 (10.9) | |
| OR | 1608 (6.4) | 89 (4.6) | 1519 (6.6) | |
| Education level, % | 0.108 | |||
| ≤11th grade | 4708 (15.8) | 356 (14.0) | 4352 (16.0) | |
| High school grade orequivalent | 4549 (22.6) | 405 (24.8) | 4144 (22.4) | |
| College or above | 10,635 (61.6) | 910 (61.2) | 9725 (61.6) | |
| Poverty income ratio, % | 0.029 | |||
| ≤130% | 6091 (21.3) | 585 (24.0) | 5506 (21.1) | |
| 131–185% | 2556 (10.5) | 226 (12.4) | 2330 (10.3) | |
| ≥186% | 11,245 (68.2) | 860 (63.6) | 10,385 (68.6) | |
| Unemployment, % | 8638 (36.4) | 939 (45.4) | 7699 (35.5) | <0.001 |
| Smoking, % | <0.001 | |||
| Current | 4014 (20.4) | 406 (24.5) | 3608 (20.0) | |
| Ever | 5006 (24.6) | 523 (30.0) | 4483 (24.1) | |
| Never | 10,872 (55.0) | 742 (45.5) | 10,130 (55.8) | |
| Physical activity ≥ 600 MET-min/week, % | 6754 (39.0) | 445 (29.3) | 6309 (39.9) | <0.001 |
| Sleep duration (hours)2 | 6.9 (1.3) | 6.5 (1.6) | 7.0 (1.3) | <0.001 |
| Energy intake (kcal/day)2, 3 | 2092.9 (765.6) | 2073.7 (776.0) | 2094.6 (764.7) | 0.511 |
| Waist circumference (cm)2 | 98.6 (16.4) | 108.5 (18.7) | 97.7 (15.9) | <0.001 |
| BMI (kg/m2)2 | 28.8 (6.7) | 32.5 (7.9) | 28.5 (6.5) | <0.001 |
| Quartile of Dietary Pattern Scores | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p for Trend | |
| “high fats, refined grains, and meat” pattern | |||||
| Sleep disorder/Total | 367/5202 | 433/5149 | 412/4863 | 459/4678 | |
| Crude | 1.0 (Ref.) | 1.23 (0.97, 1.55) | 1.13 (0.91, 1.40) | 1.41 (1.12, 1.77) ** | 0.009 |
| Model 1 | 1.0 (Ref.) | 1.29 (1.02, 1.62) * | 1.26 (1.02, 1.56) * | 1.66 (1.31, 2.11) *** | <0.001 |
| Model 2 | 1.0 (Ref.) | 1.18 (0.93, 1.51) | 1.14 (0.92, 1.40) | 1.43 (1.12, 1.84) ** | 0.009 |
| “low whole grains, vegetables, and fruits” pattern | |||||
| Sleep disorder/Total | 323/4313 | 360/4663 | 454/5177 | 534/5739 | |
| Crude | 1.0 (Ref.) | 0.99 (0.82, 1.20) | 1.16 (0.93, 1.45) | 1.18 (0.96, 1.44) | 0.084 |
| Model 1 | 1.0 (Ref.) | 1.03 (0.84, 1.27) | 1.25 (0.99, 1.58) | 1.33 (1.05, 1.68) * | 0.013 |
| Model 2 | 1.0 (Ref.) | 0.99 (0.80, 1.22) | 1.18 (0.92, 1.51) | 1.18 (0.92, 1.51) | 0.130 |
| Quartile of Dietary Pattern Scores | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p for Trend | |
| “high fats, refined grains, and meat” pattern | |||||
| Crude | 1.0 (Ref.) | −0.04 (−0.11, 0.04) | −0.07 (−0.14, 0) | −0.21 (−0.29, −0.13) *** | <0.001 |
| Model 1 | 1.0 (Ref.) | −0.04 (−0.12, 0.03) | −0.08 (−0.15, 0) * | −0.23 (−0.31, −0.15) *** | <0.001 |
| Model 2 | 1.0 (Ref.) | −0.02 (−0.10, 0.06) | −0.04 (−0.11, 0.04) | −0.17 (−0.26, −0.08) *** | 0.001 |
| “low whole grains, vegetables, and fruits” pattern | |||||
| Crude | 1.0 (Ref.) | −0.01 (−0.08, 0.05) | −0.09 (−0.16, −0.02) * | −0.21 (−0.29, −0.12) *** | <0.001 |
| Model 1 | 1.0 (Ref.) | −0.10 (−0.17, −0.03) ** | −0.23 (−0.31, −0.16) *** | −0.40 (−0.51, −0.29) *** | <0.001 |
| Model 2 | 1.0 (Ref.) | −0.05 (−0.12, 0.02) | −0.15 (−0.23, −0.07) *** | −0.26 (−0.37, −0.15) *** | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Fan, C.; Zhu, Y.; Tang, X.; Ling, L. The Obesity-Related Dietary Pattern Is Associated with Higher Risk of Sleep Disorders: A Cross-Sectional Study from NHANES. Nutrients 2022, 14, 3987. https://doi.org/10.3390/nu14193987
Wang S, Fan C, Zhu Y, Tang X, Ling L. The Obesity-Related Dietary Pattern Is Associated with Higher Risk of Sleep Disorders: A Cross-Sectional Study from NHANES. Nutrients. 2022; 14(19):3987. https://doi.org/10.3390/nu14193987
Chicago/Turabian StyleWang, Shanze, Chaonan Fan, Yingying Zhu, Xijia Tang, and Li Ling. 2022. "The Obesity-Related Dietary Pattern Is Associated with Higher Risk of Sleep Disorders: A Cross-Sectional Study from NHANES" Nutrients 14, no. 19: 3987. https://doi.org/10.3390/nu14193987
APA StyleWang, S., Fan, C., Zhu, Y., Tang, X., & Ling, L. (2022). The Obesity-Related Dietary Pattern Is Associated with Higher Risk of Sleep Disorders: A Cross-Sectional Study from NHANES. Nutrients, 14(19), 3987. https://doi.org/10.3390/nu14193987






