Quantity and Source of Protein during Complementary Feeding and Infant Growth: Evidence from a Population Facing Double Burden of Malnutrition
Abstract
1. Introduction
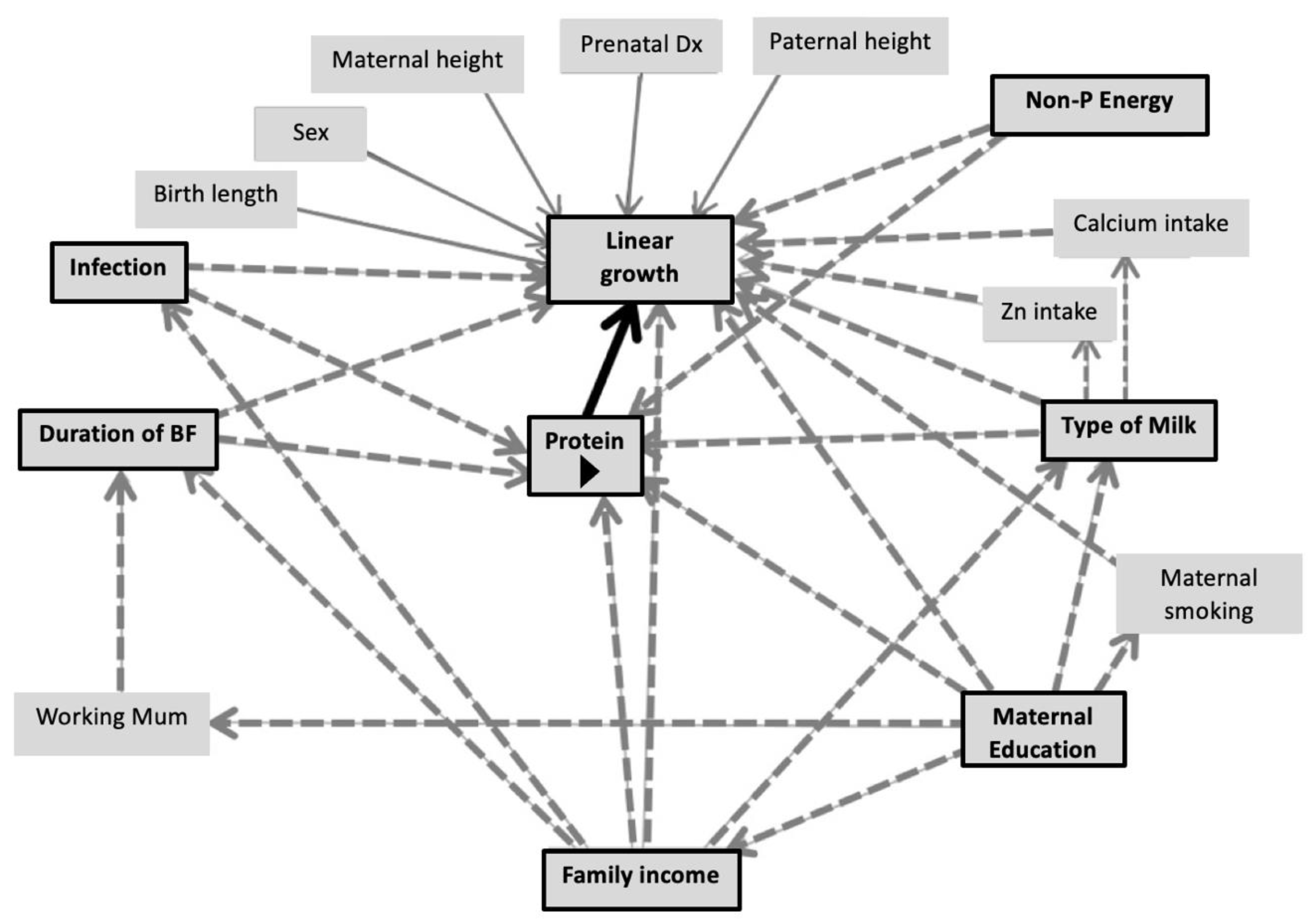
2. Subjects and Methods
3. Results
3.1. Demographic Data
3.2. Prevalence of Malnutrition in the Study Population
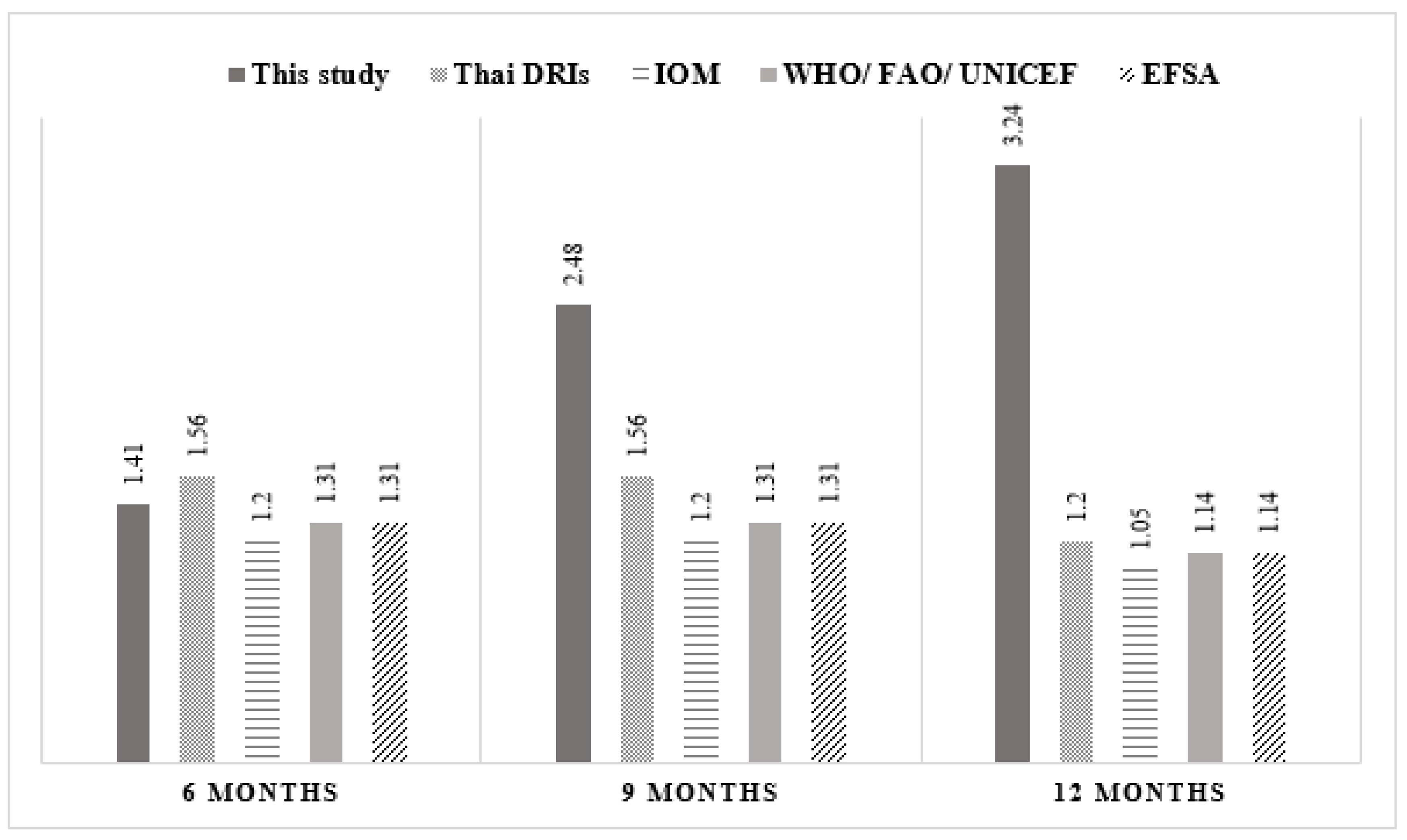
3.3. Complementary Feeding Practices and Nutrient Intakes
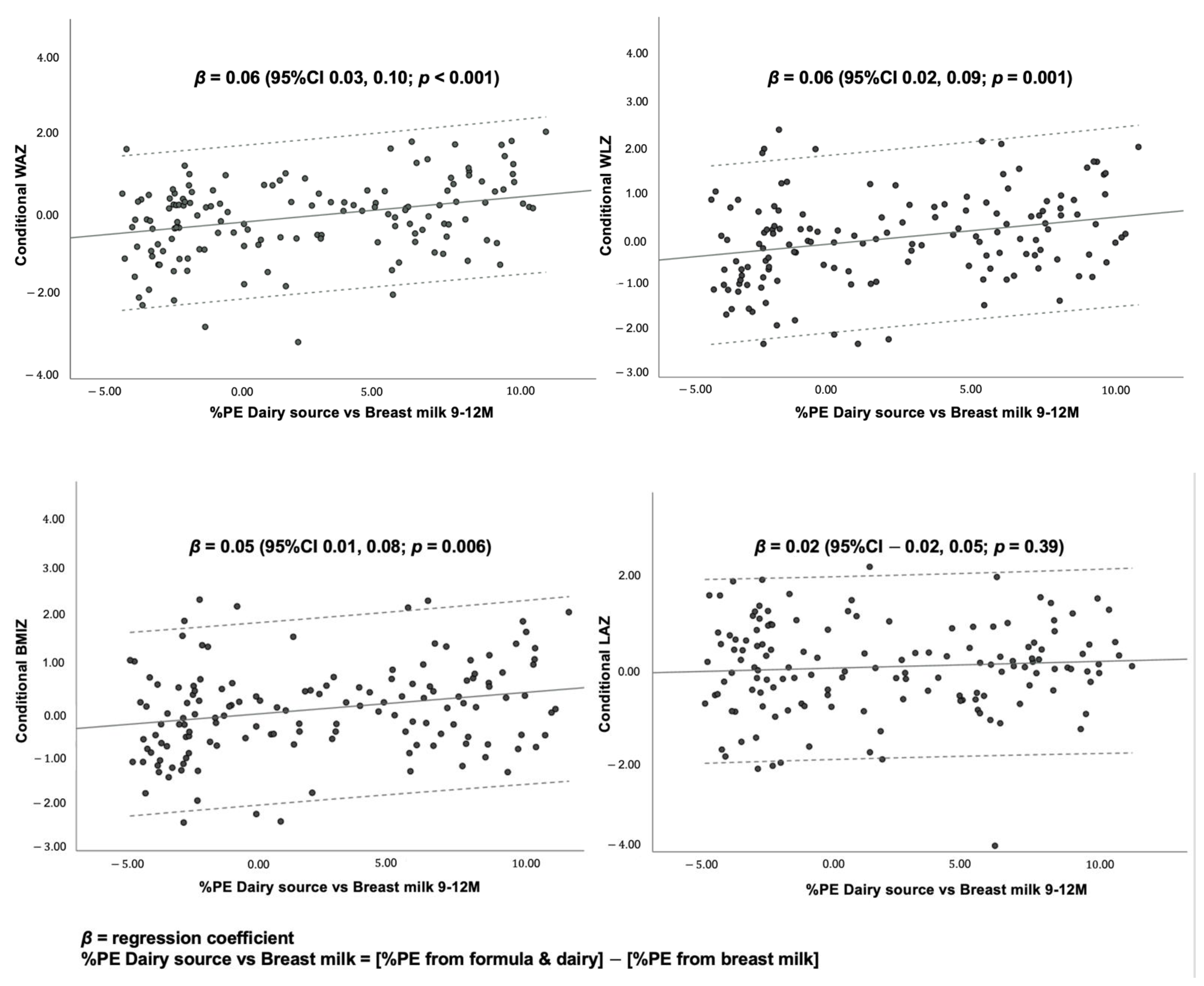
3.4. Association between Dietary Protein and Growth Outcomes
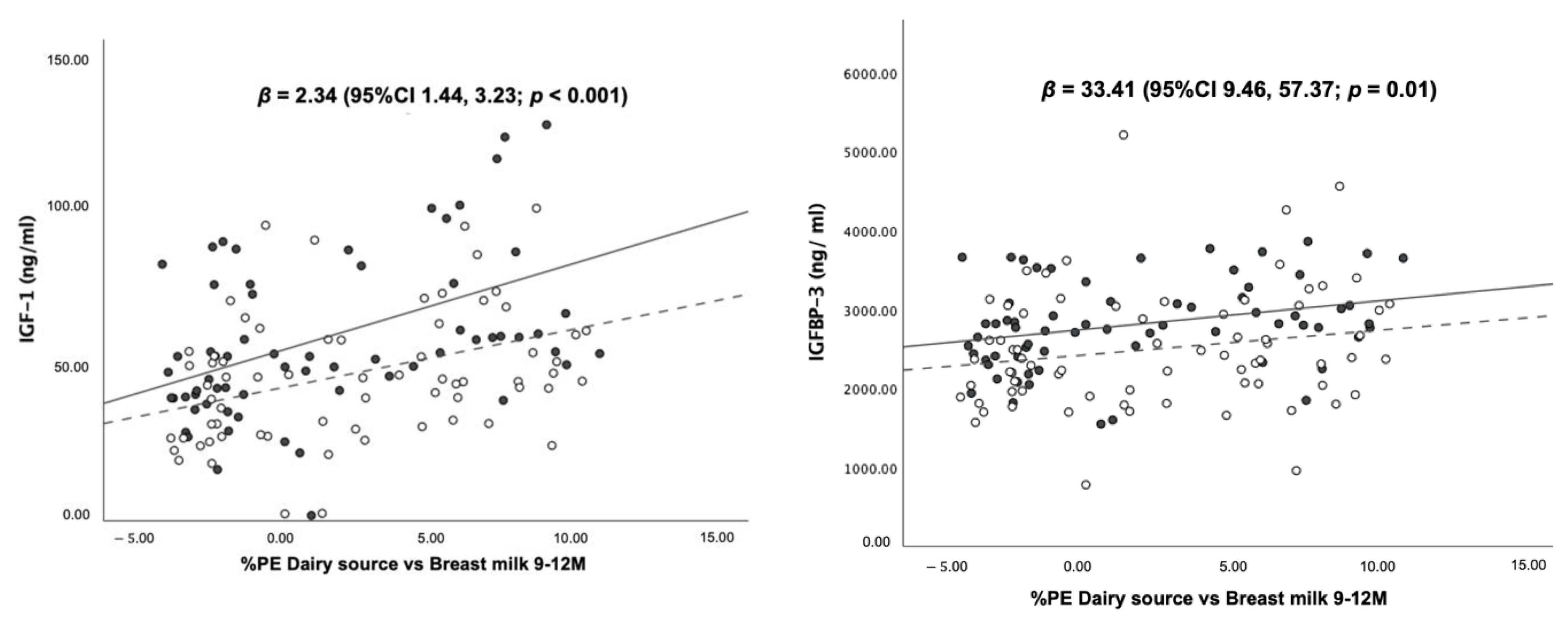
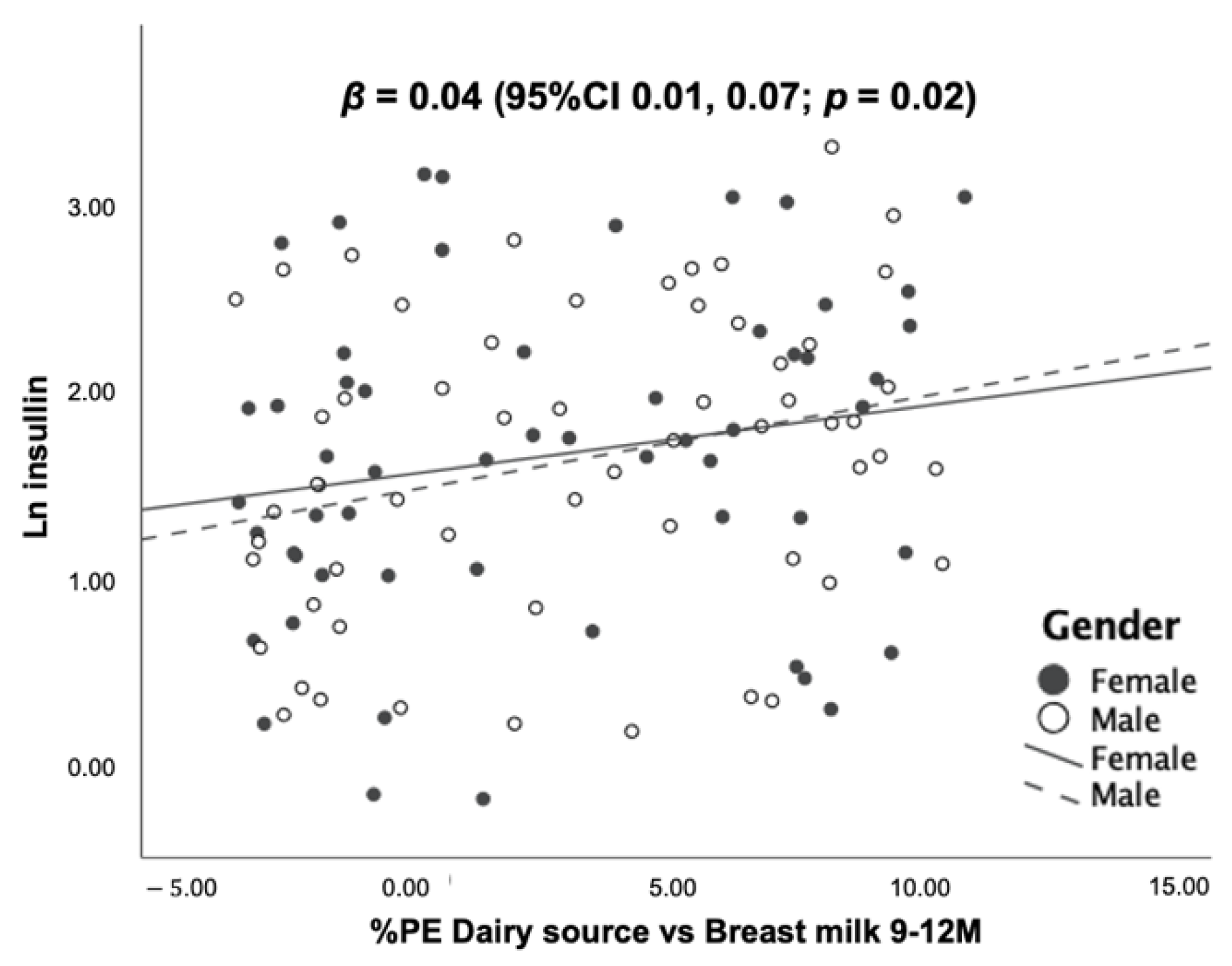
3.5. Association between Dietary Protein Intake and Blood Levels of IGF-1, IGFBP-3 and Insulin at 12 Months of Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Winichagoon, P. Thailand nutrition in transition: Situation and challenges of maternal and child nutrition. Asia Pac. J. Clin. Nutr. 2013, 22, 6–15. [Google Scholar] [PubMed]
- Dietz, W.H. Double-duty solutions for the double burden of malnutrition. Lancet 2017, 390, 2607–2608. [Google Scholar] [CrossRef]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Hoffman, D.; Arts, M.; Begin, F. The “First 1,000 Days+” as Key Contributor to the Double Burden of Malnutrition. Ann. Nutr. Metab. 2019, 75, 99–102. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Grummer-Strawn, L.; Begin, F. Emerging issues in complementary feeding: Global aspects. Matern. Child Nutr. 2017, 13, e12444. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef]
- Scaglioni, S.; Agostoni, C.; De Notaris, R.; Radaelli, G.; Radice, N.; Valenti, M.; Giovannini, M.; Riva, E. Early macronutrient intake and overweight at five years of age. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 777–781. [Google Scholar] [CrossRef]
- Hoppe, C.; Rovenna Udam, T.; Lauritzen, L.; Mølgaard, C.; Juul, A.; Fleischer Michaelsen, K. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am. J. Clin. Nutr. 2004, 80, 447–452. [Google Scholar] [CrossRef]
- Morgan, J.; Taylor, A.; Fewtrell, M. Meat consumption is positively associated with psychomotor outcome in children up to 24 months of age. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Thorisdottir, B.; Gunnarsdottir, I.; Palsson, G.I.; Halldorsson, T.I.; Thorsdottir, I. Animal protein intake at 12 months is associated with growth factors at the age of six. Acta Paediatr. 2014, 103, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Grote, V.; Lattka, E.; Weber, M.; Gruszfeld, D.; Socha, P.; Closa-Monasterolo, R.; Escribano, J.; Giovannini, M.; Verduci, E.; et al. Associations of IGF-1 gene variants and milk protein intake with IGF-I concentrations in infants at age 6 months-results from a randomized clinical trial. Growth Horm. IGF Res. 2013, 23, 149–158. [Google Scholar] [CrossRef]
- Wan, X.; Wang, S.; Xu, J.; Zhuang, L.; Xing, K.; Zhang, M.; Zhu, X.; Wang, L.; Gao, P.; Xi, Q.; et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARgamma activation. PLoS ONE 2017, 12, e0173174. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, L.; Feng, Z.; Ren, W.; Yin, J.; Duan, J.; Li, T.; Yin, Y. Dietary L-arginine supplementation protects weanling pigs from deoxynivalenol-induced toxicity. Toxins 2015, 7, 1341–1354. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Nissen, S.L.; Abumrad, N.N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar] [CrossRef]
- Mogami, H.; Yura, S.; Itoh, H.; Kawamura, M.; Fujii, T.; Suzuki, A.; Aoe, S.; Ogawa, Y.; Sagawa, N.; Konishi, I.; et al. Isocaloric high-protein diet as well as branched-chain amino acids supplemented diet partially alleviates adverse consequences of maternal undernutrition on fetal growth. Growth Horm. IGF Res. 2009, 19, 478–485. [Google Scholar] [CrossRef]
- World Health Organization. WHO Anthro for Personal Computers Version 3.2.2: Software for Assessing Growth and Development of the World’s Children 2011 [Updated 2019, Cited 2022 April]. Available online: https://www.who.int/tools/child-growth-standards/software (accessed on 1 May 2021).
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Institute of Nutrition, Mahidol University. INMUCAL-Nutrients, Version 4.0; Mahidol University: Nakorn Pathom, Thailand, 2018. [Google Scholar]
- Olaya, G.A.; Lawson, M.; Fewtrell, M.S. Efficacy and safety of new complementary feeding guidelines with an emphasis on red meat consumption: A randomized trial in Bogota, Colombia. Am. J. Clin. Nutr. 2013, 98, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Kittisakmontri, K.; Lanigan, J.; Wells, J.C.K.; Fewtrell, M. The Impact of Dietary Protein in Complementary Foods on Infant Growth and Body Composition in a Population Facing the Double Burden of Malnutrition: Protocol for a Multicenter, Prospective Cohort Study. JMIR Res. Protoc. 2020, 9, e18112. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.; Van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- Bureau of Health Promotion. Mother and Child Health Handbook; Veteran Press: Bangkok, Thailand, 2015. [Google Scholar]
- Parikh, P.; Semba, R.; Manary, M.; Swaminathan, S.; Udomkesmalee, E.; Bos, R.; Poh, B.K.; Rojroongwasinkul, N.; Geurts, J.; Sekartini, R.; et al. Animal source foods, rich in essential amino acids, are important for linear growth and development of young children in low- and middle-income countries. Matern. Child Nutr. 2022, 18, e13264. [Google Scholar] [CrossRef] [PubMed]
- Thaweekul, P.; Surapolchai, P.; Sinlapamongkolkul, P. Infant feeding practices in relation to iron status and other possible nutritional deficiencies in Pathumthani, Thailand. Asia Pac. J. Clin. Nutr. 2019, 28, 577–583. [Google Scholar] [PubMed]
- Rojroongwasinkul, N.; Kijboonchoo, K.; Wimonpeerapattana, W.; Purttiponthanee, S.; Yamborisut, U.; Boonpraderm, A.; Kunapan, P.; Thasanasuwan, W.; Khouw, I. SEANUTS: The nutritional status and dietary intakes of 0.5-12-year-old Thai children. Br. J. Nutr. 2013, 110 (Suppl. 3), S36–S44. [Google Scholar] [CrossRef]
- Brouwer, I.D.; van Liere, M.J.; de Brauw, A.; Dominguez-Salas, P.; Herforth, A.; Kennedy, G.; Lachat, C.; Omosa, E.B.; Talsma, E.F.; Vandevijvere, S.; et al. Reverse thinking: Taking a healthy diet perspective towards food systems transformations. Food Secur. 2021, 13, 1497–1523. [Google Scholar] [CrossRef]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2021, 23, e13366. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Larnkjaer, A.; Molgaard, C. Amount and quality of dietary proteins during the first two years of life in relation to NCD risk in adulthood. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 781–786. [Google Scholar] [CrossRef]
- Agostoni, C.; Scaglioni, S.; Ghisleni, D.; Verduci, E.; Giovannini, M.; Riva, E. How much protein is safe? Int. J. Obes. 2005, 29, S8–S13. [Google Scholar] [CrossRef]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2004: Integrating Nutrition and Physical Activity; Nordic Council of Ministers: Copenhagen, Denmark, 2005; p. 436.
- Food and Agricultural Organization of the United Nations, World Health Organization and United Nations University. Protein and Amino Acids Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. In Institute of Medicine, Editor; The National Academic Press: Washington, DC, USA, 2005. [Google Scholar]
- European Food Safety Authority. Dietary Reference Values for Nutrients Summary Report. EFSA Supporting Publ. 2017, 14, e15121E. [Google Scholar]
- Osmond, C.; Fall, C.H.D. Conditional Growth Models: An Exposition and Some Extensions. Disease Modelling and Public Health, Part B. In Handbook of Statistics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–300. [Google Scholar]
- Benyi, E.; Savendhal, L. The physiology of childhood growth: Hormonal regulation. Horm. Res. Paediatr. 2017, 88, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Kouwenhoven, S.M.; Fleddermann, M.; Finken, M.J.; Twisk, J.W.; van der Beek, E.M.; Abrahamse-Berkeveld, M.; van de Heijning, B.J.; van Harskamp, D.; van Goudoever, J.B.; Koletzko, B.V. Early-life metabolic and hormonal markers in blood and growth until age 2 years: Results from a randomized controlled trial in healthy infants fed a modified low-protein infant formula. Nutrients 2021, 13, 1159. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk—A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation. Int. J. Mol. Sci. 2015, 16, 17048–17087. [Google Scholar] [CrossRef]
- Melnik, B.C. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J. Diabetes 2012, 3, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Bärebring, L.; Nwaru, B.; Dierkes, J.; Ramel, A.; Åkesson, A. Protein intake in children and growth and risk of overweight or obesity: A systematic review and meta-analysis. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Thai Nationa Statistical Office (NSO). Thailand Multiple Indicator Cluster Survey 2019, Survey Findings Report; National Statistical Office of Thailand: Bangkok, Thailand, 2019. [Google Scholar]
- Mo-suwan, L.; Akeplakorn, W. Nutritional Status of Thai Children; Health System Research Institute: Bangkok, Thailand, 2009. [Google Scholar]
- UNICEF; Thai NSO. Thailand Multiple Indicator Cluster Survey 2015–2016, Final Report; NSO and UNICEF: Bangkok, Thailand, 2016. [Google Scholar]
- Ministry of Public Health. Thai Recommended Daily Intakes; Ministry of Public Health: Bangkok, Thailand, 2020.
- Kittisakmontri, K. The Impact of Dietary Protein in Complementary Foods on Infant Growth and Iron Status in a Population Facing Double-Burden of Malnutrition. Doctoral Thesis, University College London, London, UK, 2022. Available online: https://discovery.ucl.ac.uk/id/eprint/10141347/ (accessed on 8 April 2022).
- Allen, L. Comparing the value of protein sources for maternal and child nutrition. Food Nutr. Bull. 2013, 34, 263–266. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Lutter, C.K.; Stewart, C.P.; Gallegos Riofrío, C.A.; Malo, C.; Reinhart, G.; Palacios, A.; Karp, C.; Chapnick, M.; Cox, K.; et al. Eggs in Early Complementary Feeding and Child Growth: A Randomized Controlled Trial. Pediatrics 2017, 140. [Google Scholar] [CrossRef]
- Semba, R.D.; Shardell, M.; Ashour, F.A.S.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child Stunting is Associated with Low Circulating Essential Amino Acids. EBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Shapiro, M.J.; Downs, S.M.; Swartz, H.J.; Parker, M.; Quelhas, D.; Kreis, K.; Kraemer, K.; West, K.P., Jr.; Fanzo, J. A Systematic Review Investigating the Relation Between Animal-Source Food Consumption and Stunting in Children Aged 6-60 Months in Low and Middle-Income Countries. Adv. Nutr. 2019, 10, 827–847. [Google Scholar] [CrossRef]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Totzauer, M. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin. Perinatol. 2019, 43, 151153. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hendricks, A.E.; Krebs, N.F. A meat- or dairy-based complementary diet leads to distinct growth patterns in formula-fed infants: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.L.; Remer, T.; Kroke, A.; Buyken, A.E. Early protein intake and later obesity risk: Which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 y of age? Am. J. Clin. Nutr. 2007, 86, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.C.; Bach, C.C.; Matthiesen, N.B.; Henriksen, T.B.; Gagliardi, L. Directed acyclic graphs: A tool for causal studies in paediatrics. Pediatr. Res. 2018, 84, 487–493. [Google Scholar] [CrossRef] [PubMed]

| Demographic Data | Results |
|---|---|
| Infants | |
| Sex, female (n, %) | 72 (49.7) |
| Gestational age, weeks (means ± SD) | 38.8 ± 1.0 |
| Route of delivery (n, %) | |
| - Vaginal delivery | 96 (66.2%) |
| - Caesarean section | 49 (33.8%) |
| Child order, first born (n, %) | 93 (64.1%) |
| Birth anthropometry (means ± SD) | |
| - Body weight, kg | 3.2 ± 0.4 |
| - Length, cm | 49.3 ± 1.9 |
| - Head circumference, cm | 33.3 ± 1.4 |
| Parents | |
| Parental age, years old (means ± SD) | |
| - Mothers | 29.8 ± 5.7 |
| - Fathers | 32.0 ± 5.9 |
| Parental BMI, kg/m2 (means ± SD) | |
| - Mothers | 22.8 ± 4.0 |
| - Fathers | 24.7 ± 3.6 |
| Maternal educational attainment (n, %) | |
| - Did not receive formal education | 2 (1.4) |
| - Below bachelor’s degree | 74 (51.0) |
| - Bachelor’s degree and above | 69 (47.6) |
| Family characteristics | Results |
| Main caregivers (n, %), choose more than 1 | |
| - Mothers | 134 (92.4) |
| - Fathers | 6 (4.1) |
| - Grandparents | 17 (11.7) |
| - Others | 2 (1.4) |
| Family type (n, %) | |
| - Nuclear family | 50 (34.5) |
| - Extended family | 95 (65.5) |
| Main financial providers (n, %), choose more than 1 | |
| - Mother | 92 (63.5) |
| - Father | 140 (96.6) |
| - Grandparents | 13 (9.0) |
| - Others | 2 (1.4) |
| Family income per month 1, 2, THB (n, %) | |
| - less than 10,000 | 11 (7.6) |
| - 10,000–29,999 | 65 (44.8) |
| - 30,000–49,999 | 51 (35.2) |
| - ≥50,000 | 18 (12.4) |
| Variable | Results |
|---|---|
| Age of first introduction of complementary foods (months), mean ± SD | 5.7 ± 0.6 |
| Age of introduction of each food group (months), mean ± SD | |
| - Rice | 5.7 ± 0.6 |
| - Fruits | 5.8 ± 0.6 |
| - Vegetables | 5.9 ± 0.5 |
| - Eggs | 6.0 ± 0.5 |
| - Meats | 6.3 ± 0.9 |
| - Dairy products (excluding infant/follow-on formula) | 9.9 ± 2.2 |
| Breastfeeding practices | |
| - Exclusive breastfeeding until 6 months of age, n (%) | 64 (44.1%) |
| - Receiving only breast milk alongside complementary foods until 12 months of age, n (%) | 53 (36.6%) |
| - Duration of exclusive breastfeeding (months), mean ± SD | 4.4 ± 2.0 |
| - Duration of predominant breastfeeding (months), mean ± SD | 8.4 ± 4.4 |
| Formula and dairy products | |
| - Receiving formula feeding, n (%) | 87 (60.0) |
| - Receiving unfortified cow’s milk before 12 months of age, n (%) | 21 (14.5) |
| - Duration of formula feeding (months), median (IQR) | 3 (0, 9) |
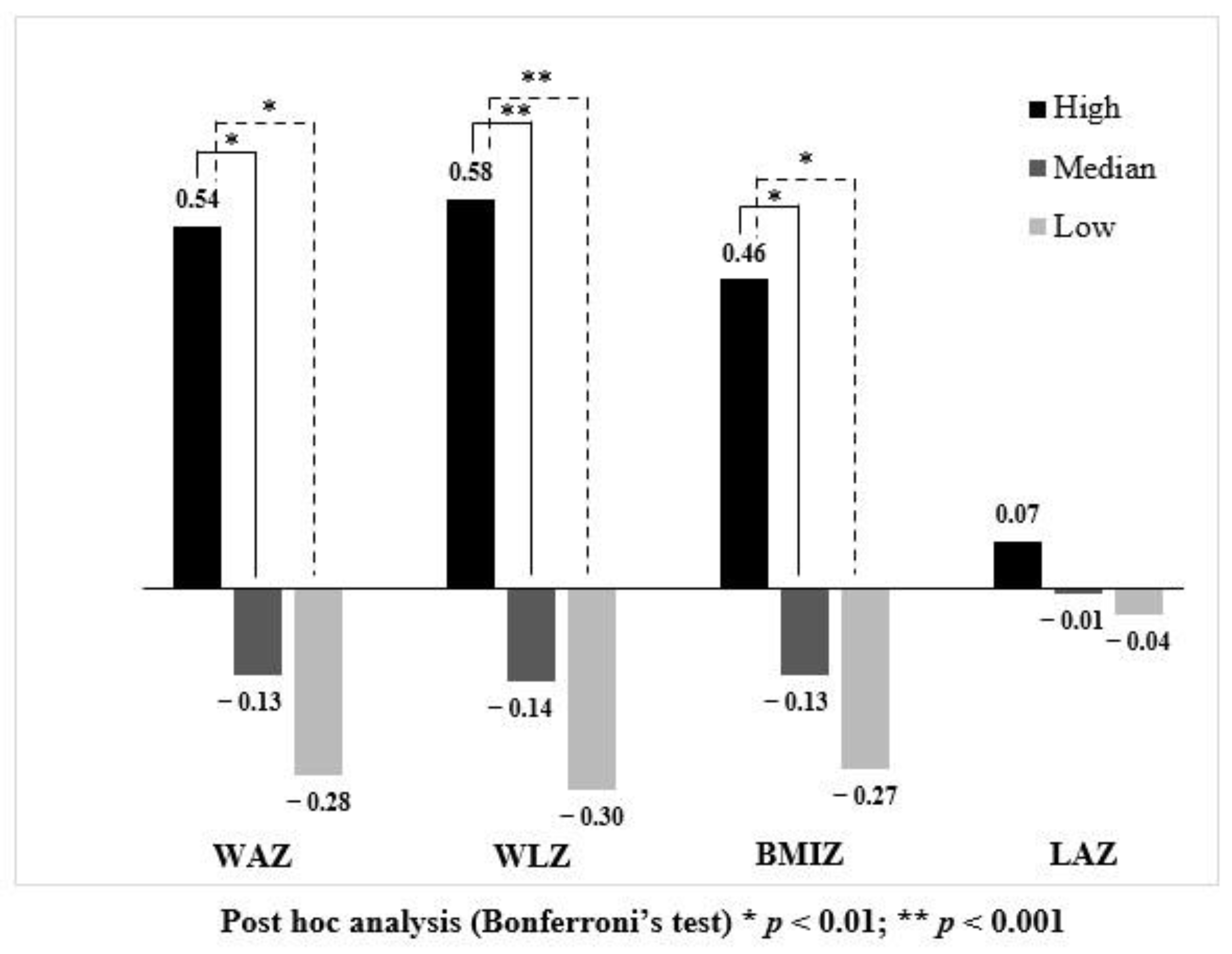
| Growth Parameters | High (n = 36) | Median (n = 73) | Low (n = 36) | Mean Difference 2 (95%CI) | ||
|---|---|---|---|---|---|---|
| H vs. L 3 | H vs. M 3 | M vs. L 3 | ||||
| 6M | ||||||
| WAZ | −0.14 | −0.40 | −0.50 | 0.36 (−0.13, 0.85) | 0.27 (−0.16, 0.69) | 0.09 (−0.33, 0.52) |
| WLZ | 0.02 | −0.06 | −0.05 | 0.07 (−0.46, 0.59) | 0.08 (−0.37, 0.54) | −0.02 (−0.47, 0.44) |
| BMIZ | −0.08 | −0.14 | −0.16 | 0.08 (−0.46, 0.59) | 0.06 (−0.40, 0.52) | 0.02 (−0.44, 0.48) |
| LAZ | −0.15 | −0.55 | −0.66 | 0.50 (−0.01, 1.01) | 0.40 (−0.05, 0.84) | 0.11 (−0.34, 0.55) |
| 9M | ||||||
| WAZ | 0.03 | −0.46 | −0.59 | 0.62 (0.16, 1.08 5) | 0.49 (0.09, 0.89 4) | 0.13 (−0.27, 0.53) |
| WLZ | 0.14 | −0.22 | −0.24 | 0.38 (−0.10, 0.86) | 0.36 (−0.06, 0.77) | 0.02 (−0.39, 0.43) |
| BMIZ | 0.09 | −0.24 | −0.26 | 0.34 (−0.14, 0.83) | 0.32 (−0.10, 0.74) | 0.02 (−0.40, 0.44) |
| LAZ | −0.17 | −0.48 | −0.69 | 0.52 (−0.01, 1.05) | 0.32 (−0.14, 0.77) | 0.20 (−0.24, 0.65) |
| 12M | ||||||
| WAZ | 0.10 | −0.45 | −0.60 | 0.70 (0.24, 1.17 4) | 0.55 (0.15, 0.96 4) | 0.15 (−0.26, 0.56) |
| WLZ | 0.25 | −0.30 | −0.39 | 0.64 (0.14, 1.16 4) | 0.55 (0.11, 0.99 4) | 0.10 (−0.35, 0.54) |
| BMIZ | 0.29 | −0.19 | −0.31 | 0.60 (0.07, 1.13 5) | 0.48 (0.02, 0.94 5) | 0.12 (−0.34, 0.58) |
| LAZ | −0.19 | −0.55 | −0.64 | 0.45 (−0.07, 0.96) | 0.35 (−0.09, 0.80) | 0.10 (−0.35, 0.54) |
| Conditional | Average %PE 6–9 M | Average %PE 9–12 M | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| WAZ | 0.17 | 0.04 | 0.26 | 0.002 |
| WLZ | 0.16 | 0.06 | 0.23 | 0.006 |
| BMIZ | 0.12 | 0.16 | 0.20 | 0.02 |
| LAZ | 0.09 | 0.26 | 0.07 | 0.39 |
| Predictor and Co-Variates | Conditional WAZ | Conditional WLZ | ||
|---|---|---|---|---|
| β | 95%CI | β | 95%CI | |
| %PE 9–12 M | 0.11 | 0.03, 0.18 1 | 0.12 | 0.05, 0.20 1 |
| Duration of predominant BF | 0.02 | −0.05, 0.08 | 0.02 | −0.05, 0.09 |
| Type of milk 9–12 M | 0.10 | −0.25, 0.45 | 0.19 | −0.16, 0.55 |
| Non-protein energy 6–9 M | 0.002 | 0, 0.004 | 0.002 | 0, 0.004 |
| Non-protein energy 9–12 M | <0.001 | −0.001, 0.002 | −0.001 | −0.002, 0.001 |
| Maternal education | 0.06 | −0.07, 0.18 | 0.05 | −0.08, 0.17 |
| Frequency of illness | −0.02 | −0.16, 0.12 | −0.03 | −0.17, 0.12 |
| Maternal BMI | −0.02 | −0.06, 0.03 | −0.01 | −0.05, 0.03 |
| Maternal age | 0.001 | −0.03, 0.03 | 0.001 | −0.03, 0.03 |
| Predictor and Co-Variates | Conditional BMIZ | Conditional LAZ | ||
| β | 95%CI | β | 95%CI | |
| %PE 9–12 M | 0.10 | 0.02, 0.18 2 | 0.01 | −0.07, 0.09 |
| Duration of predominant BF | 0.03 | −0.04, 0.10 | −0.02 | −0.08, 0.05 |
| Type of milk 9–12 M | 0.23 | −0.13, 0.59 | −0.19 | −0.56, 0.18 |
| Non-protein energy 6–9 M | 0.002 | −0.01. 0.004 | <0.001 | −0.002, 0.003 |
| Non-protein energy 9–12 M | <0.001 | −0.002, 0.001 | 0.001 | −0.001, 0.003 |
| Maternal education | 0.08 | −0.05, 0.20 | −0.05 | −0.18, 0.09 |
| Frequency of illness | −0.01 | −0.15, 0.14 | 0.001 | −0.15, 0.15 |
| Maternal BMI | −0.01 | −0.05, 0.03 | N/A | N/A |
| Maternal age | 0.01 | −0.02, 0.04 | N/A | N/A |
| Family income | N/A | N/A | 0.12 | −0.10. 0.34 |
| 2 Multiple linear regression analyses investigating associations between protein intakes from different food sources at age 9–12 months and conditional growth. | ||||
| Predictor and Co-Variates | Conditional WAZ | Conditional WLZ | ||
| β | 95%CI | β | 95%CI | |
| %PE Milk/dairy | 0.18 | 0.03, 0.32 2 | 0.16 | 0.01, 0.30 2 |
| %PE Non-dairy ASFs | 0.10 | 0.02, 0.18 2 | 0.12 | 0.04, 0.20 1 |
| %PE Plant-based foods | 0.15 | −0.15, 0.45 | 0.16 | −0.15, 0.46 |
| Duration of predominant BF | 0.02 | −0.05, 0.09 | 0.02 | −0.05, 0.09 |
| Type of milk 9–12 M | −0.04 | −0.46, 0.09 | 0.13 | −0.30, 0.56 |
| Non-protein energy 6–9 M | 0.002 | 0, 0.004 | 0.002 | 0, 0.004 |
| Non-protein energy 9–12 M | <0.001 | −0.001, 0.002 | −0.001 | −0.002, 0.001 |
| Maternal education | 0.05 | −0.07, 0.18 | 0.05 | −0.08, 0.17 |
| Frequency of illness | −0.02 | −0.16, 0.12 | −0.02 | −0.16, 0.12 |
| Maternal BMI | −0.01 | −0.06, 0.03 | −0.01 | −0.05, 0.03 |
| Maternal age | 0.01 | −0.03, 0.04 | 0.003 | −0.03, 0.04 |
| Predictor and Co-Variates | Conditional BMIZ | Conditional LAZ | ||
| β | 95%CI | β | 95%CI | |
| %PE Milk/dairy | 0.13 | −0.02, 0.28 | 0.07 | −0.08, 0.21 |
| %PE Non-dairy ASFs | 0.10 | 0.01, 0.18 2 | <0.001 | −0.09, 0.09 |
| %PE Plant-based foods | 0.14 | −0.16, 0.45 | 0.001 | −0.31, 032 |
| Duration of predominant BF | 0.03 | −0.04, 0.10 | −0.01 | −0.08, 0.06 |
| Type of milk 9–12 M | 0.17 | −0.26, 0.60 | −0.32 | −0.76, 0.12 |
| Non-protein energy 6–9 M | 0.002 | −0.01. 0.004 | <0.001 | −0.002, 0.003 |
| Non-protein energy 9–12 M | <0.001 | −0.002, 0.001 | 0.001 | −0.001, 0.003 |
| Maternal education | 0.07 | −0.06, 0.20 | −0.05 | −0.19, 0.08 |
| Frequency of illness | −0.002 | −0.15, 0.14 | −0.002 | −0.15, 0.14 |
| Maternal BMI | −0.01 | −0.05, 0.03 | N/A | N/A |
| Maternal age | 0.01 | −0.02, 0.05 | N/A | N/A |
| Family income | N/A | N/A | 0.13 | −0.10. 0.35 |
| Protein Intake (%PE) from | Correlation Coefficients (r) | ||
|---|---|---|---|
| IGF-1 (ng/mL) | IGFBP-3 (ng/mL) | Insulin (µU/mL) | |
| All food sources | 0.11 | 0.13 | 0.03 |
| 0.331 0.381 | 0.202 0.212 | 0.202 0.202 |
| Non-dairy ASFs | −0.16 | −0.04 | −0.14 |
| Plant-based foods | −0.11 | −0.09 | −0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittisakmontri, K.; Lanigan, J.; Wells, J.C.K.; Manowong, S.; Kaewarree, S.; Fewtrell, M. Quantity and Source of Protein during Complementary Feeding and Infant Growth: Evidence from a Population Facing Double Burden of Malnutrition. Nutrients 2022, 14, 3948. https://doi.org/10.3390/nu14193948
Kittisakmontri K, Lanigan J, Wells JCK, Manowong S, Kaewarree S, Fewtrell M. Quantity and Source of Protein during Complementary Feeding and Infant Growth: Evidence from a Population Facing Double Burden of Malnutrition. Nutrients. 2022; 14(19):3948. https://doi.org/10.3390/nu14193948
Chicago/Turabian StyleKittisakmontri, Kulnipa, Julie Lanigan, Jonathan C. K. Wells, Suphara Manowong, Sujitra Kaewarree, and Mary Fewtrell. 2022. "Quantity and Source of Protein during Complementary Feeding and Infant Growth: Evidence from a Population Facing Double Burden of Malnutrition" Nutrients 14, no. 19: 3948. https://doi.org/10.3390/nu14193948
APA StyleKittisakmontri, K., Lanigan, J., Wells, J. C. K., Manowong, S., Kaewarree, S., & Fewtrell, M. (2022). Quantity and Source of Protein during Complementary Feeding and Infant Growth: Evidence from a Population Facing Double Burden of Malnutrition. Nutrients, 14(19), 3948. https://doi.org/10.3390/nu14193948






