Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
2.4. Risk of Bias (RoB) Assessment and Quality of Evidence
2.5. Data Analysis
3. Results
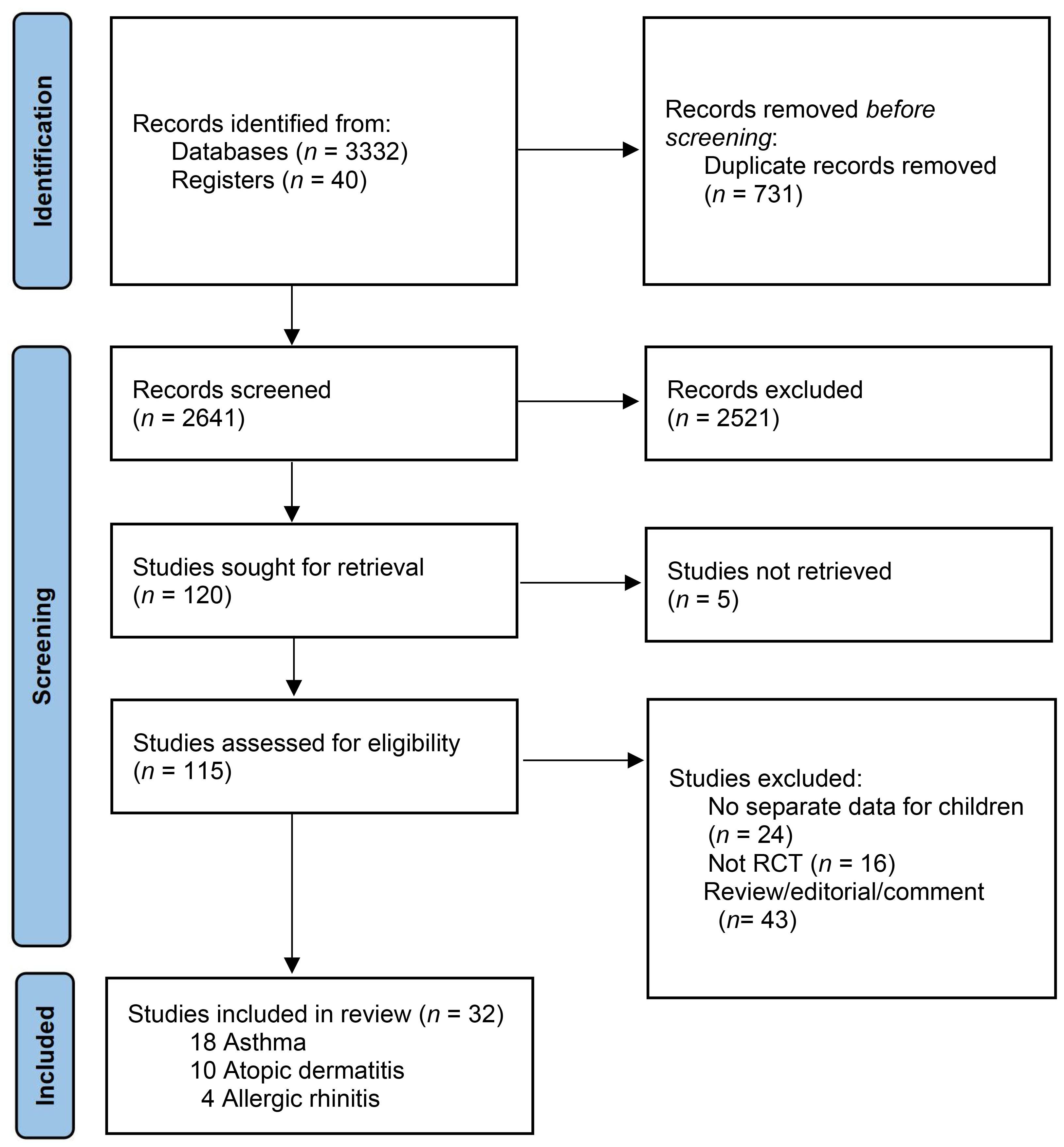
3.1. Study Selection and Characteristics
3.2. Risk of Bias
3.3. Childhood Asthma
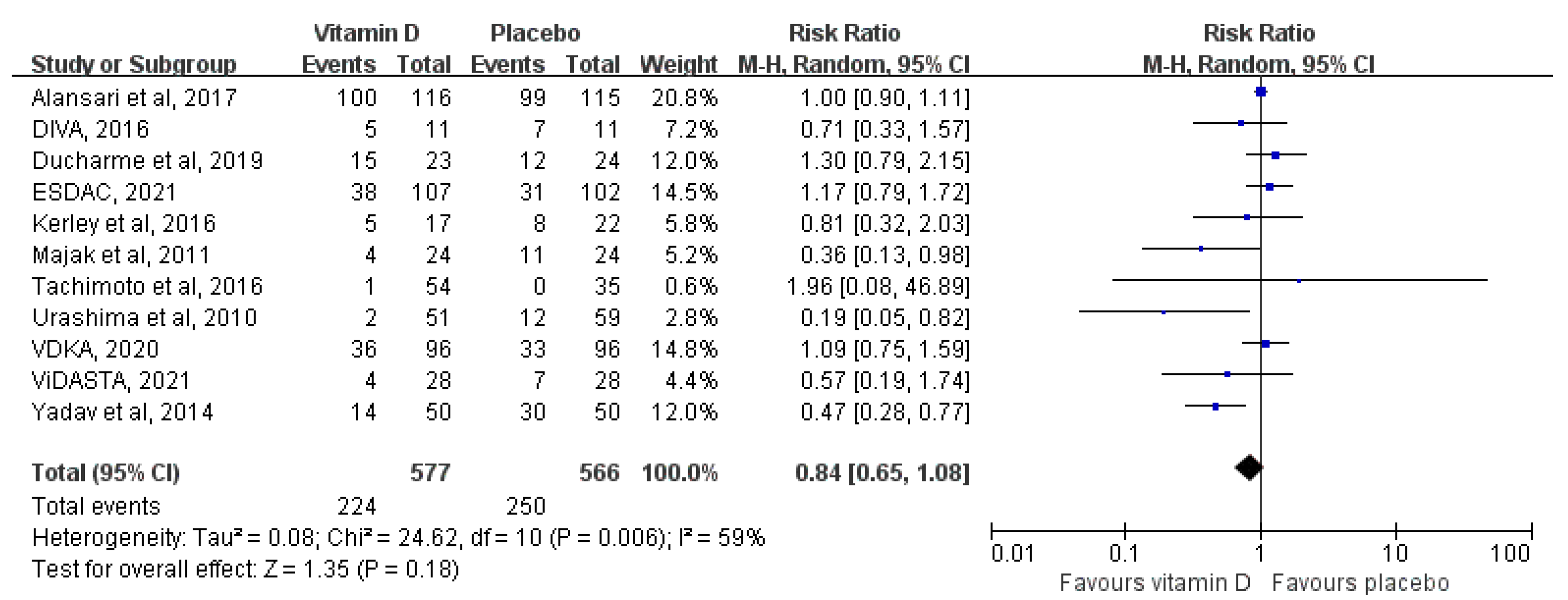
3.3.1. Overall Analysis
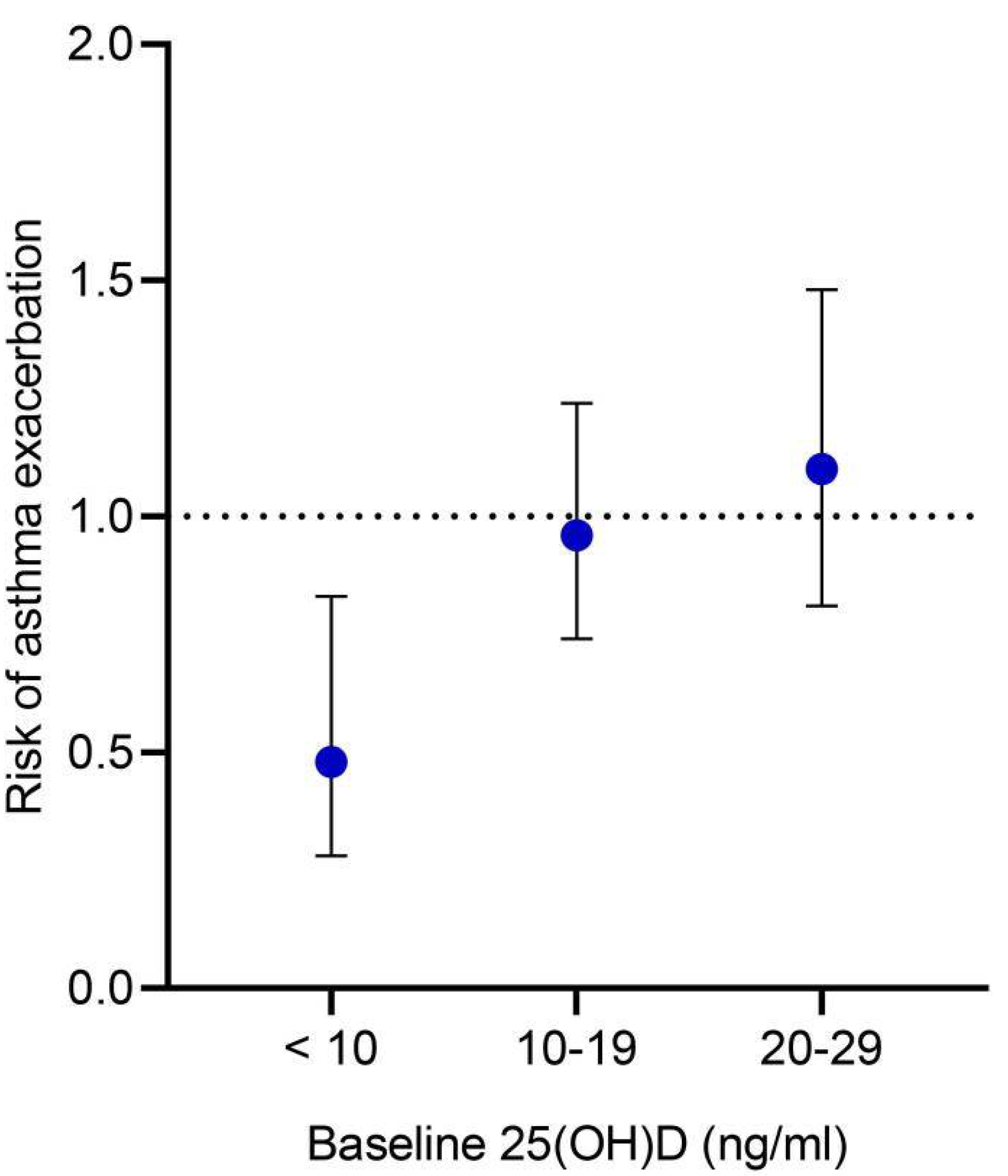
3.3.2. Additional Analysis
3.4. Atopic Dermatitis
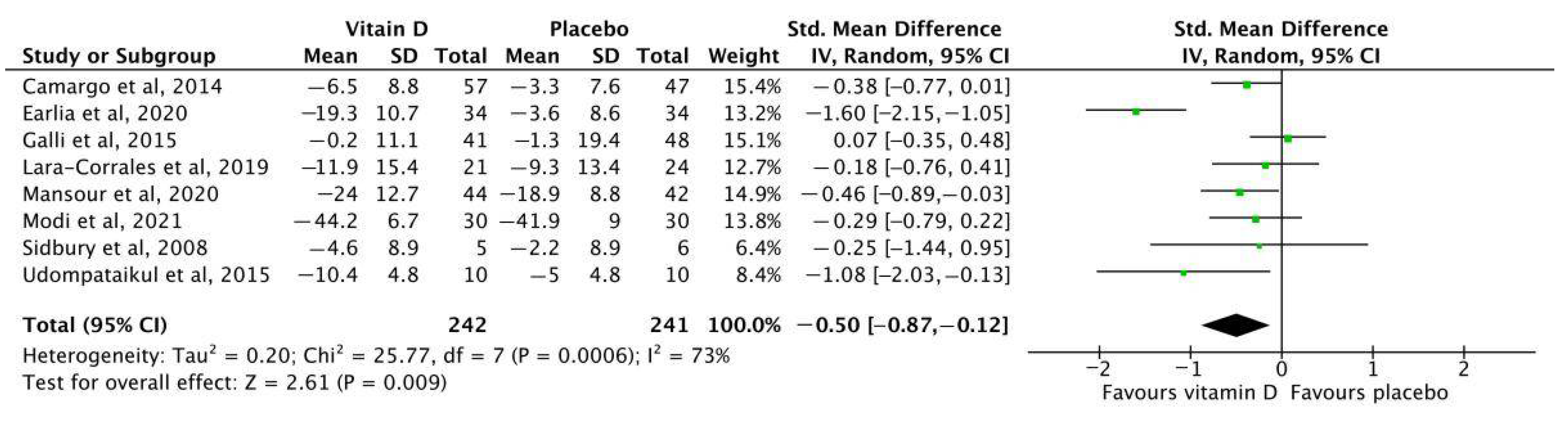
3.4.1. Overall Analysis
3.4.2. Additional Analysis
3.5. Allergic Rhinitis
3.6. Serum 25(OH)D Concentration Levels
3.7. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dierick, B.J.H.; van der Molen, T.; Flokstra-de Blok, B.M.J.; Muraro, A.; Postma, M.J.; Kocks, J.W.H.; van Boven, J.F.M. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharm. Outcomes Res. 2020, 20, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Al-Garawi, A.; Weiss, S.T.; Litonjua, A.A. Vitamin D and the development of allergic disease: How important is it? Clin. Exp. Allergy 2015, 45, 114–125. [Google Scholar] [CrossRef]
- Schrumpf, J.A.; van der Does, A.M.; Hiemstra, P.S. Impact of the Local Inflammatory Environment on Mucosal Vitamin D Metabolism and Signaling in Chronic Inflammatory Lung Diseases. Front. Immunol. 2020, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, J.E.; Lui, S.; Walker, S.A.; Chohan, V.; Xystrakis, E.; Bush, A.; Hawrylowicz, C.M.; Saglani, S.; Lloyd, C.M. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy 2014, 69, 1380–1389. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.H.; Hupperts, R.; Damoiseaux, J. Vitamin D effects on B cell function in autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Han, Y.Y.; Forno, E.; Celedón, J.C. Vitamin D Insufficiency and Asthma in a US Nationwide Study. J. Allergy Clin. Immunol. Pract. 2017, 5, 790–796.e1. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo, C.A., Jr.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J. Allergy Clin. Immunol. 2016, 137, 1063–1070.e2. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Mohamed, A.; Salah Ahmed, E.M.; Farag, Y.M.K.; Bedair, N.I.; Nassar, N.A.; Ghanem, A.I.M. Dose-response association between vitamin D deficiency and atopic dermatitis in children, and effect modification by gender: A case-control study. J. Dermatolog. Treat. 2021, 32, 174–179. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A., Jr.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef]
- Martineau, A.R.; Cates, C.J.; Urashima, M.; Jensen, M.; Griffiths, A.P.; Nurmatov, U.; Sheikh, A.; Griffiths, C.J. Vitamin D for the management of asthma. Cochrane Database Syst. Rev. 2016, 9, CD011511. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, P.; Goyal, J.P.; Thakur, C.; Choudhary, P.; Meena, J.; Charan, J.; Singh, K.; Gupta, A. Vitamin D supplementation in childhood asthma: A systematic review and meta-analysis of randomised controlled trials. ERJ Open Res. 2021, 8, 00662–02021. [Google Scholar] [CrossRef]
- Hidayati, A.N.; Sawitri, S.; Sari, D.W.; Prakoeswa, C.R.S.; Indramaya, D.M.; Damayanti, D.; Zulkarnain, I.; Citrashanty, I.; Widia, Y.; Anggraeni, S. Efficacy of vitamin D supplementation on the severity of atopic dermatitis in children: A systematic review and meta-analysis [version 1; peer review: 1 approved]. F1000Research 2022, 11, 274. [Google Scholar] [CrossRef]
- Tran, M.M.; Lefebvre, D.L.; Dharma, C.; Dai, D.; Lou, W.Y.W.; Subbarao, P.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Sears, M.R. Predicting the atopic march: Results from the Canadian Healthy Infant Longitudinal Development Study. J. Allergy Clin. Immunol. 2018, 141, 601–607.e8. [Google Scholar] [CrossRef] [PubMed]
- Giovannini-Chami, L.; Paquet, A.; Sanfiorenzo, C.; Pons, N.; Cazareth, J.; Magnone, V.; Lebrigand, K.; Chevalier, B.; Vallauri, A.; Julia, V.; et al. The “one airway, one disease” concept in light of Th2 inflammation. Eur. Respir. J. 2018, 52, 1800437. [Google Scholar] [CrossRef]
- Lenaeus, M.J.; Hirschmann, J. Primary Care of the Patient with Asthma. Med. Clin. N. Am. 2015, 99, 953–967. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Davidson, B.L. Administration of placebo vitamin D to non-consenting children. Lancet Respir. Med. 2018, 6, e23–e24. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Santesso, N.; Helfand, M.; Vist, G.; Kunz, R.; Brozek, J.; Norris, S.; Meerpohl, J.; Djulbegovic, B.; et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J. Clin. Epidemiol. 2013, 66, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0, an update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Cashman, K.D.; Fitzgerald, A.P.; Kiely, M.; Seamans, K.M. A systematic review and meta-regression analysis of the vitamin D intake-serum 25-hydroxyvitamin D relationship to inform European recommendations. Br. J. Nutr. 2011, 106, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the healthy European paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef]
- Alansari, K.; Davidson, B.L.; Yousef, K.I.; Mohamed, A.N.H.; Alattar, I. Rapid vs. Maintenance Vitamin D Supplementation in Deficient Children With Asthma to Prevent Exacerbations. Chest 2017, 152, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Bar Yoseph, R.; Livnat, G.; Schnapp, Z.; Hakim, F.; Dabbah, H.; Goldbart, A.; Bentur, L. The effect of vitamin D on airway reactivity and inflammation in asthmatic children: A double-blind placebo-controlled trial. Pediatr. Pulmonol. 2015, 50, 747–753. [Google Scholar] [CrossRef]
- Baris, S.; Kiykim, A.; Ozen, A.; Tulunay, A.; Karakoc-Aydiner, E.; Barlan, I.B. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy 2014, 69, 246–253. [Google Scholar] [CrossRef]
- Jensen, M.E.; Mailhot, G.; Alos, N.; Rousseau, E.; White, J.H.; Khamessan, A.; Ducharme, F.M. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): A pilot randomised controlled trial. Trials 2016, 17, 353. [Google Scholar] [CrossRef]
- Ducharme, F.M.; Jensen, M.; Mailhot, G.; Alos, N.; White, J.; Rousseau, E.; Tse, S.M.; Khamessan, A.; Vinet, B. Impact of two oral doses of 100,000 IU of vitamin D3 in preschoolers with viral-induced asthma: A pilot randomised controlled trial. Trials 2019, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- El-korashi, L.A.; Nafea, O.E.; Zake, L.G.; Arab, F.; Anis, R.H. Effect of Vitamin D Adjuvant and Allergen Specific Immunotherapy on Serum IL-10 and IL-17 Levels in Childhood Asthma: A Controlled Clinical Trial. Egypt J. Med. Microbiol. 2021, 30, 175–181. [Google Scholar] [CrossRef]
- Jat, K.R.; Goel, N.; Gupta, N.; Gupta, C.P.; Datta, S.; Lodha, R.; Kabra, S.K. Efficacy of vitamin D supplementation in asthmatic children with vitamin D deficiency: A randomized controlled trial (ESDAC trial). Pediatr. Allergy Immunol. 2021, 32, 479–488. [Google Scholar] [CrossRef]
- Kerley, C.P.; Hutchinson, K.; Cormican, L.; Faul, J.; Greally, P.; Coghlan, D.; Elnazir, B. Vitamin D3 for uncontrolled childhood asthma: A pilot study. Pediatr. Allergy Immunol. 2016, 27, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.; Fernandez, C.; Nella, A.; Hopp, R.; Gallagher, J.C.; Casale, T.B. Relationship of 25-hydroxyvitamin D and asthma control in children. Ann. Allergy Asthma Immunol. 2012, 108, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Rychlik, B.; Stelmach, I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clin. Exp. Allergy 2009, 39, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Najmuddin, F.; Lahiri, K. Vitamin D in pediatric asthma and allergic rhinitis: Benefits beyond skeletal health. Insights Allergy Asthma Bronchitis 2017, 3, 1. [Google Scholar] [CrossRef][Green Version]
- Swangtrakul, N.; Manuyakorn, W.; Mahachoklertwattana, P.; Kiewngam, P.; Sasisakulporn, C.; Jotikasthirapa, W.; Kamchaisatian, W.; Benjaponpitak, S. Effect of vitamin D on lung function assessed by forced oscillation technique in asthmatic children with vitamin D deficiency: A randomized double-blind placebo-controlled trial. Asian Pac. J. Allergy Immunol. 2022, 40, 22–30. [Google Scholar] [PubMed]
- Tachimoto, H.; Mezawa, H.; Segawa, T.; Akiyama, N.; Ida, H.; Urashima, M. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: A randomized, double-blind, placebo-controlled trial. Allergy 2016, 71, 1001–1009. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef]
- Forno, E.; Bacharier, L.B.; Phipatanakul, W.; Guilbert, T.W.; Cabana, M.D.; Ross, K.; Covar, R.; Gern, J.E.; Rosser, F.J.; Blatter, J. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels: The VDKA Randomized Clinical Trial. JAMA 2020, 324, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C.; Kumar, J.; Kumar, P.; Goyal, J.P.; Singh, K.; Gupta, A. Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: A randomized controlled trial (ViDASTA Trial). Pediatr. Pulmonol. 2021, 56, 1427–1433. [Google Scholar] [CrossRef]
- Yadav, M.; Mittal, K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J. Pediatr. 2014, 81, 650–654. [Google Scholar] [CrossRef]
- Aldaghi, M.; Tehrani, H.; Karrabi, M.; Abadi, F.S.; Sahebkar, M. The effect of multistrain synbiotic and vitamin D3 supplements on the severity of atopic dermatitis among infants under 1 year of age: A double-blind, randomized clinical trial study. J. Dermatol. Treat. 2022, 33, 812–817. [Google Scholar] [CrossRef]
- Camargo, C.A., Jr.; Ganmaa, D.; Sidbury, R.; Erdenedelger Kh Radnaakhand, N.; Khandsuren, B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J. Allergy Clin. Immunol. 2014, 134, 831–835.e1. [Google Scholar] [CrossRef] [PubMed]
- Earlia, N.; Maulida, M.; Hidayati, A.; Pratama, R. Pengaruh Pemberian Vitamin D Terhadap Perbaikan Gejala Klinis Pada Penderita Dermatitis Atopik Di Poliklinik Kulit Kelamin RSUD Dr. Zainoel Abidin Banda Aceh Tahun 2018: Uji Klinis Ketersamaran Ganda. J. Med. Sci. 2020, 1, 33–42. [Google Scholar] [CrossRef]
- Galli, E.; Rocchi, L.; Carello, R.; Giampietro, P.G.; Panei, P.; Meglio, P. Serum Vitamin D levels and Vitamin D supplementation do not correlate with the severity of chronic eczema in children. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 41–47. [Google Scholar] [PubMed]
- Lara-Corrales, I.; Huang, C.M.; Parkin, P.C.; Rubio-Gomez, G.A.; Posso-De Los Rios, C.J.; Maguire, J.; Pope, E. Vitamin D Level and Supplementation in Pediatric Atopic Dermatitis: A Randomized Controlled Trial. J. Cutan. Med. Surg. 2019, 23, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Mansour, N.O.; Mohamed, A.A.; Hussein, M.; Eldemiry, E.; Daifalla, A.; Hassanin, S.; Nassar, N.; Ghaith, D.; Mohamed Salah, E. The impact of vitamin D supplementation as an adjuvant therapy on clinical outcomes in patients with severe atopic dermatitis: A randomized controlled trial. Pharmacol. Res. Perspect. 2020, 8, e00679. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.P.; Dash, A.K. Clinico-biochemical relation of Vitamin D3 with the severity of atopic dermatitis and response to supplementation of Vitamin D3: A randomized controlled trial. Ind. J. Child. Health 2021, 8, 412–415. [Google Scholar] [CrossRef]
- Sidbury, R.; Sullivan, A.F.; Thadhani, R.I.; Camargo, C.A., Jr. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: A pilot study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Udompataikul, M.; Huajai, S.; Chalermchai, T.; Taweechotipatr, M.; Kamanamool, N. The Effects of Oral Vitamin D Supplement on Atopic Dermatitis: A Clinical Trial with Staphylococcus aureus Colonization Determination. J. Med. Assoc. Thail. 2015, 98 (Suppl. 9), S23–S30. [Google Scholar]
- Zulkarnain, I.; Rahmawati, Y.W.; Setyaningrum, T.; Citrashanty, I.; Aditama, L.; Avanti, C. Vitamin D3 supplementation reduced Staphylococcus aureus colonization in the skin of pediatric patients with atopic dermatitis. Eur. J. Pediatr. Dermatol. 2019, 29, 143–149. [Google Scholar]
- Akram, S.; Khan, M.A.; Fazil, M.; Kiramatullah Khan, M.Q.; Shah, H.B.U. Effect of Vitamin-D Supplementation in Children with Moderate-Severe Persistent Allergic Rhinitis. Int. J. Pathol. 2020, 18, 92–98. [Google Scholar]
- Hassan, S.M.; Elmageed, M.M.A.; Senosy, M.G.E. Vitamin D Intervention in Children with Allergic rhinitis: A Pilot Randomized Controlled Trial. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 544–550. [Google Scholar] [CrossRef][Green Version]
- Jerzynska, J.; Stelmach, W.; Rychlik, B.; Lechańska, J.; Podlecka, D.; Stelmach, I. The clinical effect of vitamin D supplementation combined with grass-specific sublingual immunotherapy in children with allergic rhinitis. Allergy Asthma Proc. 2016, 37, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Jerzyńska, J.; Stelmach, W.; Rychlik, B.; Majak, P.; Podlecka, D.; Woicka-Kolejwa, K.; Stelmach, I. Clinical and immunological effects of vitamin D supplementation during the pollen season in children with allergic rhinitis. Arch. Med. Sci. 2018, 14, 122–131. [Google Scholar] [CrossRef]
- Zhao, D.D.; Yu, D.D.; Ren, Q.Q.; Dong, B.; Zhao, F.; Sun, Y.H. Association of vitamin D receptor gene polymorphisms with susceptibility to childhood asthma: A meta-analysis. Pediatr. Pulmonol. 2017, 52, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Nanzer, A.M.; Chambers, E.S.; Ryanna, K.; Freeman, A.T.; Colligan, G.; Richards, D.F.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; et al. The effects of calcitriol treatment in glucocorticoid-resistant asthma. J. Allergy Clin. Immunol. 2014, 133, 1755–1757.e4. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Diette, G.B.; Woo, H.; Koehler, K.; Romero, K.; Rule, A.M.; Detrick, B.; Brigham, E.; McCormack, M.C.; Hansel, N.N. Vitamin D Status Modifies the Response to Indoor Particulate Matter in Obese Urban Children with Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1815–1822.e2. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sun, Y.; Zeng, Z.; Liu, Y.; Peng, S. Vitamin D supplementation in pregnant women or infants for preventing allergic diseases: A systematic review and meta-analysis of randomized controlled trials. Chin. Med. J. 2022, 135, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L.; Drezner, M.K.; Binkley, N.C. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J. Steroid Biochem. Mol. Biol. 2007, 103, 631–634. [Google Scholar] [CrossRef]
- Hattangdi-Haridas, S.R.; Lanham-New, S.A.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Bae, J.H. Vitamin D and atopic dermatitis: A systematic review and meta-analysis. Nutrition 2016, 32, 913–920. [Google Scholar] [CrossRef] [PubMed]
| First Author (year) | Sample Size | Country | Disease | Disease Severity | Age, Years, Range | Male (%) | BMI, kg/m2, Mean (SD) | Baseline 25(OH)D, ng/mL, Mean (SD) | Co-Treatment | Dose of Vitamin D | Treatment Duration | Primary Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alansari et al. (2017) [29] | 231 | USA | Asthma | Moderate to severe | 2–14 | 64.9 | NR | 15.4 (5.3) | None | 300,000–600,000 IU, statim followed by 400 IU daily | 12 months | Unplanned visit for asthma exacerbation |
| Bar Yoseph et al. (2015) [30] | 39 | Israel | Asthma | Mild | 6–18 | 64.1 | 20.6 (3.7) | 20.4 (6.7) | None | 14000 IU weekly | 6 weeks | Lung functions |
| Baris et al. (2014) [31] | 32 | Turkey | Asthma | Mild to moderate | 5–15 | 37.5 | NR | 19.5 (10.3) | SCIT | 650 IU daily | 12 months | Asthma control assessed by TASS |
| DIVA (2016) [32] | 22 | Canada | Asthma | All severity | 1–5 | 31.8 | NR | 25.3 (8.2) | None | 100,000 IU statim followed by 400 IU daily | 6 months | Incidence of asthma exacerbation |
| Ducharme et al. (2019) [33] | 47 | Canada | Asthma | All severity | 1–5 | 63.8 | NR | NR | ICS | 100,000 IU × 2 doses, 14 weeks apart | 7 months | Incidence of asthma exacerbation |
| EI-Korashi et al. (2021) [34] | 46 | Egypt | Asthma | Mild to moderate | 1–18 | 52.2 | NR | 13.0 (4.0) | SCIT | 600 IU daily | 6 months | Change in serum level of IL-10 |
| ESDAC (2021) [35] | 250 | India | Asthma | All severity | 4–12 | 72 | NR | 11.2 (4.5) | None | 1000 IU daily | 9 months | Asthma control assessed by C-ACT |
| Kerley et al. (2016) [36] | 39 | Ireland | Asthma | All severity | 6–16 | 61.5 | 18.8 (3.7) | 21.2 (8.7) | None | 2000 IU daily | 15 weeks | Lung functions |
| Lewis et al. (2012) [37] | 30 | USA | Asthma | All severity | 6–17 | NR | NR | NR | None | 1000 IU daily | 12 months | Asthma control assessed by ACT |
| Majak et al. (2009) [38] | 36 | Poland | Asthma | All severity | 6–12 | 61.1 | NR | 31.7 (3.2) | ICS + SCIT | 1000 IU weekly | 3 months | ICS dose reduction |
| Majak et al. (2011) [39] | 48 | Poland | Asthma | All severity | 5–18 | 66.7 | 18.7 (4.1) | 35.6 (15.3) | ICS | 500 IU daily | 6 months | Incidence of asthma exacerbation |
| Najmuddin et al. (2017) [40] | 66 | India | Asthma | All severity | 6–12 | 63.6 | NR | NR | None | 60,000 IU weekly | 10 weeks | Lung functions |
| Swangtrakul et al. (2022) [41] | 41 | Thailand | Asthma | All severity | 3–18 | 48.8 | 19.7 (4.3) | 16.4 (2.2) | None | <30 kg: 300,000 IU; >30 kg: 600,000 IU | 3 months | Lung functions |
| Tachimoto et al. (2016) [42] | 89 | Japan | Asthma | All severity | 6–15 | 56.2 | 17.5 (2.7) | 29.4 (6.8) | None | 800 IU daily | 2 months | Asthma control assessed by GINA |
| Urashima et al. (2010) [43] | 110 | Japan | Asthma | All severity | 6–15 | 56.3 | NR | NR | None | 1200 IU daily | 4 months | Incidence of asthma exacerbation |
| VDKA (2020) [44] | 192 | USA | Asthma | Mild to moderate | 6–16 | 59.9 | NR | 22.7 (4.6) | ICS | 4000 IU daily | 12 months | Time to a severe asthma exacerbation |
| ViDASTA (2021) [45] | 60 | India | Asthma | Moderate | 6–11 | 56.7 | NR | 16.2 (9.0) | ICS | 2000 IU daily | 3 months | Asthma control assessed by C-ACT |
| Yadav et al. (2014) [46] | 100 | India | Asthma | Moderate to severe | 5–13 | 49 | NR | NR | None | 60,000 IU monthly | 6 months | Incidence of asthma exacerbation |
| Aldaghi et al. (2021) [47] | 54 | Iran | Atopic dermatitis | All severity | 0–1 | 51.9 | NR | NR | Corticosteroids, emollient, and antihistamines | 1000 IU daily | 2 months | Disease severity assessed by SCORAD |
| Camargo et al. (2014) [48] | 107 | Mongolia | Atopic dermatitis | All severity | 2–17 | 58.9 | NR | NR | Emollient, patient education, and basic skin care | 1000 IU daily | 1 month | Disease severity assessed by EASI |
| Earlia et al. (2020) [49] | 68 | Indonesia | Atopic dermatitis | All severity | 1–18 | 50 | NR | NR | Corticosteroids, emollient, and antihistamines | 600 IU daily | 28 days | Disease severity assessed by SCORAD |
| Galli et al. (2015) [50] | 89 | Italy | Atopic dermatitis | All severity | 0.5–16.25 | 53.9 | NR | 48.3 (40.6) | None | 2000 IU daily | 3 months | Disease severity assessed by SCORAD score |
| Lara-Corrales et al. (2019) [51] | 45 | Canada | Atopic dermatitis | All severity | 0–18 | 53.3 | NR | 17.8 (6.2) | None | 1000 IU daily | 3 months | Disease severity assessed by SCORAD |
| Mansour et al. (2020) [52] | 92 | Egypt | Atopic dermatitis | All severity | 5–16 | 51.2 | 26.9 (5.0) | 24.1 (7.3) | Corticosteroids | 1600 IU daily | 3 months | Disease severity assessed by EASI |
| Modi et al. (2021) [53] | 60 | India | Atopic dermatitis | Moderate to severe | 1–14 | 55 | NR | 17.5 (2.8) | Regular treatment | 60,000 IU weekly | 6 weeks | Disease severity assessed by SCORAD |
| Sidbury et al. (2008) [54] | 11 | USA | Atopic dermatitis | Mild | 2–13 | 54.5 | NR | NR | Previously prescribed therapies | 1000 IU daily | 1 month | Disease severity assessed by EASI |
| Udompataikul et al. (2015) [55] | 20 | Thailand | Atopic dermatitis | Mild to moderate | 1–18 | 35 | NR | 17.0 (1.6) | Antihistamine, sunscreen, skin moisturizer, and cleanser | 2000 IU daily | 1 month | Disease severity assessed by SCORAD |
| Zulkarnain et al. (2019) [56] | 20 | Indonesia | Atopic dermatitis | All severity | 2–12 | 60 | NR | NR | None | 400 IU daily | 28 days | Staphylococcus aureus colonization |
| Akram et al. (2020) [57] | 120 | Pakistan | Allergic rhinitis | Moderate to severe | 5–15 | 53.3 | NR | 23.3 (11.9) | Standard treatment | 800 IU daily | 1 month | Symptom score |
| Hassan et al. (2016) [58] | 100 | Egypt | Allergic rhinitis | All severity | 6–12 | 50 | 27.9 (4.9) | 18.4 (6.1) | None | 1000 IU daily | 6 months | Symptoms score |
| Jerzynska et al. (2016) [59] | 45 | Poland | Allergic rhinitis | Moderate to severe | 5–12 | 57.8 | NR | 45.9 (5.2) | SLIT and standard treatment | 1000 IU daily | 5 months | Symptom-medication score |
| Jerzynska et al. (2018) [60] | 38 | Poland | Allergic rhinitis | Moderate to severe | 5–12 | NR | NR | 60.9 (36.7) | Standard treatment | 1000 IU daily | 5 months | Symptom-medication score |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhou, Q.; Zhang, G.; Tian, X.; Li, Y.; Wang, Z.; Zhao, Y.; Chen, Y.; Luo, Z. Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3947. https://doi.org/10.3390/nu14193947
Li Q, Zhou Q, Zhang G, Tian X, Li Y, Wang Z, Zhao Y, Chen Y, Luo Z. Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(19):3947. https://doi.org/10.3390/nu14193947
Chicago/Turabian StyleLi, Qinyuan, Qi Zhou, Guangli Zhang, Xiaoyin Tian, Yuanyuan Li, Zhili Wang, Yan Zhao, Yaolong Chen, and Zhengxiu Luo. 2022. "Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis" Nutrients 14, no. 19: 3947. https://doi.org/10.3390/nu14193947
APA StyleLi, Q., Zhou, Q., Zhang, G., Tian, X., Li, Y., Wang, Z., Zhao, Y., Chen, Y., & Luo, Z. (2022). Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis. Nutrients, 14(19), 3947. https://doi.org/10.3390/nu14193947






