Dietary Vitamin B Complex: Orchestration in Human Nutrition throughout Life with Sex Differences
Abstract
1. Introduction
2. B Vitamins Orchestration in Different Life Stages
2.1. Thiamine
2.2. Riboflavin
2.3. Niacin
2.4. Pantothenic Acid
2.5. Pyridoxine
2.6. Biotin
2.7. Folic Acid
2.8. Cobalamin
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5HT | Serotonin |
| Ach | Acetylcholine |
| AD | Alzheimer’s disease |
| AI | Adequate intake |
| ALP | Alkaline phosphatase |
| CoA | Coenzyme A |
| DFE | Dietary Folate Equivalents |
| FAD | Flavin adenine dinucleotide |
| FMN | Flavin mononucleotide |
| Hcy | Homocysteine |
| HG | Hyperemesis gravidarum |
| HLCS | holocarboxylase synthetase (HLCS) |
| KS | Korsakoff syndrome |
| LBW | Low birth weight |
| Mg | Magnesium |
| NAD | Nicotinamide adenine dinucleotide |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NE | Niacin equivalents |
| NTG | normal-tension glaucoma |
| OCAs | Oral contraceptive agents |
| PDH | Pyruvate dehydrogenase |
| PL | Pyridoxal |
| PM | Pyridoxamine |
| PMS | Premenstrual syndrome |
| PN | Pyridoxine |
| PNG | Pyridoxine-59-b-D-glucoside |
| RBC | Red blood cell |
| RDA | Recommended dietary allowance |
| RDI | Recommended dietary intake |
| SIDS | Sudden infant death syndrome |
| TDP | Thiamine diphosphate |
| TPP | Thiamine pyrophosphate |
| WE | Wernicke’s encephalopathy |
| α-KGDH | α-ketoglutarate dehydrogenase |
References
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy-A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Smith, A.G.; Croft, M.T.; Moulin, M.; Webb, M.E. Plants need their vitamins too. Curr. Opin. Plant Biol. 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Hans, K.B.; Jana, T. Micronutrients in the life cycle: Requirements and sufficient supply. NFS J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Herrmann, W.; Obeid, R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch. Arztebl. Int. 2008, 105, 680–685. [Google Scholar] [CrossRef] [PubMed]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Singh, A.; Trumpff, C.; Genkinger, J.; Davis, A.; Spann, M.; Werner, E.; Monk, C. Micronutrient dietary intake in Latina pregnant adolescents and its association with level of depression, stress, and social support. Nutrients 2017, 9, 1212. [Google Scholar] [CrossRef]
- Robinson, S.; Fall, C. Infant nutrition and later health: A review of current evidence. Nutrients 2012, 4, 859–874. [Google Scholar] [CrossRef]
- Agostoni, C.; Baselli, L.; Mazzoni, M.B. Early nutrition patterns and diseases of adulthood: A plausible link? Eur. J. Intern. Med. 2013, 24, 5–10. [Google Scholar] [CrossRef]
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef]
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Oudman, E.; Wijnia, J.W.; Oey, M.; van Dam, M.; Painter, R.C.; Postma, A. Wernicke’s encephalopathy in hyperemesis gravidarum: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 84–93. [Google Scholar] [CrossRef]
- Allen, L.H. B vitamins in breast milk: Relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 2012, 3, 362–369. [Google Scholar] [CrossRef]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef]
- Nazir, M.; Lone, R.; Charoo, B.A. Infantile thiamine deficiency: New insights into an old disease. Indian Pediatr. 2019, 56, 673–681. [Google Scholar] [CrossRef]
- Bhat, J.I.; Ahmed, Q.I.; Ahangar, A.A.; Charoo, B.A.; Sheikh, M.A.; Syed, W.A. Wernicke’s encephalopathy in exclusive breastfed infants. World J. Pediatr. 2017, 13, 485–488. [Google Scholar] [CrossRef]
- Lonsdale, D.; Shamberger, R.J.; Audhya, T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: A pilot study. Neuro Endocrinol. Lett. 2002, 23, 303–308. [Google Scholar]
- Thomson, A.D.; Guerrini, I.; Marshall, E.J. The Evolution and Treatment of Korsakoff’s Syndrome Out of Sight, Out of Mind? Neuropsychol. Rev. 2012, 22, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Polegato, B.F.; Pereira, A.G.; Azevedo, P.S.; Costa, N.A.; Zornoff, L.A.M.; Paiva, S.A.R.; Minicucci, M.F. Role of thiamin in health and disease. Nutr. Clin. Pract. 2019, 34, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Latifiyan, Z.; Torkashvand, R.; Saatchi, M. The effect of vitamin B1 on heavy menstrual bleeding. Prog. Nutr. 2019, 21, 843–848. [Google Scholar]
- Wang, C.; Fei, G.; Pan, X.; Sang, S.; Wang, L.; Zhong, C.; Jin, L. High thiamine diphosphate level as a protective factor for Alzheimer’s disease. Neurol. Res. 2018, 40, 658–665. [Google Scholar] [CrossRef]
- Pourhassan, M.; Biesalski, H.K.; Angersbach, B.; Lueg, G.; Klimek, C.; Wirth, R. Prevalence of thiamine deficiency in older hospitalized patients. Clin. Interv. Aging 2018, 13, 2247–2250. [Google Scholar] [CrossRef]
- Wacker, J.; Frühauf, J.; Schulz, M.; Chiwora, F.M.; Volz, J.; Becker, K. Riboflavin deficiency and preeclampsia. Obstet. Gynecol. 2000, 96, 38–44. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Adair, L.S.; Bentley, M.E.; Flax, V.L.; Jamieson, D.J.; Ellington, S.R.; Tegha, G.; Chasela, C.S.; Kamwendo, D.; et al. Thiamin and Riboflavin in Human Milk: Effects of Lipid-Based Nutrient Supplementation and Stage of Lactation on Vitamer Secretion and Contributions to Total Vitamin Content. PLoS ONE 2016, 11, e0149479. [Google Scholar]
- Sangermani, R.; Boncimino, A. The use of nutraceutics in children‘s and adolescent’s headache. Neurol. Sci. 2017, 38, 121–124. [Google Scholar] [CrossRef]
- Thompson, D.F.; Saluja, H.S. Prophylaxis of migraine headaches with riboflavin: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 394–403. [Google Scholar] [CrossRef]
- Chocano-Bedoya, P.O.; Manson, J.E.; Hankinson, S.E.; Willett, W.C.; Johnson, S.R.; Chasan-Taber, L.; Ronnenberg, A.G.; Bigelow, C.; Bertone-Johnson, E.R. Dietary B vitamin intake and incident premenstrual syndrome. Am. J. Clin. Nutr. 2011, 93, 1080–1086. [Google Scholar] [CrossRef]
- Yu, L.; Tan, Y.; Zhu, L. Dietary vitamin B2 intake and breast cancer risk: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 721–729. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Muraki, I.; Tamakoshi, A. Among the water-soluble vitamins, dietary intakes of vitamins C, B2 and folate are associated with the reduced risk of diabetes in Japanese women but not men. Br. J. Nutr. 2019, 121, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Enriquez, A.; Rapadas, M.; Martin, E.M.M.A.; Wang, R.; Moreau, J.; Lim, C.K.; Szot, J.O.; Ip, E.; Hughes, J.N.; et al. NAD deficiency, congenital malformations, and niacin supplementation. N. Engl. J. Med. 2017, 377, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Penke, M.; Kiess, W. Paediatric endocrinology: Can niacin supplementation protect against congenital malformations? Nat. Rev. Endocrinol. 2017, 13, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dang, S.; Cheng, Y.; Qiu, H.; Mi, B.; Jiang, Y.; Qu, P.; Zeng, L.; Wang, Q.; Li, Q.; et al. Dietary intakes and dietary patterns among pregnant women in Northwest China. Public Health Nutr. 2017, 20, 282–293. [Google Scholar] [CrossRef]
- Daniels, L.; Gibson, R.S.; Diana, A.; Haszard, J.J.; Rahmannia, S.; Luftimas, D.E.; Hampel, D.; Shahab-Ferdows, S.; Reid, M.; Melo, L.; et al. Micronutrient intakes of lactating mothers and their association with breast milk concentrations and micronutrient adequacy of exclusively breastfed Indonesian infants. Am. J. Clin. Nutr. 2019, 110, 391–400. [Google Scholar] [CrossRef]
- Committee on Nutrition. Composition of Human Milk: Normative Data. In Pediatric Nutrition Handbook, 2nd ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 1985; pp. 363–368. [Google Scholar]
- Litwack, G. Vitamins and Nutrition. In Human Biochemistry; Academic Press: Cambridge, MA, USA, 2018; pp. 645–680. [Google Scholar]
- Drucker, A.M.; Li, W.Q.; Park, M.K.; Li, T.; Qureshi, A.A.; Cho, E. Niacin intake and incident adult-onset atopic dermatitis in women. J. Allergy Clin. Immunol. 2017, 139, 2020–2022.e2. [Google Scholar] [CrossRef][Green Version]
- Ito, M.; Morita, T.; Okazaki, S.; Koto, M.; Ichikawa, Y.; Takayama, R.; Hoashi, T.; Saeki, H.; Kanda, N. Dietary habits in adult Japanese patients with atopic dermatitis. J. Dermatol. 2019, 46, 515–521. [Google Scholar] [CrossRef]
- Qin, B.; Xun, P.; Jacobs, D.R.; Zhu, N.; Daviglus, M.L.; Reis, J.P.; Steffen, L.M.; Van Horn, L.; Sidney, S.; He, K. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Clin. Nutr. 2017, 106, 1032–1040. [Google Scholar] [CrossRef]
- Kaplon, R.E.; Gano, L.B.; Seals, D.R. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J. Appl. Physiol. 2014, 116, 156–163. [Google Scholar] [CrossRef]
- Majewski, M.; Kozłowska, A.; Thoene, M.; Lebiedzińska, A. Variations of niacin content with regard to carbohydrates in energy-rich diets of elite European athletes and their relation with dietary RDA. J. Elem. 2016, 21, 745–755. [Google Scholar] [CrossRef]
- Fenech, M.; Baghurst, P.; Luderer, W.; Turner, J.; Record, S.; Ceppi, M.; Bonassi, S. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, beta-carotene and high intake of pantothenic acid, biotin and riboflavin are significantly associated with increased genome instability—Results from a dietary intake and micronucleus index survey in South Australia. Carcinogenesis 2005, 26, 991–999. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; McClung, J.P. Pantothenic Acid. In The Vitamins; Academic Press: Cambridge, MA, USA, 2017; pp. 387–398. [Google Scholar]
- Lee, J.H.; Ahn, S.Y.; Lee, H.A.; Won, K.S.; Chang, H.W.; Oh, J.S.; Kim, H.W. Dietary intake of pantothenic acid is associated with cerebral amyloid burden in patients with cognitive impairment. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.K.; Choi, B.Y. The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 806–816. [Google Scholar] [CrossRef]
- Hisano, M.; Suzuki, R.; Sago, H.; Murashima, A.; Yamaguchi, K. Vitamin B6 deficiency and anemia in pregnancy. Eur. J. Clin. Nutr. 2010, 64, 221–223. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Dietary Reference Values for vitamin B6. EFSA J. 2016, 14, e04485. [Google Scholar] [CrossRef]
- Barnard, H.C.; Dekock, J.J.; Vermaak, W.J.H.; Potgieter, G.M. A new perspective in the assessment of vitamin B-6 nutritional status during pregnancy in humans. J. Nutr. 1987, 117, 1303–1306. [Google Scholar] [CrossRef]
- Roepke, J.L.; Kirksey, A. Vitamin B6 nutriture during pregnancy and lactation. I. Vitamin B6 intake, levels of the vitamin in biological fluids, and condition of the infant at birth. Am. J. Clin. Nutr. 1979, 32, 2249–2256. [Google Scholar] [CrossRef]
- Roepke, J.L.; Kirksey, A. Vitamin B6 nutriture during pregnancy and lactation. II. The effect of long-term use of oral contraceptives. Am. J. Clin. Nutr. 1979, 32, 2257–2264. [Google Scholar] [CrossRef]
- Martner-Hewes, P.M.; Hunt, I.F.; Murphy, N.J.; Swendseid, M.E.; Settlage, R.H. Vitamin B-6 nutriture and plasma diamine oxidase activity in pregnant Hispanic teenagers. Am. J. Clin. Nutr. 1986, 44, 907–913. [Google Scholar] [CrossRef]
- Serapinas, D.; Boreikaite, E.; Bartkeviciute, A.; Bandzeviciene, R.; Silkunas, M.; Bartkeviciene, D. The importance of folate, vitamins B6 and B12 for the lowering of homocysteine concentrations for patients with recurrent pregnancy loss and MTHFR mutations. Reprod. Toxicol. 2017, 72, 159–163. [Google Scholar] [CrossRef]
- Reinken, L.; Gant, H. Vitamin B6 nutrition in women with hyperemesis gravidarum during the first trimester of pregnancy. Clin. Chim. Acta 1974, 55, 101–102. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Interventions with Vitamins B6, B12 and C in Pregnancy. Paediatr. Perinat. Epidemiol. 2012, 26, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Shrim, A.; Boskovic, R.; Maltepe, C.; Navios, Y.; Garcia-Bournissen, F.; Koren, G. Pregnancy outcome following use of large doses of vitamin B6 in the first trimester. J. Obstet. Gynaecol. 2006, 26, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Khodadad, M.; Kheirabadi, G.; Bahadoran, P. Efficacy of vitamin B6 on pregnancy outcomes: A randomized clinical trial. J. Pharm. Res. Int. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Lumeng, L.; Cleary, R.E.; Wagner, R.; Yu, P.L.; Li, T.K. Adequacy of vitamin B6 supplementation during pregnancy: A prospective study. Am. J. Clin. Nutr. 1976, 29, 1376–1383. [Google Scholar] [CrossRef]
- Heiskanen, K.; Siimes, M.A.; Salmenperä, L.; Perheentupa, J. Low vitamin B6 status associated with slow growth in healthy breast-fed infants. Pediatr. Res. 1995, 38, 740–746. [Google Scholar] [CrossRef]
- Kang-Yoon, S.A.; Kirksey, A.; Giacoia, G.; West, K. Vitamin B-6 status of breast-fed neonates: Influence of pyridoxine supplementation on mothers and neonates. Am. J. Clin. Nutr. 1992, 56, 548–558. [Google Scholar] [CrossRef]
- Husami, N.; Idriss, W.; Jewelewicz, R.; Ferin, M.; Vande Wiele, R.L. Lack of acute effects of pyridoxine on prolactin secretion and lactation. Fertil. Steril. 1978, 30, 393–397. [Google Scholar] [CrossRef]
- Schulze-Bonhage, A.; Kurthen, M.; Walger, P.; Elger, C.E. Pharmacorefractory status epilepticus due to low vitamin B6 levels during pregnancy. Epilepsia 2004, 45, 81–84. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, B.; Millman, S.E.; Chen, C.; Li, X.; Morris, J.P., IV; Mayle, A.; Ho, Y.J.; Loizou, E.; Liu, H.; et al. Vitamin B6 addiction in acute myeloid leukemia. Cancer Cell 2020, 37, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Benke, P.J.; Duchowny, M.; McKnight, D. Biotin and Acetazolamide for Treatment of an Unusual Child With Autism Plus Lack of Nail and Hair Growth. Pediatric Neurol. 2018, 79, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Martineau, J.; Barthelemy, C.; Garreau, B.; Lelord, G. Vitamin B6, magnesium, and combined B6-Mg: Therapeutic effects in childhood autism. Biol. Psychiatry 1985, 20, 467–478. [Google Scholar] [CrossRef]
- Marino, S.; Vitaliti, G.; Marino, S.D.; Pavone, P.; Provvidenti, S.; Romano, C.; Falsaperla, R. Pyridoxine add-On treatment for the control of behavioral adverse effects induced by levetiracetam in children: A case-control prospective study. Ann. Pharmacother. 2018, 52, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; Dubray, C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.W.; Whittington, R.M.; Weisman, R.; Horrigan, D.L. Pyridoxine responsive anemia in the human adult. Proc. Soc. Exp. Biol. Med. 1956, 91, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Ribayamercado, J.D.; Russell, R.M.; Sahyoun, N.; Morrow, F.D.; Gershoff, S.N. Vitamin B-6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am. J. Clin. Nutr. 1991, 53, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, Y.; Shin, H.; Kang, K.; Park, J.M.; Kim, B.K.; Kwon, O.; Lee, J.J. Seizures related to vitamin B6 deficiency in adults. J. Epilepsy Res. 2015, 5, 23–24. [Google Scholar] [CrossRef]
- Lee, J.E.; Li, H.; Giovannucci, E.; Lee, I.M.; Selhub, J.; Stampfer, M.; Ma, J. Prospective study of plasma vitamin B6 and risk of colorectal cancer in men. Cancer Epidemiol Biomark. Prev. 2009, 18, 1197–1202. [Google Scholar] [CrossRef]
- Hlais, S.; Abou Reslan, D.R.; Sarieddine, H.K.; Nasreddine, L.; Taan, G.; Azar, S.; Obeid, O.A. Effect of lysine, vitamin B(6), and carnitine supplementation on the lipid profile of male patients with hypertriglyceridemia: A 12-week, open-label, randomized, placebo-controlled trial. Clin. Ther. 2012, 34, 1674–1682. [Google Scholar] [CrossRef]
- Zhao, M.; Lamers, Y.; Ralat, M.A.; Coats, B.S.; Stacpoole, P.W.; Chi, Y.Y.; Muller, K.E.; Bain, J.R.; Newgard, C.B. Marginal vitamin B6 deficiency affects fatty acid profiles in healthy men and women. FASEB J. 2011, 25, 586.3. [Google Scholar] [CrossRef]
- Kjeldby, I.K.; Fosnes, G.S.; Ligaarden, S.C.; Farup, P.G. Vitamin B6 deficiency and diseases in elderly people-a study in nursing homes. BMC Geriatr. 2013, 13, 13. [Google Scholar] [CrossRef]
- Avcin, M. Dermatitis seborrheica and biotin deficiency in infants. Zdrav. Vestn. 1952, 21, 15–21. [Google Scholar] [PubMed]
- Fujimoto, W.; Inaoki, M.; Fukui, T.; Inoue, Y.; Kuhara, T. Biotin deficiency in an infant fed with amino acid formula. J. Dermatol. 2005, 32, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Noda, E.; Koyama, Y.; Shirai, T.; Horino, A.; Juri, T.; Koike, M. Biotin deficiency in an infant fed with amino acid formula and hypoallergenic rice. Acta Paediatr. 1996, 85, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Mamada, Y.; Murata, T.; Taniguchi, A.; Hasegawa, Y.; Suzuki, T.; Kohda, K.; Nasuno, K.; Watanabe, T.; Yamaguchi, S.; Ishiguro, A. Biotin deficiency in amino acid formula nutrition for an infant with milk protein allergy. Arerugi 2008, 57, 552–557. [Google Scholar] [PubMed]
- Heard, G.S.; Hood, R.L.; Johnson, A.R. Hepatic biotin and the sudden infant death syndrome. Med. J. Aust. 1983, 2, 305–306. [Google Scholar] [CrossRef]
- Johnson, A.R.; Hood, R.L.; Emery, J.L. Biotin and the sudden infant death syndrome. Nature 1980, 285, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Mohrenschlager, M.; Schmidt, T.; Ring, J.; Abeck, D. Recalcitrant trachyonychia of childhood—Response to daily oral biotin supplementation: Report of two cases. J. Dermatol. Treat. 2000, 11, 113–115. [Google Scholar] [CrossRef]
- Ferreira, P.; Chan, A.; Wolf, B. Irreversibility of Symptoms with Biotin Therapy in an Adult with Profound Biotinidase Deficiency. In JIMD Reports; Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Eds.; Springer: Berlin, Germany, 2017; Volume 36, pp. 117–120. [Google Scholar]
- Watanabe, T.; Yasumura, S.; Shibata, H.; Fukui, T. Biotin status and its correlation with other biochemical parameters in the elderly people of Japan. J. Am. Coll. Nutr. 1998, 17, 48–53. [Google Scholar] [CrossRef]
- Hure, A.J.; Collins, C.E.; Smith, R. A longitudinal study of maternal folate and vitamin B12 status in pregnancy and postpartum, with the same infant markers at 6 months of age. Matern. Child Health J. 2012, 16, 792–801. [Google Scholar] [CrossRef]
- Vinaykumar, N.; Kumar, A.; Quadros, L.S.; Prasanna, L.C. Determining the effect of folate diets during pregnancy and lactation on neurobehavioural changes in the adult life of offspring. J. Taibah Univ. Med. Sci. 2019, 14, 523–530. [Google Scholar] [CrossRef]
- Hay, G.; Johnston, C.; Whitelaw, A.; Trygg, K.; Refsum, H. Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. Am. J. Clin. Nutr. 2008, 88, 105–114. [Google Scholar] [CrossRef]
- Stamm, R.A.; Houghton, L.A. Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients 2013, 5, 3920–3947. [Google Scholar] [CrossRef]
- Furness, D.; Fenech, M.; Dekker, G.; Khong, T.Y.; Roberts, C.; Hague, W. Folate, vitamin B12, vitamin B6 and homocysteine: Impact on pregnancy outcome. Matern. Child Nutr. 2013, 9, 155–166. [Google Scholar] [CrossRef]
- Mishra, J.; Tomar, A.; Puri, M.; Jain, A.; Saraswathy, K.N. Trends of folate, vitamin B 12, and homocysteine levels in different trimesters of pregnancy and pregnancy outcomes. Am. J. Hum. Biol. 2020, 32, e23388. [Google Scholar] [CrossRef]
- Reynolds, E.H. Folic acid, ageing, depression, and dementia. BMJ 2002, 324, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Murphy, M.; Solé-Navais, P.; Yajnik, C. Cobalamin status from pregnancy to early childhood: Lessons from global experience. Adv. Nutr. 2017, 8, 971–979. [Google Scholar] [CrossRef]
- Bjørke-Monsen, A.L.; Ueland, P.M. Cobalamin status in children. J. Inherit. Metab. Dis. 2011, 34, 111–119. [Google Scholar] [CrossRef]
- Fanjiang, G.; Kleinman, R.E. Nutrition and performance in children. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 342–347. [Google Scholar] [CrossRef]
- Dali-Youcef, N.; Andrès, E. An update on cobalamin deficiency in adults. QJM 2009, 102, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef] [PubMed]

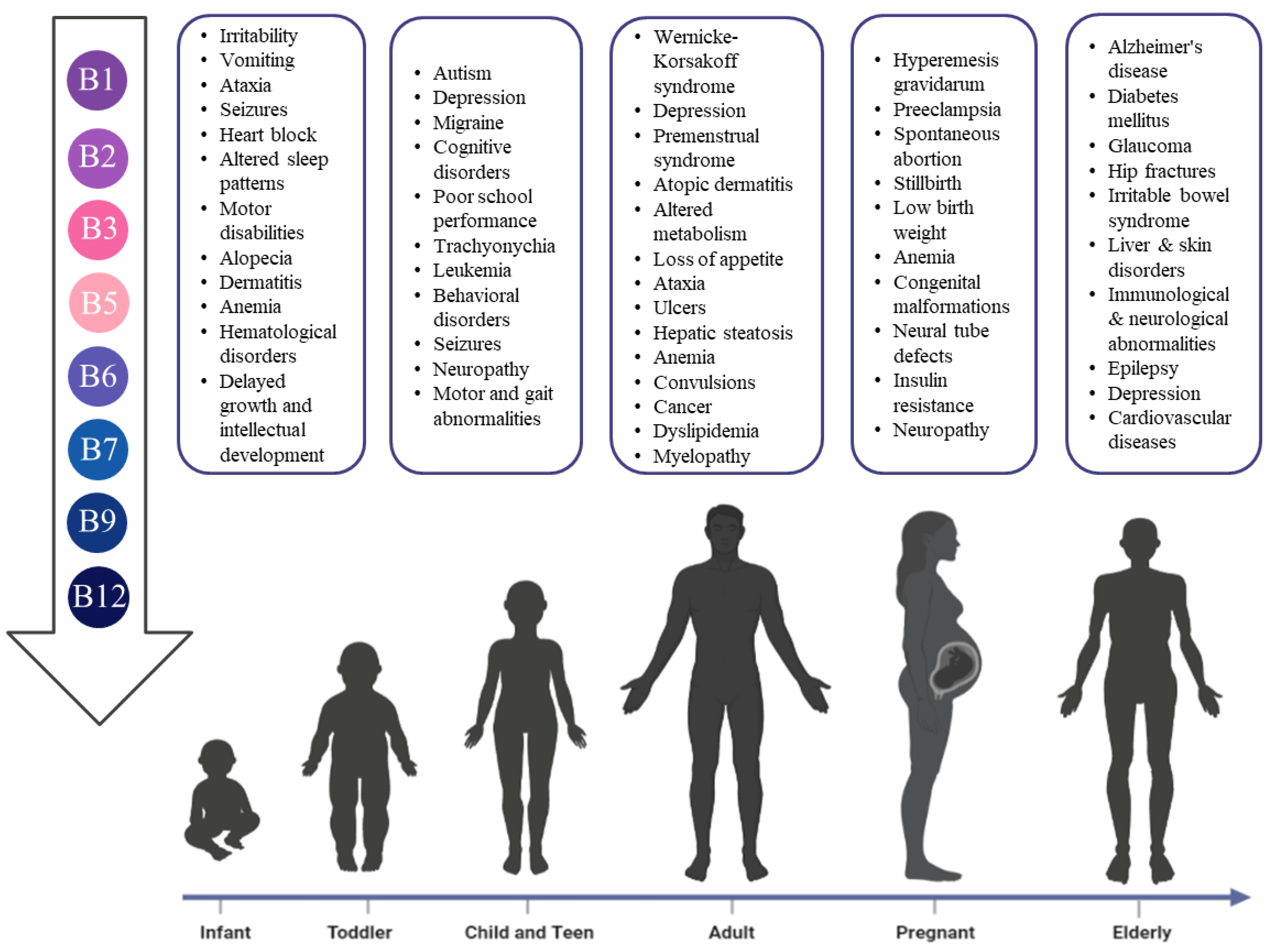
| Life Stage | RDI (mg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 0.2 | Involved in carbohydrates and amino acids metabolism as well as neurotransmitters biosynthesis | Deficiency may cause irritability, vomiting, ataxia, altered sleep patterns, and may cause infant encephalopathy | Maternal Milk |
| 7–12 Months | 0.2 | ||||
| Children | 1–3 Years | 0.5 | Deficiency may cause anorexia, irritability, agitation, muscle pain, diminished deep tendon reflexes, ataxia, paralysis, and pediatric depression | Meat, vegetables, whole grains, and legumes | |
| 4–9 Years | 0.9 | ||||
| Teen | Girl | 1.1 | |||
| Boy | 1.2 | ||||
| Adult | Men | 1.2 | The deficiency is often associated with alcohol abuse causing Wernicke–Korsakoff syndrome | ||
| Women | Same as above Used to lessen the symptoms of premenstrual syndrome (PMS) and menorrhagia | ||||
| Non-pregnant | 1.0 | ||||
| Pregnant | 1.3 | Same as above | Deficiency cause Hyperemesis gravidarum | ||
| Lactating | 1.5 | Same as above Maternal supplements are recommended to avoid impaired infant growth | |||
| Elderly | 1.1–1.2 | Same as above | |||
| Life Stage | RDI (mg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 0.4 | Early postnatal brain development Coenzyme in mitochondrial energy production in the flavoprotein redox reactions | Deficiency may cause abnormal brain development | Maternal Milk |
| 7–12 Months | 0.3 | ||||
| Children | 1–3 Years | 0.5 | Same as above Treat migraines | Migraine, pain, and attention deficit hyperactivity disorder (ADHD) | Beef, organ meats—mostly in calf liver, egg, salmon, mushrooms, dark green leafy vegetables such as spinach. |
| 4–9 Years | 0.9 | ||||
| Teen | Girl | 1.0 | |||
| Boy | 1.3 | ||||
| Adult | Men | 1.3 | Same as above | Same as above | |
| Women | Same as above Decrease incidence of PMS Cofactor in one-carbon metabolism | ||||
| Non-pregnant | 1.1 | Risk of breast cancer | |||
| Pregnant | 1.4 | Same as above Reduce risk of preeclampsia Prevent mitochondrial dysfunction and oxidative stress Stabilize nitric oxide release | Preeclampsia | ||
| Lactating | 1.6 | ||||
| Elderly | 1.1–1.3 | Same as above Reduce the risk of Type 2 diabetes Antioxidant activity Reduce iron overload | Deficiency may elevate the risk of Type 2 diabetes | ||
| Life Stage | RDI (mg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 3 | Play a major role in normal metabolic pathways Essential for normal development and growth Important for healthy cognitive function | Deficiency may cause Multiple congenital malformations | Maternal Milk |
| 7–12 Months | 4-5 | ||||
| Children | 1–3 Years | 6 | Yeast, poultry, meat, Redfish (e.g., tuna and salmon), beans, and coffee. | ||
| 4–9 Years | 8 | ||||
| Teen | Girl | 12 | |||
| Boy | 16 | ||||
| Adult | Men | 16 | Same as above Prevent the occurrence of pellagra-associated dermatitis | Deficiency may cause pellagra-associated dermatitis | |
| Women | |||||
| Non-pregnant | 14 | ||||
| Pregnant | 18 | Same as above Reduce the risk of multiple congenital malformations in the fetus | |||
| Lactating | 17 | Same as above | Same as above | ||
| Elderly | 14–16 | Has neuroprotective effects against age-related disorders such as hearing loss and myelination | Deficiency may increase the incidence of hip fractures | ||
| Life Stage | AI (mg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 1.7 | Important for normal development and growth | Loss of appetite, growth impairment, dermatitis, weakness, ataxia, paralysis, adrenal hypertrophy, ulcers, and hepatic steatosis | Maternal Milk |
| 7–12 Months | 3 | ||||
| Children | 1–3 Years | 4 | Organ meats (particularly liver and heart), broccoli, avocados, mushrooms, and some yeasts | ||
| 4–9 Years | 4 | ||||
| Teen | Girl | 5 | |||
| Boy | 5 | ||||
| Adult | Men | 5 | Inversely related to the levels of C-reactive protein (CRP) | ||
| Women | |||||
| Non-pregnant | NA | ||||
| Pregnant | 5 | Third-trimester pregnant women need to consume more than the average intake to maintain a blood vitamin level | |||
| Lactating | 7 | Same as above | |||
| Elderly | 5 | Same as above | |||
| Life Stage | RDI (mg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 0.1 | Essential for average infant growth in height and weight Lifelong treatment is essential for infants with an autosomal recessive defect in the glutamic acid decarboxylase enzyme | Deficiency may cause non-responsive polymorphic seizures | Maternal Milk |
| 7–12 Months | 0.3 | ||||
| Children | 1–3 Years | 0.5–0.6 | Required for thymidine biosynthesis and host immunocompetence Treat the symptoms of behavioral disorders associated with autism, hyperkinetic syndrome, and schizophrenia. Adjuvant to anti-epileptic drugs Exert beneficial effects on stress accompanying adolescence phase | Cereals, fishes, meats, starchy vegetables such as potatoes, legumes; nuts, bananas, avocados, and non-citrus fruits | |
| 4–9 Years | 0.6 | ||||
| Teen | Girl | 1–1.2 | |||
| Boy | 1–1.3 | ||||
| Adult | Men | 1.3 | Extremely beneficial against colorectal cancer in adult males Reduce plasma cholesterol level | microcytic hypochromic anemia Lymphopenia convulsions | |
| Women | |||||
| Non-pregnant | 1.3 | Necessary for estrogen metabolism Prescribed for women with breast cysts Effective during PMS | |||
| Pregnant | 5.5–7.6 | Pregnancy stabilization Prevent any miscarriages Improve hyperemesis gravidarum Essential for heme and porphyrin synthesis and the proper iron utilization by red blood cells Maintain normal fetal/infant development. | Hyperemesis gravidarum, anemia nausea, vomiting, spontaneous abortion | ||
| Lactating | 5.5–7.6 | Same as above Mood elevation Anemia improvement | Same as above | ||
| Elderly | 1.5–1.7 | Reduce the risk of irritable bowel syndrome | Deficiency may cause irritable bowel syndrome | ||
| Life Stage | AI (µg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 5 | Critical role in maintaining healthy hair, skin, and nails Prevent serious brain abnormalities | Insufficiency is linked to alopecia and dermatitis around body orifices | Maternal Milk |
| 7–12 Months | 5–6 | ||||
| Children | 1–3 Years | 8–12 | Efficient in both shiny and opaque types of trachyonychia | Deficiency is linked to Trachyonychia | Red meat, eggs, nuts, seeds, and certain vegetables |
| 4–9 Years | 12 | ||||
| Teen | Girl | 20–25 | |||
| Boy | 20–25 | ||||
| Adult | Men | 30 | Treat the symptoms of inherited disorder biotinidase deficiency | Deficiency may cause myelopathy and irreversible neurological damages | |
| Women | |||||
| Non-pregnant | 30 | ||||
| Pregnant | 35 | ||||
| Lactating | 35 | ||||
| Elderly | 30 | It has a critical role in bone mineral homeostasis Has a role in allergic and autoimmune disorders | Deficiency is related to specific diseases such as diabetes mellitus, liver and skin disorders, immunological and neurological abnormalities, and epilepsy | ||
| Life Stage | RDI (mg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 0.05 | Important for the proper development Protect from asthma in later stages of life. | Deficiency may cause long-term impairment of cognitive function, learning, and memory deficits and detected brain atrophy | Maternal Milk |
| 7–12 Months | 0.08 | ||||
| Children | 1–3 Years | 0.15 | Essential for normal cognitive, motor, behavioral and vascular development | Deficiency may cause developmental delay, cognitive deterioration, motor and gait abnormalities, behavioral or psychiatric symptoms, seizures, signs of demyelination or failure of myelination, vascular changes, megaloblastic anemia and infant neural tube defects | Beans, lentils, leafy green vegetables, and lemons |
| 4–9 Years | 0.2 | ||||
| Teen | Girl | 0.4 | |||
| Boy | 0.4 | ||||
| Adult | Men | 0.4 | Modify the adverse effects of smoking regarding one-carbon metabolism and redox balance. | ||
| Women | |||||
| Non-pregnant | 0.4 | ||||
| Pregnant | 0.6 | It has a significant role in the normal neural and physical development of the fetus Prevent megaloblastic anemia and neural tube defects | In mothers, a deficiency might cause metabolic effects such as insulin resistance, glomerular sclerosis, neuropathy in the extremities, and megaloblastic anemia. Also, severe fetal adverse effects, including congenital neural tube defects, cardiac and urinary tract defects, and even cancer | ||
| Lactating | 0.5 | Same as above | |||
| Elderly | 0.4 | Reduces the risk of depression, dementia, and vascular disease. | |||
| Life Stage | AI (µg/Day) | Importance and Health Effects | Deficiency Symptoms | Source | |
|---|---|---|---|---|---|
| Infant | 0–6 Months | 0.4 | Maintain normal physical growth Prevent neurological and hematological disorders | Deficiency may cause neurological, insufficient physical growth (failure to thrive), and hematological disorders | Maternal Milk |
| 7–12 Months | 0.5 | ||||
| Children | 1–3 Years | 0.7 | Essential for healthy cognitive function, motor development, speech and language skills | Fish, meat, dairy products such as cheese and eggs | |
| 4–9 Years | 1.2 | ||||
| Teen | Girl | 1.8–2.4 | |||
| Boy | 1.8–2.4 | ||||
| Adult | Men | 2.4 | Reduces the deficiency manifestation ranging from fatigue to severe disorders | Deficiency may cause fatigue, common sensory neuropathy, neuropsychiatric symptoms, atrophic glossitis, isolated macrocytosis, neutrophil hypersegmentation, combined sclerosis of the spinal cord, hemolytic anemia, and pancytopenia | |
| Women | |||||
| Non-pregnant | 2.4 | ||||
| Pregnant | 2.6 | Cobalamin status of mothers during gestation highly affects the infant’s status | Deficiency may cause high body mass indexes, recurrent miscarriages, preterm delivery, and intrauterine growth restriction | ||
| Lactating | 2.8 | Same as above | Same as above | ||
| Elderly | 2.4 | Reduces the risk of disrupted cellular metabolism, age-related disease, and functional decline, including cognition, cardiovascular disease, and bone health. | Deficiency may contribute to age-related cognitive decline, subtle deficits, frank dementia, cardiovascular disease, and bone health. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.A.; Hafez, H.A.; Kamel, M.A.; Ghamry, H.I.; Shukry, M.; Farag, M.A. Dietary Vitamin B Complex: Orchestration in Human Nutrition throughout Life with Sex Differences. Nutrients 2022, 14, 3940. https://doi.org/10.3390/nu14193940
Ali MA, Hafez HA, Kamel MA, Ghamry HI, Shukry M, Farag MA. Dietary Vitamin B Complex: Orchestration in Human Nutrition throughout Life with Sex Differences. Nutrients. 2022; 14(19):3940. https://doi.org/10.3390/nu14193940
Chicago/Turabian StyleAli, Mennatallah A., Hala A. Hafez, Maher A. Kamel, Heba I. Ghamry, Mustafa Shukry, and Mohamed A. Farag. 2022. "Dietary Vitamin B Complex: Orchestration in Human Nutrition throughout Life with Sex Differences" Nutrients 14, no. 19: 3940. https://doi.org/10.3390/nu14193940
APA StyleAli, M. A., Hafez, H. A., Kamel, M. A., Ghamry, H. I., Shukry, M., & Farag, M. A. (2022). Dietary Vitamin B Complex: Orchestration in Human Nutrition throughout Life with Sex Differences. Nutrients, 14(19), 3940. https://doi.org/10.3390/nu14193940









