Abstract
Background: In preconception and pregnancy, women are encouraged to take folic acid-based supplements over and above food intake. The upper tolerable limit of folic acid is 1000 mcg per day; however, this level was determined to avoid masking a vitamin B12 deficiency and not based on folic acid bioavailability and metabolism. This review’s aim is to assess the total all-source intake of folate in women of childbearing age and in pregnancy in high-income countries with folate food fortification programs. Methods: A systematic search was conducted in five databases to find studies published since 1998 that reported folate and folic acid intake in countries with a mandatory fortification policy. Results: Women of childbearing age do not receive sufficient folate intake from food sources alone even when consuming fortified food products; however, almost all women taking a folic acid-based supplement exceed the upper tolerable limit of folic acid intake. Conclusions: Folic acid supplement recommendations and the upper tolerable limit of 1000 mcg set by policy makers warrant careful review in light of potential adverse effects of exceeding the upper tolerable limit on folic acid absorption and metabolism, and subsequent impacts on women’s health during their childbearing years.
1. Introduction
Governments worldwide encourage women of childbearing age to take folic acid (FA) prior to pregnancy to avoid neural tube defects (NTDs) [1,2,3,4,5,6,7,8,9,10] and some governments (e.g., Australia, Canada and United States) have implemented a FA food fortification program to ensure that women who unintentionally conceive or are not using a folic acid supplement have sufficient folate to prevent neural tube defects [11,12]. Since the mandatory folic acid fortification program began in 2009, Australian policy makers have recommended 400 mcg of FA in addition to fortification for individuals considering conception [6].
While the fortification policy has prevented NTDs, emerging research has documented the presence of unmetabolized folic acid (UMFA) in the serum in people in countries that have a FA fortification program and/or take a FA supplement [13,14,15]. While the effect of this is unclear, some have raised concerns regarding UMFA and adverse health effects [16,17,18,19,20,21].
1.1. Folate Metabolism
Folate-dependent biochemical processes are influenced by the form and bioavailability of folate [22]. Folate is a generic term for vitamin B9 that includes several forms, including that naturally found in food (natural folate). All folate forms have a common structure but differ based on the pteridine ring and whether it is reduced or oxidized [23]. Folate derived from food such as leafy green vegetables are polyglutamates (Figure 1), whereas synthetic FA is a monoglutamate (Figure 2) [21].
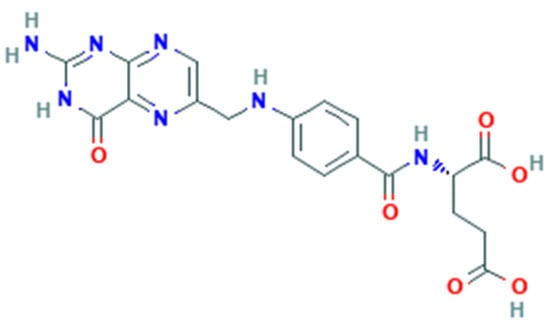
Figure 1.
Folic acid structure.
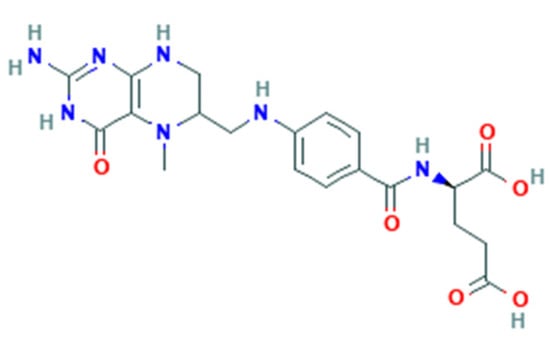
Figure 2.
5-MTHF structure.
Food folate is naturally present in foods such as leafy green vegetables and is in a reduced form [13]. This natural folate exists in a polyglutamate form and needs to be further hydrolyzed to a monoglutamate form by the intestinal lumen to be transported [24]. The intestinal absorption of polyglutamate derivatives of tetrahydrofolate (THF) into monoglutamates is carried out by the brush border enzyme glutamate carboxypeptidase II (GPCII) [25]. This crossing of the apical brush border is carried out via two transporters, the proton-coupled folate transporter (PCFT) and the reduced folate carrier (RFC) [26,27]. Most of the absorbed natural folate is metabolized to 5-methyltetrahydrofolate (5-MTHF) in the intestine and/or liver [26] because the RFC has a higher affinity for reduced folates such as 5-MTHF compared to FA [26]. The fate of both natural folate and FA is to be metabolized to 5-MTHF [28]. The RFC transporter is found throughout the intestinal tract and is highly expressed in the liver and placenta [26]. The PCFT’s key role is as the major transporter of dietary folate in the proximal small intestine, specifically in the brush border of the duodenum and jejunum and has an optimal function with an acidic PH but has a higher affinity to FA than it does to reduced folates such as 5-MTHF [16,26,29,30]. Once absorbed by the RFC or PCFT, the folate is transported into the hepatic portal vein by multidrug resistance-associated protein 3 (MRP3) and then to the liver, where it is taken up via hepatocytes [26]. The intestine can convert reduced folates to 5-MTHF quite well; however, it is the first-pass metabolism and intestinal absorption where the difference between the metabolism of FA and the reduced folate forms exists as its reduction and methylation are dose dependent [14,28].
While natural folates do not need to be converted to 5-MTHF to cross the mucosal cell (Figure 3) and enter the portal vein to the blood, they do need to be metabolized to 5-MTHF in the liver or released into the blood or bile [27]. One small study showed that the majority of FA (at a physiologic dose) passes into the portal vein in an unmodified form, whilst monoglutamates are almost all converted to 5-MTHF [31]. All forms of folate must be converted to 5-methyltetrahydrofolate (5-MTHF), which is the main form of folate found in plasma, accounting for approximately 98% of total serum folate [16,23]. Where natural folates can be converted directly to 5-MTHF directly, FA relies on the dihydrofolate reductase (DHFR) enzyme to be reduced [21,24]. The activity of DHFR is slow and easily saturated in the first step conversion to dihydrofolate and therefore when the dose exceeds the capacity of the enzyme or there is a DHFR polymorphism, an increase in free FA in plasma may be seen [27]. The main circulating form of folate in the blood, 5-MTHF, is in high demand during fetal development as DNA synthesis and cell division are increased [26]. The folate receptor-α (encoded by the folate receptor 1 (FOLR1) gene) is expressed on epithelial cells and ensures uptake of 5-MTHF by the placenta and the brain during development. This receptor is upregulated in pregnancy due to the rapid increase in folate-dependent activities such as tissue growth, placental development, and enlargement of the uterus [26,32]. 5-MTHF donates its methyl group to support DNA methylation via DNA methyltransferase enzymes and 5-MTHF is directly linked to the viability of the embryo and the ability to maintain DNA methylation patterns in replicating cells [26,33]. A lack of 5-MTHF may therefore compromise DNA methylation, cause uracil misincorporation, chromosomal breaks, impaired ovarian follicle development and increased risk of pregnancy loss [32]. Folate metabolism can be impaired in people who carry the methylenetetrahydrofolate gene (MTHFR) polymorphisms, resulting in reduced enzyme activity and therefore a reduction in 5-MTHF [34,35,36]. The MTHFR gene has been linked to infertility in both men and women [37,38,39,40,41,42,43]. This is particularly pertinent given that studies in mice show that excess FA reduces MTHFR activity irrespective of MTHFR polymorphisms thereby reducing methylation capacity. Research has identified 5-MTHF as a possible alternative for FA in those with MTHFR polymorphisms as doses of 800 mcg have been shown to bypass the MTHFR enzyme [23,24,25,44,45,46,47].
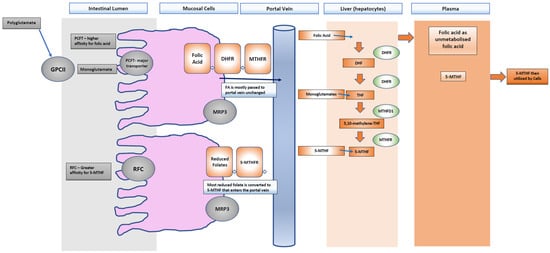
Figure 3.
Polyglutamates are converted to monoglutamates by glutamate carboxypeptidase II GPCII and absorbed by the reduced folate carrier (RFC) or the proton-coupled folate transporter (PCFT). Almost all the reduced folates are converted into 5-MTHF and transported to the portal vein by multidrug resistance-associated protein 3 (MRP3) to the liver and blood. Folic acid is mostly passed unchanged to the portal vein, where it needs to be metabolized in the liver to dihydrofolate (DHF) by the enzyme dihydrofolatereductase (DHFR) and to tetrahydrofolate (THF) also by DHFR and finally to 5-methyltetrahdyrofolate (5-MTHF). What is not converted will pass into the plasma.
1.2. Folic Acid Dose
Although mandatory fortification exists in some countries, the fortification levels per 100 mg food differ across each country, with no upper limit on the number of foods that may be fortified voluntarily, easily leading to an intake level above the original estimated level of 100 mcg per day [10,11,12,48,49,50,51,52,53].
FA is widely used in supplements and food fortification as it is relatively inexpensive, stable, and freely available [7,54,55,56]. Women are encouraged by governments to take FA supplements during preconception and pregnancy in addition to any folate they may consume through natural folate and fortified foods. Doses of 400–500 mcg are recommended and included in prenatal multivitamins by the Department of Health in Australia [57], whereas the United States, the Centers for Disease Control and Prevention recommends 800 mcg per day [5,6,7] and in Canada at least 1000 mcg of FA are typically found in a prenatal multivitamin [51,58,59].
Women at high risk of pregnancy loss or who had a previous pregnancy affected by a NTD, are routinely prescribed FA doses of 4–5 mg in Australia, Canada, and the United States [6,7,9,46]. FA is considered to be better absorbed and therefore a measurement, known as total food folate daily folate equivalent (DFE) (see Box 1), has been devised by the Institute of Medicine and is the recommended method to provide comparability between synthetic FA and natural food folates whereby the folate from fortified food is weighted 1.7-fold greater than naturally occurring folate [10]. For example, a serving of food from leafy greens may provide 100 mcg of folate, which equals 100 mcg DFE; however, 100 mcg of folic acid in a fortified food is estimated to be 170 mcg DFE.
Box 1. Daily folate equivalent (DFE).
Total food folate DFE = mcg natural food folate mcg + synthetic folic acid from fortified foods and supplements times a bioavailability factor of 1.7 = mcg DFE/day (natural food folate) + (folic acid mcg × 1.7) [1,2] or 1 DFE equals 1 mcg food folate, 0.6 mcg synthetic folic acid consumed with food or 0.5 mcg consumed on an empty stomach with fortified food [3,4,5].
1.3. Upper Tolerable Limit of Folic Acid
Unlike 5-MTHF (and natural folate), FA has an upper tolerable limit (UL), which the US’ Institute of Medicine sets at 1000 mcg due to the potential for high FA intake to mask a vitamin B12 deficiency in the user [10]. The UL does not include naturally occurring folate from food or 5-MTHF as there is no recognized UL for these forms [28]. The studies used to determine this upper limit do not consider the potential impact of elevated FA intake on folate metabolism [16]. Apart from masking a vitamin B12 deficiency, the only documented risk factor for excessive FA intake is the presence of unmetabolized folic acid (UMFA) [13,16,19,21,60,61]. UMFA is the FA that cannot be metabolized to 5-MTHF due to limited DHFR activity and, therefore, starts to appear in the circulation even with 200 mcg of FA acid intake per day [62,63]. This UMFA has been linked to adverse health effects including reduced brain function and motor and somatosensory processing in offspring [64], cleft lip [65], asthma [52,66], autism [67] anemia [68], natural killer cell cytotoxicity [61], adverse cardiac events and cancer [21] and cognitive impairment [68].
There is a risk that women may be exposed to unintended adverse health outcomes through excess FA intake; for this reason, it is imperative that preconception and pregnancy folate intake from all sources for women of childbearing age is more clearly understood.
2. Materials and Methods
This review aimed to assess the total all-source intake of folate in women of childbearing age and in pregnancy in high-income countries with folate food fortification programs.
This systematic review was designed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.
We conducted a detailed search of the following electronic databases: MEDLINE (OVID), Embase, CINAHL (EBSCO), Scopus, and BiblioMAP. The search strategy is attached (File S1, Search Strategy) and was conducted on the seventh and eighth of October 2021. It included countries identified by the World Bank as high-income countries, pregnant women or women in preconception, folate exposure and source of folate.
Studies published from 1998 until the search date of 8 October 2021 were included. We wanted to include only studies that reflected total folate intake and therefore the start date of 1998 was selected as this is the date that mandatory FA fortification began in the U.S. and other countries around the world [10,63,69]. Scientific posters and conference abstracts were not included. There were no language limits. A PICO(T) format was used to formulate the search strategy and help design the research question. The population of interest (P) was identified as women of childbearing age either in preconception or pregnancy period from high-income countries (as identified by the World Bank [69] that have a mandatory fortification policy of fortifying foods with FA. The intervention/exposure (I) was folate intake of any form, that is dietary folate from non-fortified foods, dietary intake from fortified foods and/or supplements. A comparator (C) was not included. We excluded control studies and intervention studies except where the control group was representative of the study group and nutritional/supplemental data were available. The outcome (O) measured was intake of folate in any form in women of childbearing age, whether in preconception or pregnancy. This included folate from natural food sources such as leafy green vegetables, FA from fortified foods and all folate forms in supplementary dietary products and multivitamins.
Titles and abstracts were reviewed by one reviewer (CL). Manuscripts were included if they were from epidemiological observational studies that explicitly report on folate intake in women of reproductive age—specifically in preconception and pregnancy. There were no language restrictions. Studies were excluded if they did not report on folate intake (rather than folate status), included women who were breastfeeding and were not from countries with a mandatory fortification policy.
Full texts were reviewed and assessed based on the inclusion and exclusion criteria above. If full texts could not be accessed, we contacted the authors and/or publisher and if the full text could not be located they were excluded. Any uncertainty about the inclusion of a study was discussed by the other authors (AS, VS and AM) and consensus was gained.
Figure 4 presents the flowchart of study selection and inclusion. A total of 1885 studies were identified. After removing duplicates (n = 780) and excluded citations by title and abstract (n = 648), 457 full-text studies were assessed for eligibility. Of those, 421 were excluded because they were either not measuring folate intake, not from a country that had a mandatory fortification policy, not an academic paper, or had an incorrect study design or date range. This left a total of 36 studies for inclusion.
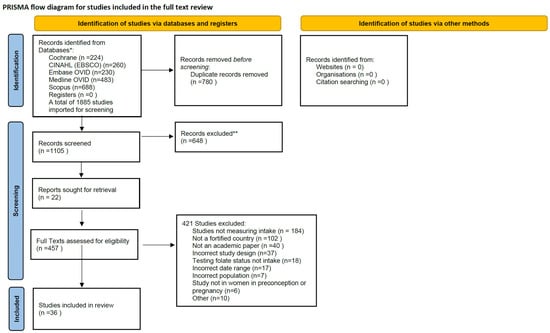
Figure 4.
PRISMA-P flowchart of study selection [70]. Quality Assessment of Study. * p < 0.01; ** p < 0.001.
Critical appraisal of the studies was conducting using the Axis tool for cross-sectional studies (Table S1). The studies were assessed for key issues such as suitability of the study to answer the research question, and possibility of bias being introduced into the study. All studies except five [2,3,66,71,72] detailed funding sources and sources of conflict.
All studies stated clear aims and objectives and the study design was appropriate for the aim.
The few areas that data were lacking was in the sample size justification with twenty [2,3,33,53,58,59,62,72,73,74,75,76,77,78,79,80,81,82,83,84] of the thirty-six studies giving data on sample size justification. Another question that was not answered by many studies was the area of non-responders; few studies (n = 9) [3,33,59,62,66,71,77,80,83] gave information about non-responders. Twenty one of the thirty six studies addressed and categorized non-responders. Clarity around statistical significance and/or precision estimates was also lacking.
3. Results
The 36 studies included in this review were separated into two categories, those sampling women of childbearing age (n = 18) and those sampling pregnant women (n = 21). Three studies were included in both categories. The study designs were predominantly cross-sectional or cohort and provided data about the folate intake from all sources in the sample populations. Our analysis of these studies identified four key areas of discussion or themes: natural folate intake, food fortification intake, supplement intake and the rate of women exceeding the upper tolerable limit of 1000 mcg FA.
3.1. Study Characteristics
3.1.1. Women of Childbearing Age
Table 1 shows summarized data from the 18 studies sampling non-pregnant women of childbearing age [1,2,3,4,5,33,53,72,77,78,81,82,84,85,86,87,88,89]. The age of women included in the studies was 17–49 years of age and sample sizes ranged from 51 participants to 9707, with a total of 52,580 women being sampled across all studies. All studies were from the United States (n = 15), Canada (n = 2) and Australia (n = 1).

Table 1.
Included studies sampling women of childbearing age, not pregnant.
3.1.2. Pregnant Women
Table 2 shows summarized data from 21 studies sampling pregnant women [50,51,52,58,59,62,66,71,73,74,75,76,78,79,80,83,88,90,91,92,93]. Two studies included women in their first trimester [71,75], nine studies included women in either their first or second trimester [50,59,62,66,74,76,80,92,93], one study included women with less than 26 weeks gestation [52], one study less than 27 weeks gestation [58], three studies in their second or third trimester [51,88,91] and five studies at any stage of gestation [73,78,79,83,90]. The sample size ranged from 51 participants to 2146, with a total of 16,314 sampled across all studies. All studies were from Canada (n = 8), United States (n = 7) or Australia (n = 7). One study was conducted before the mandatory folic acid fortification program commenced [76].

Table 2.
Included studies sampling pregnant women.
3.2. Natural Food Folate and Fortification
In women of childbearing age, the recommended intake of 400 mcg folate per day was not met by any study from natural folate sources (see Table 1). One U.S study found that fortified cereals and grains contributes approximately 70.8% of dietary folate [5]. The studies showed that the mean intake of folate from natural food folate ranged from 228.5 to 324.3 mcg/day after fortification [72], contributing to a 50% increase in serum folate concentrations and a 59% increase in RBC concentrations [53]. Another study showed that 43% of folate came from both natural and fortified foods [86]; however, food alone did not achieve the recommended 400 mcg/day for women in childbearing years. One study that investigated only food sources of folate found that 80% of women of reproductive age did not meet the recommended daily allowance (RDA) of 400 mcg per day [88], while another study showed that only 23% of women achieved the RDA of 400 mcg per day [83].
In Australia, two studies by Dorise and Beringer found that only 20% [74] and 55% [90] of pregnant women met the folate recommendations from diet alone (including natural food folate and fortified foods), respectively. In another study, folate was consistently below the estimated average requirement (EAR) of 520 mcg [78] particularly in the periconceptional period [80]. In Canada, 70% of women did not meet the EAR of 520 mcg of folate in first trimester of pregnancy [75] nor was the diet from food or fortification sufficient to achieve the minimum 400 mcg/day FA recommendation [50]. Food fortification and natural folates contributed approximately 303 mcg/day preconceptionally [92] and in pregnancy ranged from 282.2 mcg dietary folate equivalents (DFE)/day [83], 483 mcg DFE/day in early pregnancy [62] to 331 mcg/day in women 20–38 weeks gestation [88], and 465 mcg DFE/day in late pregnancy [62].
Table 3 outlines women’s folate intake by folate source or type. Of those studies that only measured natural food folate intake, women of childbearing age consumed between 170.92 and 259 mcg per day and pregnant women consumed between 140 and 483 mcg DFE. The average intake of folate from natural food folate was 205.48 mcg [1,4,5,33,89] in these studies.

Table 3.
Folate intake per day by natural food intake, fortified food intake, supplements and total.
Synthetic folic acids from fortified foods contributed 181.7 to 239 mcg per day in non-pregnant women and 96 to 768 mcg per day in pregnant women.
The combined food folate of natural and synthetic folic acid based on the conversion of folates to daily folate equivalents was as high as 500.5 mcg DFE (369.9 mcg) in women of preconception age [5] and 627.6 mcg DFE per day in pregnant women [83].
3.3. Supplementation and Upper Tolerable Limit
In women of childbearing age, supplements contributed 47.5% [53] to 57% of folate intake [85] and were the main reason why 2.4–7% of women exceeded the upper tolerable limit of 1000 mcg per day [4,53,84]. The intake of FA varied throughout the studies but was typically between 400 and 1000 mcg per day through the intake of supplements with folic acid. The percentage of women taking 400 mcg of FA per day ranged from 24% in one study [77] to approximately 78% in another [85], while women taking 1000 mcg of FA via supplements represented approximately 20% of the sample population [85,86].
The level of FA intake from vitamin supplements was much higher in pregnant women than in non-pregnant women due to the high rate of supplementation in pregnant populations. Specifically, FA supplements represented between 77% [79] and 92% [92] of FA intake in pregnant women with an estimation of supplementation contributing to 84% of FA intake in early pregnancy and 63% in late pregnancy [66]. While the intake of folic acid-based supplements was high in pregnant women, it was not in women in the preconception stage with one study reporting that 63% of women were not taking a prenatal with folate in the three months before falling pregnant [80].
In all studies reporting pregnant women exceeding the UL of folic acid, it was identified that FA-based supplements were the cause of their excess FA [50,52,58,59,66,73,75,76,79,80,93]. The percentage of women exceeding this UL ranged from 25% [58], 33.4% [73], 40% [79], 83–85% [59,62], 87% [75], 96% [50] to 100% [76]. It was estimated that supplements contributed to a mean daily intake of 878 mcg FA per day [79], 1000 mcg/day [59] to over 2000 mcg/day [50,66]. In one study, this overconsumption was more likely to occur in the first trimester [80].
It was difficult to evaluate a consistent method of overall intake of folate (food folate, fortified food, and supplements) as not all studies provide intake in DFEs and therefore only DFE levels will be reported. In women of childbearing age the total level of intake ranged from 864 mcg DFE [1] to 1778 DFE [86]. In pregnant women, total intake was much higher, ranging from 1451 mcg DFE [73] to 2181 mcg DFE [75]. The total DFE intake in pregnant women was recorded predominantly in Trimester 1 of pregnancy [50,62].
The overall intake of FA throughout pregnancy including supplements, not only caused an increased red blood cell folate [51,76] but contributed to total folate intakes up to 200% above the RDA [58], sometimes with a FA intake of up to 2948 mcg/day [66]. The studies measuring UMFA showed that it was measurable in all pregnant women [51] and even early first-trimester maternal plasma UMFA was detected in 97% of women and 93% of cord blood samples [62].
4. Discussion
4.1. Natural Food Intake and Fortification
There is a general consensus that women of childbearing age require a minimum of 400 mcg of FA per day in addition to their normal dietary intake to reduce the risk of neural tube defects [10] and, based on studies such as the ones included in this review, it is clear that women of childbearing age, are not receiving sufficient dietary folate to protect against neural tube defects, but rather they are receiving on average, half the recommended dose of folate from food. In response to studies that supported this finding of low folate intake from the diet, mandatory fortification of food products was introduced in the United States in 1998, at a level of 140 mcg per 100 g in all cereal grain products [11]. In Australia, mandatory fortification in wheat flour commenced in 2009 with 200 mcg per 100 g of wheat flour [94]. This regulation was based on modelling studies that showed that the increase in FA was expected to be approximately 100 mcg of additional FA per day; however, it is clear from the studies in this review that the intake of FA from foods alone is closer to 239 mcg or DFE 582, with the combined folate intake from natural food folate and FA fortification reaching close to the required intake of 400 mcg in women of childbearing age 500 mcg DFE. The fortification of folic acid was introduced to prevent neural tube defects in populations that were vulnerable to low levels of folate and by all accounts FA has been successful in achieving this [95,96,97,98,99,100]. The fortification program, however, did not cap the number of foods being fortified, nor did it consider populations that may be vulnerable to large amounts of FA like children and older adults [21,30,101] or tailor the intake levels via supplementation suggested for women in preconception and pregnancy.
4.2. Folate Measurement
The assessment of folate intake from food (natural folate and fortified foods) in the studies included in this review is based on the assumption that FA has better bioavailability than natural folates. However, these assumptions were arguably based on limited research do not align with emerging evidence. Natural folates are in a reduced form and are assumed to have nearly half the bioavailability of foods fortified with FA (synthetic FA fortified foods) [10]. This assumption is based on one study [102] with only ten female participants [10,102]. Researchers have since suggested that this finding may be incorrect [30] with studies investigating the bioavailability of natural folate to be anything from 78% of that of FA in food [103], and 98% for other foods [104]. A very detailed report on folate chemistry and metabolism suggests that folate polyglutamates (natural food folate) are not less bioavailable than monoglutamates (synthetic folic acid) [27]. DFE assumes that synthetic FA has a higher bioavailability compared with natural folate [33]. The measurement of food folate is difficult as confounding factors like loss of folate due to cooking, brush border enzyme activity, pH of the intestinal lumen, certain nutrients affecting absorption, and poor compliance in studies where participants are faced with difficult interventions [27,104,105,106], contribute to the accuracy of the study. It is important, therefore, that we gain a better understanding of folate metabolism because if we are underestimating the amount of natural folate that is bioavailable, this may have a bearing on FA fortification rates currently and future directions for many governments around the world. Further bioavailability research should also compare FA to the more active form, 5-MTHF, given studies have shown that bioavailability of both folinic acid and 5-MTHF are equivalent to FA as measured by changes in plasma folate levels [30,107].
4.3. Folate Metabolism
This review has highlighted the extent to which women of childbearing age, particularly in pregnancy, are exceeding the UL of FA, and this may be impacting on their folate metabolism. Bioavailability is very different to metabolism; although FA may be more bioavailable, it is not metabolized the same was as natural folate. Natural folate uptake by the brush boarder enzymes does depend on the type of food, the pH of intestinal tract and quality of the mucosa; however, there is no solid evidence that it is 50% less absorbed than FA and the studies show huge variation in percentage uptake of natural folates versus FA. Bioavailability is key because if FA is not metabolized sufficiently to 5-MTHF, which is the main circulating form of folate used by the placenta and brain, then the increased amount may be inhibiting the production of 5-MTHF and its re-uptake. It has been found that synthetic FA has no coenzyme activity until it can be reduced by dihydrofolate reductase (DHFR) [28] and that the DHFR enzyme is easily saturated which means that above a dose of 200–300 mcg the normal intestinal absorption of is saturated and rather than being converted by DHFR to dihydrofolate (DHF) or tetrahydrofolate (THF), we begin to see an accumulation of unmetabolized folic acid in the serum [14,62,108,109]. This then becomes an important question regarding both fortification and supplementation. If as studies in this review suggest, that the level of FA from fortification alone is reaching this saturation point for FA metabolism, and women, particularly in pregnancy are almost always exceeding this threshold, should the recommendation of further FA in preconception and pregnancy be changed to 5-MTHF to achieve the recommended folate level? 5-MTHF cannot accumulate [26,27] and is considered to be as good as FA, if not better in raising folate levels and preventing neural tube defects [23,24,25,46,110,111]. The studies in this review confirm that food-based folate is not sufficient to achieve recommended levels of folate in preconception and pregnancy; however, they do highlight that although FA may be considered to be absorbed better, it is not metabolized into 5-MTHF like natural folates and is accumulating instead.
Studies that have investigated 5-MTHF as a possible substitute for FA found that 5-MTHF at a dose of 7.5 mg every 12 h over four days rapidly increased serum total folate and has an advantage over FA as it can replete body stores in folate insufficient women within a few days [110]. 5-MTHF compared to folic acid was found to increase plasma folate levels more effectively [111]. Safety studies of high-dose 5-MTHF in rats showed no adverse events [112] and dosages as high as 17 mg of 5-MTHF daily for 12 weeks have been shown to have no side effects [113]. In pregnancy, there have been reported no side effects with 1.13 mg [114] and 7.5 mg and 15 mg 5-MTHF have been used without side effects in patients with depression [115,116]. 5-MTHF is more effective at improving folate status and may therefore be a better alternative to folic acid in those with infertility and recurrent pregnancy loss [24,46].
4.4. Unmetabolized Folic Acid
The fact that previous studies show people exposed to 200 mcg per day or less of FA are unlikely to have unmetabolized folic acid in serum, yet 300 to 400 mcg or above could lead to UMFA [16,109], suggest that all the women in the studies in this review in preconception or pregnancy would have levels of UMFA in their serum. Murphy et al. in their study, showed that all the women had the presence of UMFA irrespective of the dose [51] and Plumptre et al. found that 93% of cord blood samples had UMFA detected [62]. UMFA has been implicated in reduced natural killer cell activity in post-menopausal women [61], various forms of cancer, cardiovascular disease [21], autism [117] and orofacial clefts [118,119]. This has serious implications, given the studies in this review show that many women in preconception and almost all women in pregnancy have the presence of UMFA and are exceeding 400 mcg of FA per day. UMFA has also been found to cause a pseudo-MTHFR deficiency in rats [120] and reduce 5-MTHF. This effect has concerning implications as low folate is associated with uracil misincorporation [121], reduced DNA methylation [120] and changes in epigenetic patterns [122]. So too, the presence of UMFA has been linked to aberrant DNA methylation [123]. Methylation and the one carbon metabolism cycle (Figure 5) is a critical pathway required for oogenesis [124] and spermatogenesis [125,126] and is required for epigenesis and imprinting [127] and folate is required for the synthesis of purines and thymidylate for RNA and DNA synthesis [28], thus having implications for preconception, pregnancy, birth defects and the outcome of the offspring. The folic acid UTL of 1000 mcg has been set based on the potential for high folic acid intake to mask a vitamin B12 deficiency [16,28,62] and not the level at which serum UMFA appears. Based on the studies in this review, every single woman may exceed the level of FA intake where UMFA would appear. This is not the case with 5-MTHF which cannot accumulate and has a feedback loop to ensure that there is a homeostatic mechanism that prevents excessive levels of folate in tissues [28]. Previous research has shown that high-dose folic acid results in a global loss of methylation across the sperm methylome and leads to altered sperm epigenome and sperm DNA methylation, lower pregnancy rates, infertility rates and abnormal embryos [128,129].
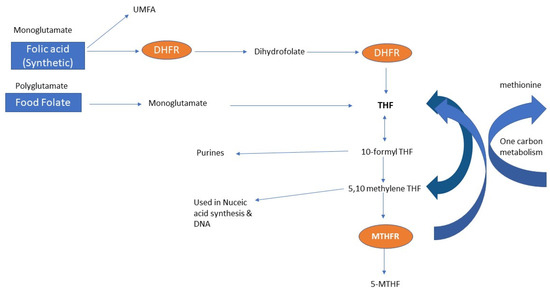
Figure 5.
Folate metabolism leading to one carbon metabolism—the end goal is for folates to be metabolized to 5-MTHF, where it is used by methionine synthase reductase (MTR) for one carbon metabolism and methylation reactions: 5-MTHF = 5-methyltetrahydrofolate reductase, DHFR = dihydrofolate reductase, THF = tetrahydrofolate, MTHFR: methylenetetrahydrofolate reductase, and UMFA: unmetabolized folic acid.
A lack of 5-MTHF contributes to an elevation in follicular levels of homocysteine [35,130] which correlates to adverse pregnancy outcomes as folate receptor-α has a high affinity for 5-MTHF and ensures cellular uptake where the folate receptor is expressed in the ovary, oocytes and human embryonic stem cells [32]. These biochemical mechanisms may underpin the reported links between MTHFR polymorphism and infertility [37,38,40,41,131], implantation failure [22] and recurrent pregnancy loss [24,36,46,130,132]. Such mechanisms may also explain preliminary research that has found replacing FA supplements with folinic acid or MTHF supplements may increase pregnancy success in women with MTHFR polymorphisms who have experienced recurrent pregnancy loss [133].
4.5. Upper Tolerable Limit
The studies in this review confirm that many women preconceptionally and almost all women at some stage in pregnancy are exceeding the UL of 1000 mcg/day. The upper limit seems to be almost inconsequential as it is simply a consideration of vitamin B12 masking and does not take into account the consideration of metabolism. If metabolism is a concern, then all women in pregnancy are exceeding the threshold and most women taking a prenatal multivitamin most certainly are as well. Given the documented concerns regarding the presence of UMFA, this upper tolerable limit needs to be re-evaluated.
5. Conclusions
Natural folates may not deliver the optimal amount of folate to reduce the risk of NTDs or methylate DNA sufficiently to prevent miscarriage. Women may be at risk of producing UMFA due to their intake of FA from food fortification. Any supplementation of FA over and above that has the potential to cause adverse health outcomes, reduce MTHFR activity, mask vitamin B12 deficiency, reduce methylation of DNA and therefore contribute to adverse pregnancy outcomes such as pregnancy loss. The results of this review warrant close attention from public health researchers and policy makers and may justify a review of the current approach to ensure folate sufficiency in the general population and avoidance of UMFA levels that may influence fertility and pregnancy outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14132715/s1, File S1: detailed search strategy; Table S1: Quality assessment of studies.
Author Contributions
Conceptualization, C.L., A.S., A.M. and V.S.; methodology, C.L. and A.S.; software, C.L.; validation, C.L., A.S., A.M. and V.S.; formal analysis, A.S.; investigation, C.L.; resources, A.S.; data curation, C.L.; writing—original draft preparation, C.L.; writing—review and editing, A.S., A.M. and V.S.; visualization, C.L.; supervision, A.S., A.M. and V.S.; project administration, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
NTDs: neural tube defects, FA: folic acid, UMFA: unmetabolized folic acid, THF: tetrahydrofolate, 5-MTHF: 5-methyltetrahydrofolate, MTHFR: methylenetetrahdyrofolate reductase, GPCII: glutamate carboxypeptidase II, PCFT: proton-coupled folate transporter, RFC: reduced folate carrier, DHFR: dihydrofolate reductase, MRP3: multidrug resistant-associated protein 3, EAR: estimated average requirement, RDA: recommended daily allowance, RDI: recommended daily intake, DFE: daily folate equivalents; FOLR1: folate receptor 1, DHF: dihydrofolate, and THF: tetrahydrofolate.
References
- Cena, E.R.; Joy, A.B.; Heneman, K.; Espinosa-Hall, G.; Garcia, L.; Schneider, C.; Wooten Swanson, P.C.; Hudes, M.; Zidenberg-Cherr, S. Folate Intake and Food-Related Behaviors in Nonpregnant, Low-Income Women of Childbearing Age. J. Am. Diet. Assoc. 2008, 108, 1364–1368. [Google Scholar] [CrossRef]
- Tinker, S.C.; Hamner, H.C.; Berry, R.J.; Bailey, L.B.; Pfeiffer, C.M. Does obesity modify the association of supplemental folic acid with folate status among nonpregnant women of childbearing age in the United States? Birth Defects Res. 2012, 94, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Brown, C.J.P.; Block, G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J. Am. Coll. Nutr. 2005, 24, 266–274. [Google Scholar] [CrossRef] [PubMed]
- French, M.R.; Barr, S.I.; Levy-Milne, R. Folate intakes and awareness of folate to prevent neural tube defects: A survey of women living in Vancouver, Canada. J. Am. Diet. Assoc. 2003, 103, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Mumford, S.L.; Chavarro, J.E.; Zhang, C.; Pollack, A.Z.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. The Impact of Dietary Folate Intake on Reproductive Function in Premenopausal Women: A Prospective Cohort Study. PLoS ONE 2012, 7, e46276. [Google Scholar] [CrossRef]
- Department of Health and Ageing. Nutrient Reference Values for Australia and New Zealand; Australian National Health and Medical Research Council, Ed.; Department of Health and Ageing: Canberra, Australia, 2006. [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. Morb. Mortal. Wkly. Rep. 1992, 41, 1–7. [Google Scholar]
- Bailey, L.B. Folate and vitamin B12 recommended intakes and status in the United States...including summary. Nutr. Rev. 2004, 62, S14–S21. [Google Scholar] [CrossRef]
- Czeizel, A.E. Primary prevention of neural-tube defects and some other major congenital abnormalities: Recommendations for the appropriate use of folic acid during pregnancy. Paediatr. Drugs 2000, 2, 437–449. [Google Scholar] [CrossRef]
- Institute of Medicine (US); Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- Food and Drug Administration. Food standards: Amendment of standards of identity for enriched grain products to require addition of folic acid; final rule (21 CFR Parts 136, 137, and 139). Fed. Regist. 1996, 61, 8781–8797. [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Maruvada, P.; Stover, P.J.; Mason, J.B.; Bailey, R.L.; Davis, C.D.; Field, M.S.; Finnell, R.H.; Garza, C.; Green, R.; Gueant, J.-L.; et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: A summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 2020, 112, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.R.; McPartlin, J.; Scott, J. Folic acid fortification and public health: Report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007, 7, 41. [Google Scholar] [CrossRef]
- Sweeney, M.R.; Staines, A.; Daly, L.; Traynor, A.; Daly, S.; Bailey, S.W.; Alverson, P.B.; Ayling, J.E.; Scott, J.M. Persistent circulating unmetabolised folic acid in a setting of liberal voluntary folic acid fortification. Implications for further mandatory fortification? BMC Public Health 2009, 9, 295. [Google Scholar] [CrossRef]
- Obeid, R.; Herrmann, W. The emerging role of unmetabolized folic acid in human diseases: Myth or reality? Curr. Drug Metab. 2012, 13, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Kasoha, M.; Kirsch, S.H.; Munz, W.; Herrmann, W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. Am. J. Clin. Nutr. 2010, 92, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Yang, J.; O’Connor, D.L. Unmetabolized folic acid and total folate concentrations in breast milk are unaffected by low-dose folate supplements. Am. J. Clin. Nutr. 2009, 89, 216–220. [Google Scholar] [CrossRef]
- Page, R.; Robichaud, A.; Arbuckle, T.E.; Fraser, W.D.; MacFarlane, A.J. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am. J. Clin. Nutr. 2017, 105, 1101–1109. [Google Scholar] [CrossRef]
- Page, R.; Wong, A.; Arbuckle, T.E.; MacFarlane, A.J. The MTHFR 677C>T polymorphism is associated with unmetabolized folic acid in breast milk in a cohort of Canadian women. Am. J. Clin. Nutr. 2019, 110, 401–409. [Google Scholar] [CrossRef]
- Smith, A.D.; Kim, Y.-I.; Refsum, H. Is folic acid good for everyone? Am. J. Clin. Nutr. 2008, 87, 517–533. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.O.; Shim, S.H.; Lee, Y.; Kim, J.H.; Jeon, Y.J.; Ko, J.J.; Lee, W.S.; Kim, N.K. Genetic Variation of Methylenetetrahydrofolate Reductase (MTHFR) and Thymidylate Synthase (TS) Genes Is Associated with Idiopathic Recurrent Implantation Failure. PLoS ONE 2016, 11, e0160884. [Google Scholar] [CrossRef]
- Cochrane, K.M.; Mayer, C.; Devlin, A.M.; Elango, R.; Hutcheon, J.A.; Karakochuk, C.D. Is natural (6 S)-5-methyltetrahydrofolic acid as effective as synthetic folic acid in increasing serum and red blood cell folate concentrations during pregnancy? A proof-of-concept pilot study. Trials 2020, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat. Med. 2013, 41, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. The metabolic basis for developmental disorders due to defective folate transport. Biochimie 2016, 126, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Shane, B.; Bailey, L. Folate chemistry and metabolism. Folate Health Dis. 1995, 2, 1–19. [Google Scholar]
- Pietrzik, K.; Bailey, L.; Shane, B. Folic acid and L-5-methyltetrahydrofolate. Clin. Pharmacokinet. 2010, 49, 535–548. [Google Scholar] [CrossRef]
- Qiu, A.; Jansen, M.; Sakaris, A.; Min, S.H.; Chattopadhyay, S.; Tsai, E.; Sandoval, C.; Zhao, R.; Akabas, M.H.; Goldman, I.D. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006, 127, 917–928. [Google Scholar] [CrossRef]
- Shane, B. Folate Fortification: Enough Already? Am. J. Clin. Nutr. 2003, 77, 8–9. [Google Scholar] [CrossRef]
- Patanwala, I.; King, M.J.; Barrett, D.A.; Rose, J.; Jackson, R.; Hudson, M.; Philo, M.; Dainty, J.R.; Wright, A.J.; Finglas, P.M. Folic acid handling by the human gut: Implications for food fortification and supplementation. Am. J. Clin. Nutr. 2014, 100, 593–599. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.M.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Devine, O.; Tinker, S.C.; Berry, R.J. Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: Have we reached optimal prevention? Am. J. Clin. Nutr. 2018, 107, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Yang, Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HuGE review. Am. J. Epidemiol. 2000, 151, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.K.; Shrestha, N.; Joshi, P.; Lakha, R.; Shrestha, S.; Sharma, L.; Chandra, A.; Singh, N.; Kc, Y.; Rijal, B. Association of parental methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism in couples with unexplained recurrent pregnancy loss. BMC Res. Notes 2018, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Vanilla, S.; Dayanand, C.D.; Kotur, P.F.; Moideen Kutty, A.; Vegi, P.K. Evidence of paternal N5, N10—methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism in couples with recurrent spontaneous Abortions (RSAs) in Kolar district—A south west of India. J. Clin. Diagn. Res. 2015, 9, BC15–BC18. [Google Scholar] [CrossRef] [PubMed]
- Altmae, S.; Stavreus-Evers, A.; Ruiz, J.R.; Laanpere, M.; Syvanen, T.; Yngve, A.; Salumets, A.; Nilsson, T.K. Variations in folate pathway genes are associated with unexplained female infertility. Fertil. Steril. 2010, 94, 130–137. [Google Scholar] [CrossRef]
- Gupta, N.; Sarkar, S.; David, A.; Gangwar, P.K.; Gupta, R.; Khanna, G.; Sankhwar, S.N.; Khanna, A.; Rajender, S. Significant impact of the MTHFR polymorphisms and haplotypes on male infertility risk. PLoS ONE 2013, 8, e69180. [Google Scholar] [CrossRef]
- Enciso, M.; Sarasa, J.; Xanthopoulou, L.; Bristow, S.; Bowles, M.; Fragouli, E.; Delhanty, J.; Wells, D. Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Hum. Genet. 2016, 135, 555–568. [Google Scholar] [CrossRef]
- Gava, M.M.; De Oliveira Chagas, E.; Bianco, B.; Christofolini, D.M.; Pompeo, A.C.L.; Glina, S.; Barbosa, C.P. Methylenetetrahydrofolate reductase polymorphisms are related to male infertility in Brazilian men. Genet. Test. Mol. Biomark. 2011, 15, 153–157. [Google Scholar] [CrossRef]
- Gava, M.M.; Kayaki, E.A.; Bianco, B.; Teles, J.S.; Christofolini, D.M.; Pompeo, A.C.; Glina, S.; Barbosa, C.P. Polymorphisms in folate-related enzyme genes in idiopathic infertile Brazilian men. Reprod. Sci. 2011, 18, 1267–1272. [Google Scholar] [CrossRef]
- Hong, H.H.; Hu, Y.; Yu, X.Q.; Zhou, L.; Lv, M.Q.; Sun, Y.; Ren, W.J.; Zhou, D.X. Associations of C677T polymorphism in methylenetetrahydrofolate reductase (MTHFR) gene with male infertility risk: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 101–109. [Google Scholar] [CrossRef]
- Ledowsky, C.; Steel, A.; Schloss, J. Methylenetetrahydrofolate Reductase (MTHFR) genetic polymorphisms and the risk of infertility in couples accessing Assisted Reproductive technologies. Adv. Integr. Med. 2021, 8, 220–229. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5-methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Savella, G.M.; Church, T.R.; Goez-Mogollon, L.; Sosinsky, A.Z.; Noe, O.B.; Kaimal, A.; Cohen, L.S. A prenatal supplement with methylfolate for the treatment and prevention of depression in women trying to conceive and during pregnancy. Ann. Clin. Psychiatry 2019, 31, 4–16. [Google Scholar]
- Servy, E.J.; Jacquesson-Fournols, L.; Cohen, M.; Menezo, Y.J. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: A key to pregnancy outcome: A case series. J. Assist. Reprod. Genet. 2018, 35, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Hekmatdoost, A.; Vahid, F.; Yari, Z.; Sadeghi, M.; Eini-Zinab, H.; Lakpour, N.; Arefi, S. Methyltetrahydrofolate vs folic acid supplementation in idiopathic recurrent miscarriage with respect to methylenetetrahydrofolate reductase C677T and A1298C polymorphisms: A randomized controlled trial. PLoS ONE 2015, 10, e0143569. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Vaesken Mde, L.; Alonso-Aperte, E.; Varela-Moreiras, G. Contribution of folic acid-fortified foods to fertile women’s folate Recommended Nutrient Intake through breakfast simulation models. Public Health Nutr. 2015, 18, 1960–1968. [Google Scholar] [CrossRef]
- Obeid, R.; Kirsch, S.H.; Kasoha, M.; Eckert, R.; Herrmann, W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism 2011, 60, 673–680. [Google Scholar] [CrossRef]
- Murphy, M.; Muldoon, K.A.; Harvey, A.L.J.; Rose, E.G.; Erwin, E.; Rennicks White, R.; MacFarlane, A.J.; Wen, S.W.; Walker, M.C. Gestational Folate and Folic Acid Intake among Women in Canada at Higher Risk of Pre-Eclampsia. J. Nutr. 2021, 151, 1976–1982. [Google Scholar]
- Murphy, M.S.Q.; Muldoon, K.A.; Sheyholislami, H.; Behan, N.; Lamers, Y.; Rybak, N.; White, R.R.; Harvey, A.L.J.; Gaudet, L.M.; Smith, G.N.; et al. Impact of high-dose folic acid supplementation in pregnancy on biomarkers of folate status and 1-carbon metabolism: An ancillary study of the Folic Acid Clinical Trial (FACT). Am. J. Clin. Nutr. 2021, 113, 1361–1371. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Sharma, S.; Rifas-Shiman, S.L.; Camargo, C.A.; Weiss, S.T.; Oken, E.; Gillman, M.W.; Gold, D.R.; DeMeo, D.L.; Litonjua, A.A. Folic Acid in Pregnancy and Childhood Asthma: A US Cohort. Clin. Pediatr. 2018, 57, 421–427. [Google Scholar] [CrossRef]
- Yang, Q.H.; Carter, H.K.; Mulinare, J.; Berry, R.J.; Friedman, J.M.; Erickson, J.D. Race-ethnicity differences in folic acid intake in women of childbearing age in the United States after folic acid fortification: Findings from the National Health and Nutrition Examination Survey, 2001–2002. Am. J. Clin. Nutr. 2007, 85, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control & Prevention. Knowledge and use of folic acid by women of childbearing age—United States, 1995 and 1998. MMWR—Morb. Mortal. Wkly. Rep. 1999, 48, 325–327. [Google Scholar]
- Centers for Disease Control & Prevention. Knowledge and use of folic acid among women of reproductive age—Michigan, 1998. MMWR—Morb. Mortal. Wkly. Rep. 2001, 50, 185–189. [Google Scholar]
- Centers for Disease Control & Prevention. Use of vitamins containing folic acid among women of childbearing age—United States, 2004. MMWR—Morb. Mortal. Wkly. Rep. 2004, 53, 847–850. [Google Scholar]
- Commonwealth of Australia. Reproductive Health; Department of Health, Ed.; Commonwealth of Australia: Canberra, Australia, 2020. [Google Scholar]
- Gómez, M.F.; Field, C.J.; Olstad, D.L.; Loehr, S.; Ramage, S.; McCargar, L.J.; Kaplan, B.J.; Dewey, D.; Bell, R.C.; Bernier, F.P.; et al. Use of micronutrient supplements among pregnant women in Alberta: Results from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Matern. Child Nutr. 2015, 11, 497–510. [Google Scholar] [CrossRef]
- Masih, S.P.; Plumptre, L.; Ly, A.; Berger, H.; Lausman, A.Y.; Croxford, R.; Kim, Y.I.; O’Connor, D.L. Pregnant Canadian Women Achieve Recommended Intakes of One-Carbon Nutrients through Prenatal Supplementation but the Supplement Composition, Including Choline, Requires Reconsideration. J. Nutr. 2015, 145, 1824–1834. [Google Scholar] [CrossRef]
- Kim, Y.I. Will mandatory folic acid fortification prevent or promote cancer? Am. J. Clin. Nutr. 2004, 80, 1123–1128. [Google Scholar] [CrossRef]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E.; et al. Unmetabolized Folic Acid in Plasma Is Associated with Reduced Natural Killer Cell Cytotoxicity among Postmenopausal Women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Kyoung-Jin, S.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Young-In, K. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Quinlivan, E.P.; Gregory, J.F., III. Effect of food fortification on folic acid intake in the United States. Am. J. Clin. Nutr. 2003, 77, 221–225. [Google Scholar] [CrossRef]
- Azaryah, H.; Verdejo-Román, J.; Martin-Pérez, C.; García-Santos, J.A.; Martínez-Zaldívar, C.; Torres-Espínola, F.J.; Campos, D.; Koletzko, B.; Pérez-García, M.; Catena, A.; et al. Effects of Maternal Fish Oil and/or 5-Methyl-Tetrahydrofolate Supplementation during Pregnancy on Offspring Brain Resting-State at 10 Years Old: A Follow-Up Study from the NUHEAL Randomized Controlled Trial. Nutrients 2020, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Rozendaal, A.M.; van Essen, A.J.; te Meerman, G.J.; Bakker, M.K.; van der Biezen, J.J.; Goorhuis-Brouwer, S.M.; Vermeij-Keers, C.; de Walle, H.E. Periconceptional folic acid associated with an increased risk of oral clefts relative to non-folate related malformations in the Northern Netherlands: A population based case-control study. Eur. J. Epidemiol. 2013, 28, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Whitrow, M.J.; Moore, V.M.; Rumbold, A.R.; Davies, M.J. Effect of supplemental folic acid in pregnancy on childhood asthma: A prospective birth cohort study. Am. J. Epidemiol. 2009, 170, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- DeVilbiss, E.A.; Magnusson, C.; Gardner, R.M.; Rai, D.; Newschaffer, C.J.; Lyall, K.; Dalman, C.; Lee, B.K. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: Population based cohort study. BMJ 2017, 359, j4273. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am. J. Clin. Nutr. 2010, 91, 1733–1744. [Google Scholar] [CrossRef]
- World Bank. List of High Income Countries. Available online: https://data.worldbank.org/country/XD (accessed on 21 October 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 2013, 177, 1338–1347. [Google Scholar] [CrossRef]
- Mojtabai, R. Body mass index and serum folate in childbearing age women. Eur. J. Epidemiol. 2004, 19, 1029–1036. [Google Scholar] [CrossRef]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L., III; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef]
- Dorise, B.; Byth, K.; McGee, T.; Wood, A.; Blumenthal, C. A low intensity dietary intervention for reducing excessive gestational weight gain in an overweight and obese pregnant cohort. Eat. Weight Disord. 2020, 25, 257–263. [Google Scholar] [CrossRef]
- Dubois, L.; Diasparra, M.; Bedard, B.; Colapinto, C.K.; Fontaine-Bisson, B.; Morisset, A.S.; Tremblay, R.E.; Fraser, W.D. Adequacy of nutritional intake from food and supplements in a cohort of pregnant women in Québec, Canada: The 3D Cohort Study (Design, Develop, Discover). Am. J. Clin. Nutr. 2017, 106, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Furness, D.; Fenech, M.; Dekker, G.; Khong, T.Y.; Roberts, C.; Hague, W. Folate, Vitamin B12, Vitamin B6 and homocysteine: Impact on pregnancy outcome. Matern. Child Nutr. 2013, 9, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Hamner, H.C.; Tinker, S.C.; Flores, A.L.; Mulinare, J.; Weakland, A.P.; Dowling, N.F. Modelling fortification of corn masa flour with folic acid and the potential impact on Mexican-American women with lower acculturation. Public Health Nutr. 2013, 16, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Hure, A.; Young, A.; Smith, R.; Collins, C.; Hure, A.; Young, A.; Smith, R.; Collins, C. Diet and pregnancy status in Australian women. Public Health Nutr. 2009, 12, 853–861. [Google Scholar] [CrossRef]
- Jun, S.; Gahche, J.J.; Potischman, N.; Dwyer, J.T.; Guenther, P.M.; Sauder, K.A.; Bailey, R.L. Dietary Supplement Use and Its Micronutrient Contribution During Pregnancy and Lactation in the United States. Obstet. Gynecol. 2020, 135, 623–633. [Google Scholar] [CrossRef]
- Livock, M.; Anderson, P.J.; Lewis, S.; Bowden, S.; Muggli, E.; Halliday, J. Maternal micronutrient consumption periconceptionally and during pregnancy: A prospective cohort study. Public Health Nutr. 2017, 20, 294–304. [Google Scholar] [CrossRef]
- Marchetta, C.M.; Hamner, H.C. Blood folate concentrations among women of childbearing age by race/ethnicity and acculturation, NHANES 2001–2010. Matern. Child Nutr. 2016, 12, 39–50. [Google Scholar] [CrossRef]
- Rai, D.; Bird, J.K.; McBurney, M.I.; Chapman-Novakofski, K.M. Nutritional status as assessed by nutrient intakes and biomarkers among women of childbearing age-is the burden of nutrient inadequacies growing in America? Public Health Nutr. 2015, 18, 1658–1669. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.W.; Song, W.O. Pre-Pregnancy Weight Status Is Associated with Diet Quality and Nutritional Biomarkers during Pregnancy. Nutrients 2016, 8, 162. [Google Scholar] [CrossRef]
- Tinker, S.C.; Cogswell, M.E.; Hamner, H.C.; Berry, R.J. Usual folic acid intakes: A modelling exercise assessing changes in the amount of folic acid in foods and supplements, National Health and Nutrition Examination Survey, 2003–2008. Public Health Nutr. 2012, 15, 1216–1227. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Afeiche, M.C.; Wright, D.L.; Toth, T.L.; Williams, P.L.; Gillman, M.W.; Hauser, R.; Chavarro, J.E. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet. Gynecol. 2014, 124, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Mínguez-Alarcón, L.; Fong, K.C.; Abu Awad, Y.; Di, Q.; Chavarro, J.E.; Ford, J.B.; Coull, B.A.; Schwartz, J.; Kloog, I.; et al. Supplemental Folate and the Relationship between Traffic-Related Air Pollution and Livebirth among Women Undergoing Assisted Reproduction. Am. J. Epidemiol. 2019, 188, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Rich-Edwards, J.W.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Ginsburg, E.S.; Missmer, S.A.; Chavarro, J.E. Maternal prepregnancy folate intake and risk of spontaneous abortion and stillbirth. Obstet. Gynecol. 2014, 124, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Pick, M.E.; Edwards, M.; Moreau, D.; Ryan, E.A. Assessment of diet quality in pregnant women using the Healthy Eating Index. J. Am. Diet. Assoc. 2005, 105, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Sotres-Alvarez, D.; Siega-Riz, A.M.; Herring, A.H.; Carmichael, S.L.; Feldkamp, M.L.; Hobbs, C.A.; Olshan, A.F. Maternal dietary patterns are associated with risk of neural tube and congenital heart defects. Am. J. Epidemiol. 2013, 177, 1279–1288. [Google Scholar] [CrossRef]
- Beringer, M.; Schumacher, T.; Keogh, L.; Sutherland, K.; Knox, P.; Herden, J.; Brown, L.; Rae, K. Nutritional adequacy and the role of supplements in the diets of Indigenous Australian women during pregnancy. Midwifery 2021, 93, 102886. [Google Scholar] [CrossRef]
- Hromi-Fiedler, A.; Bermúdez-Millán, A.; Segura-Pérez, S.; Pérez-Escamilla, R. Nutrient and food intakes differ among Latina subgroups during pregnancy. Public Health Nutr. 2012, 15, 341–351. [Google Scholar] [CrossRef]
- Martinussen, M.P.; Risnes, K.R.; Jacobsen, G.W.; Bracken, M.B. Folic acid supplementation in early pregnancy and asthma in children aged 6 years. Am. J. Obstet. Gynecol. 2012, 206, e71–e77. [Google Scholar] [CrossRef]
- Roy, A.; Evers, S.E.; Campbell, M.K. Dietary supplement use and iron, zinc and folate intake in pregnant women in London, Ontario. Chronic Dis. Inj. Can. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Standard 2.1.1; Australian New Zealand Food Standards Code—Cereals and Cereal Products. Australian Government, Federal Register of Legislation: Canberra, Australia, 2010.
- Božović, I.B.; Vraneković, J. Folate and folic acid: Current knowledge and gaps. Med. Flum. 2014, 50, 169–175. [Google Scholar]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folic acid and pregnancy: Properties, medical uses and health benefits. In Folic Acid: Properties, Medical Uses and Health Benefits; Nova Science Publishers, Inc.: Happauge, NY, USA, 2012; pp. 1–37. [Google Scholar]
- Fitzpatrick, A. Folate (folic acid): Implications for health and disease. Agro Food Ind. Hi-Tech 2003, 14, 45–48. [Google Scholar]
- Haggarty, P.; Campbell, D.M.; Duthie, S.; Andrews, K.; Hoad, G.; Piyathilake, C.; Fraser, I.; McNeill, G. Folic acid use in pregnancy and embryo selection. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Imbard, A.; Benoist, J.F.; Blom, H.J. Neural tube defects, folic acid and methylation. Int. J. Environ. Res. Public Health 2013, 10, 4352–4389. [Google Scholar] [CrossRef] [PubMed]
- Rossokha, Z.; Gorovenko, N. Assessment of the individual folic acid doses requirement for patients with reproductive disorders. J. Perinat. Med. 2017, 45, 493. [Google Scholar]
- Bjork, M.; Riedel, B.; Spigset, O.; Veiby, G.; Kolstad, E.; Daltveit, A.K.; Gilhus, N.E. Association of Folic Acid Supplementation during Pregnancy with the Risk of Autistic Traits in Children Exposed to Antiepileptic Drugs In Utero. JAMA Neurol. 2018, 75, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sauberlich, H.; Kretsch, M.; Skala, J.; Johnson, H.; Taylor, P. Folate requirement and metabolism in nonpregnant women. Am. J. Clin. Nutr. 1987, 46, 1016–1028. [Google Scholar] [CrossRef]
- Winkels, R.M.; Brouwer, I.A.; Siebelink, E.; Katan, M.B.; Verhoef, P. Bioavailability of food folates is 80% of that of folic acid. Am. J. Clin. Nutr. 2007, 85, 465–473. [Google Scholar] [CrossRef]
- Brouwer, I.A.; van Dusseldorp, M.; West, C.E.; Meyboom, S.; Thomas, C.M.; Duran, M.; van het Hof, K.H.; Eskes, T.K.; Hautvast, J.G.; Steegers-Theunissen, R.P. Dietary folate from vegetables and citrus fruit decreases plasma homocysteine concentrations in humans in a dietary controlled trial. J. Nutr. 1999, 129, 1135–1139. [Google Scholar] [CrossRef]
- Hannon-Fletcher, M.P.; Armstrong, N.C.; Scott, J.M.; Pentieva, K.; Bradbury, I.; Ward, M.; Strain, J.; Dunn, A.A.; Molloy, A.M.; Kerr, M.A. Determining bioavailability of food folates in a controlled intervention study. Am. J. Clin. Nutr. 2004, 80, 911–918. [Google Scholar] [CrossRef]
- McNulty, H.; Pentieva, K. Folate bioavailability. Proc. Nutr. Soc. 2004, 63, 529–536. [Google Scholar] [CrossRef]
- Pietrzik, K.; Remer, T. Zur Bioverfügbarkeitsprüfung von Mikronährstoffen. Z. Ernährungswiss. 1989, 28, 130–141. [Google Scholar] [CrossRef]
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.R.; McPartlin, J.; Weir, D.G.; Scott, J.M. Measurements of sub-nanomolar concentrations of unmetabolised folic acid in serum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 788, 187–191. [Google Scholar] [CrossRef]
- Bailey, S.W.; Ayling, J.E. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci. Rep. 2018, 8, 4096. [Google Scholar] [CrossRef]
- Prinz-Langenohl, R.; Brämswig, S.; Tobolski, O.; Smulders, Y.; Smith, D.; Finglas, P.; Pietrzik, K. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C → T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 2009, 158, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, K.E.; Dahms, I.; Broschard, T.H.; Boehni, R.; Moser, R. Safety evaluation of calcium L-methylfolate. Toxicol. Rep. 2019, 6, 1018–1030. [Google Scholar] [CrossRef]
- Bostom, A.G.; Shemin, D.; Bagley, P.; Massy, Z.A.; Zanabli, A.; Christopher, K.; Spiegel, P.; Jacques, P.F.; Dworkin, L.; Selhub, J. Controlled comparison of L-5-methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. Circulation 2000, 101, 2829–2832. [Google Scholar] [CrossRef]
- Bentley, S.; Hermes, A.; Phillips, D.; Daoud, Y.A.; Hanna, S. Comparative Effectiveness of a Prenatal Medical Food to Prenatal Vitamins on Hemoglobin Levels and Adverse Outcomes: A Retrospective Analysis. Clin. Ther. 2011, 33, 204–210. [Google Scholar] [CrossRef]
- Fava, M.; Shelton, R.C.; Zajecka, J.M. Evidence for the use of l-methylfolate combined with antidepressants in MDD. J. Clin. Psychiatry 2011, 72, 27693. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Etemad, B.; Rickels, K.; Clain, A.; Baer, L.; Dalton, E.D.; Sacco, G.R.; Schoenfeld, D. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: Results of two randomized, double-blind, parallel-sequential trials. Am. J. Psychiatry 2012, 169, 1267–1274. [Google Scholar] [CrossRef]
- Beard, C.M.; Panser, L.A.; Katusic, S.K. Is excess folic acid supplementation a risk factor for autism? Med. Hypotheses 2011, 77, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Vereczkey, A.; Bánhidy, F. Cleft lip and palate: Etiology and prevention. In Cleft Lip and Palate: Etiology, Surgery and Repair and Sociopsychological Consequences; Nova Science Publishers, Inc.: Happauge, NY, USA, 2013; pp. 1–53. [Google Scholar]
- Ray, J.G.; Meier, C.; Vermeulen, M.J.; Wyatt, P.R.; Cole, D.E. Association between folic acid food fortification and congenital orofacial clefts. J. Pediatr. 2003, 143, 805–807. [Google Scholar] [CrossRef]
- Christensen, K.E.; Mikael, L.G.; Leung, K.-Y.; Lévesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification—Its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Pickell, L.; Brown, K.; Li, D.; Wang, X.L.; Deng, L.; Wu, Q.; Selhub, J.; Luo, L.; Jerome-Majewska, L.; Rozen, R. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res. Part A—Clin. Mol. Teratol. 2011, 91, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Canovas, S.; Ross, P.J.; Kelsey, G.; Coy, P. DNA Methylation in Embryo Development: Epigenetic Impact of ART (Assisted Reproductive Technologies). Bioessays 2017, 39, 1700106. [Google Scholar] [CrossRef]
- Cisneros, F.J. DNA methylation and male infertility. Front. Biosci. 2004, 9, 1189–1200. [Google Scholar] [CrossRef]
- Santi, D.; De Vincentis, S.; Magnani, E.; Spaggiari, G. Impairment of sperm DNA methylation in male infertility: A meta-analytic study. Andrology 2017, 5, 695–703. [Google Scholar] [CrossRef]
- Kelly, T.L.J.; Trasler, J.M. Reproductive epigenetics. Clin. Genet. 2004, 65, 247–260. [Google Scholar] [CrossRef]
- Aarabi, M.; San Gabrie, M.C.; Chan, D.; Behan, N.A.; Caron, M.; Pastinen, T.; Bourque, G.; MacFarlane, A.J.; Zini, A.; Trasler, J. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum. Mol. Genet. 2015, 24, 6301–6313. [Google Scholar] [CrossRef] [PubMed]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 2013, 4, 2889. [Google Scholar] [CrossRef] [PubMed]
- Dauglait, K.; Serapinas, D. The importance of MTHFR gene mutation detection in patient with recurrent miscarriages. Genetika 2015, 47, 609–616. [Google Scholar] [CrossRef]
- Eloualid, A.; Abidi, O.; Charif, M.; El Houate, B.; Benrahma, H.; Louanjli, N.; Chadli, E.; Ajjemami, M.; Barakat, A.; Bashamboo, A.; et al. Association of the MTHFR A1298C variant with unexplained severe male infertility. PLoS ONE 2012, 7, e34111. [Google Scholar] [CrossRef]
- Khaleghparast, A.; Khaleqparast, S.; Khaleghparast, H. Association between the A1298C polymorphism of the methylenetetrahydrofolate reductase gene and recurrent spontaneous abortion. Iran. J. Neonatol. 2014, 5, 7–11. [Google Scholar] [CrossRef]
- Ledowsky, C. The different forms and dosages of folate that practitioners specialising in infertility support prescribe to patients with the MTHFR genetic polymorphisms: A case series. Aust. J. Herb. Naturop. Med. 2021, 33, 20. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).