Abstract
The importance of B complex vitamins starts early in the human life cycle and continues across its different stages. At the same time, numerous reports have emphasized the critical role of adequate B complex intake. Most studies examined such issues concerning a specific vitamin B or life stage, with the majority reporting the effect of either excess or deficiency. Deep insight into the orchestration of the eight different B vitamins requirements is reviewed across the human life cycle, beginning from fertility and pregnancy and reaching adulthood and senility, emphasizing interactions among them and underlying action mechanisms. The effect of sex is also reviewed for each vitamin at each life stage to highlight the different daily requirements and/or outcomes. Thiamine, riboflavin, niacin, pyridoxine, and folic acid are crucial for maternal and fetal health. During infancy and childhood, B vitamins are integrated with physical and psychological development that have a pivotal impact on one’s overall health in adolescence and adulthood. A higher intake of B vitamins in the elderly is also associated with preventing some aging problems, especially those related to inflammation. All supplementation should be carefully monitored to avoid toxicity and hypervitaminosis. More research should be invested in studying each vitamin individually concerning nutritional disparities in each life stage, with extensive attention paid to cultural differences and lifestyles.
1. Introduction
Vitamins are a group of organic compounds necessary for normal physiological functions. Their established daily intake must be met as they are essential elements that cannot be endogenously synthesized. The water-soluble vitamins comprise vitamin C and B complex vitamins: Thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), biotin (B7), folate (B9), and cobalamin (B12). The B vitamins are not categorized according to their chemical structural resemblance, but rather concerning their water solubility and the inter-related cellular coenzyme purposes they undergo [1]. Concerning their origins, the primary source of B vitamins, except vitamin B12, is dietary food sources. Their synthesis in chloroplasts, mitochondria, and cytosol is regulated based on the plant’s changeable needs [2]. Vitamin B12 is produced by bacteria and is typically sequestered from animal-derived foods, with synthesis to occur, for example, in the foregut of ruminant animals [1]. The structures of different B vitamins are depicted in Figure 1.
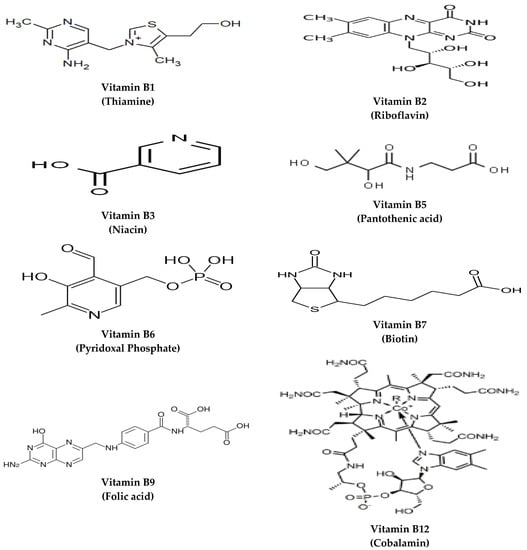
Figure 1.
The structures of B vitamins.
Generally, it is claimed that the recommended dietary allowance (RDA) of vitamins during the life cycle may differ owing to the variation in both metabolic and functional demand for vitamins at this stage. Hence, humans in different locations might be vulnerable to the risk of inadequacy, and its impact on human health varies according to the extent of deficiency [3].
For instance, vitamin B12 deficiency can be manifested as megaloblastic anemia that might progress to funicular myelosis as an end-stage disease [4]. The risk of developing such deficiency exponentially increases in stages of higher demand, such as pregnancy and early childhood. In addition, pregnancy is a critical stage in life, requiring adequate micronutrient supplementation.
Special consideration is given to folate to prevent the risk of neural tube defects [5]. Moreover, the risk of having a small-for-gestational-age child is fourfold in pregnant women with inadequate diets, especially in folate and ferritin.
Herein, the current review presents the most updated information from the scientific literature regarding B complex vitamins’ dietary requirements, metabolism, interaction, and importance in different life stages. At first, each dietary vitamin source, metabolism, and associated deficiencies are presented. Next, analysis from recent research on the diverse needs of vitamin B complex is offered at different life stages, i.e., preconception, pregnancy and lactation, pediatrics, adult, and old life, and in the context of sex type, i.e., male and female.
2. B Vitamins Orchestration in Different Life Stages
As mentioned above, during human life, the daily requirements of B vitamins vary according to the demands of each life stage.
During the critical stage of pregnancy and lactation, maternal balanced nutrition and adequate micronutrient intake have a crucial impact on the health and survival of both the mother and fetus before and during the gestational period and later through their life. This stage is characterized by profound and rapid physiological alterations that might predispose women to disorders such as obesity, insulin resistance, and calcium deficiency associated with osteopenia [6]. In addition, physical and cognitive functions during childhood and leading up to adulthood can be affected significantly due to poor maternal nutrition and inadequate intake of micronutrients such as B vitamins [7]. Insufficient nutrition and micronutrient deficiency contribute to depression and stress, affecting the mother’s health and, consequently, her baby’s status [8], which in turn obliges a nutrient-dense diet and social support. It is noteworthy that although some vitamins are crucial during these stages of life, others, such as biotin significance, were not reported during pregnancy and lactation, which is likely attributed to its endogenous synthesis, which makes biotin deficiency and complications very scarce at such life stages.
In infancy, evidence has shown that environmental factors such as diet and physical activity can affect infants’ physiological and psychological development, affecting their health and longevity in adulthood. There is also a growing interest in comparing breast and formula feeding in early infancy and their impact later in life, particularly concerning developing diseases such as obesity, hypertension, hyperlipidemia, and diabetes mellitus [9].
Later in childhood, developing good habits for living a healthy lifestyle can help improve their performance in school and other adult life stages, in addition to preventing later-life non-communicable diseases such as obesity and all related metabolic disorders, pulmonary disorders, anemia, and dental caries [5]. Addressing nutritional disparities among pediatric populations is an international endeavor. However, it can help guide future research and development of policies and programs aimed at fulfilling the daily requirements of vitamins and minerals during this stage of life, especially B vitamins.
In adulthood, good nutrition also plays a crucial role in attaining and sustaining a healthy lifestyle due to the complex link between diet and health. Nutrition at this stage is often linked to various factors such as biological, environmental, and cultural factors and is often a reflection of the early nutritional status [10]. The potential risks of weight loss, cardio-respiratory disorders, gastrointestinal-related disorders, impaired immune response, and wound healing increase with malnutrition [11].
In the elderly, it is worth mentioning that during the next five decades, the proportion of people over 60 is expected to rise from 11% to 22%, estimated to be 2 billion [12]. Hence, it is imperative nowadays to focus on the nutritional status of the elderly population to prevent the risk of Alzheimer’s disease, diabetes, obesity, insulin resistance, metabolic syndrome, and cardiovascular diseases [13], with careful attention to the recommended daily intake of B vitamins. In addition, other disorders are also related to malnutrition in the elderly such as osteoporosis, skeletal muscle wasting, and impaired wound healing. All these are related to inflammation and immunosenescence due to aging [14].
Though a sufficient quantity of the B vitamins should be attained from a healthy diet, growing evidence suggests that humans at specific locations have increasing demands of one or more of the B vitamins, such as childhood or pregnancy, which might pose a negative impact on overall health and or brain functions as explained in detail in the following subsections. The deficiency of these vitamins in different life stages is crucial and might predispose humans to a multitude of disorders, as illustrated in Figure 2.
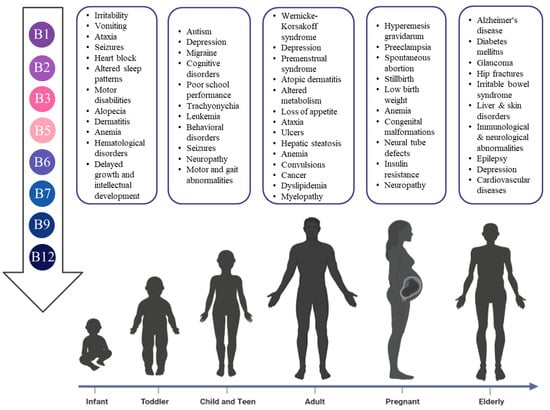
Figure 2.
The risks of B vitamins deficiency in different life stages.
2.1. Thiamine
The recommended daily intake, main action, and natural sources of vitamin B1 in different life stages are illustrated in Table 1.

Table 1.
Recommended daily intake (RDI), main action, and natural sources of vitamin B1 (thiamine) in different life stages.
Pregnancy and lactation: Thiamine deficiency in pregnancy can result from an extreme case of nausea and vomiting known as Hyperemesis gravidarum (HG), which could lead to Wernicke’s encephalopathy (WE) and Korsakoff syndrome (KS). The most observed WE symptoms are eye movement disorders, which were present in 86.4% of cases, altered mental status or cognitive abilities in 83.6%, and ataxia in 83.1%. Treatment of HG comprises 700 mg/day parenterally of thiamine and lower doses (435 mg/day) of thiamine for HG cases with altered cognitive abilities [15].
Thiamine deficiency most commonly occurs during lactation, pregnancy, and increased physical activity. The central nervous system oversees 20% of the body’s metabolic activity and therefore has high-energy requirements, with brain energy primarily coming from glucose. Impaired glucose metabolism is thus known to cause neurological illnesses, necessitating thiamine supplementation for both mother and infant health [16].
Infancy: Infantile thiamine intake for the first six months of life depends on maternal intake. Maternal supplements are recommended to avoid impaired infant growth in the case of deficiency. Thiamine in human milk exists as either free thiamine or thiamine monophosphate, with the phosphorylated forms unable to pass through membranes [16]. Exclusively breastfed infants have the risk of thiamine deficiency. The risk of thiamine deficiency increases with delayed complementary feeding. Infantile thiamine deficiency could also occur due to the consumption of soy-based formula, especially in the case of vegetarian-based diets that are thiamine-deficient [17]. In infancy, thiamine deficiency could cause irritability, vomiting, ataxia, and altered sleep patterns. In addition, long-term consequences of thiamine deficiency in infants are motor disabilities, seizures, heart block, and delayed motor skills [18].
Regional studies for women in Kashmir that relied on polished rice and meat or chicken soups were found to lead to maternal thiamine deficiency, especially in times of stress such as pregnancy and lactation, in which levels are already depleted. A study conducted in 2014–2015 in Kashmir reported infantile encephalopathy, which was related to thiamine deficiency. The study included 50 exclusively breastfed 1–6 months infants with acute encephalopathy, with 90% of the subjects of the lower socioeconomic class [19]. Infant encephalopathy could develop rickets, viral and bacterial infections, urea cycle defects, and fatty acid oxidation disorders.
Childhood: A previous study linked thiamine deficiency with autistic features in children. In contrast, a thiamine derivative (thiamine tetrahydrofurfuryl disulfide) administered 50 mg twice daily for two months improved social skills and cognitive awareness [20]. Moreover, depression in children was witnessed, as mood swings or laziness were also suggested to be linked to thiamine deficiency [17].
Adulthood: Thiamine deficiency is often associated with alcohol abuse. Alcohol metabolism leads to reduced transport of thiamine to areas in the body, including the blood–brain barrier. The enzymes involved in brain cell metabolism, such as α-ketoglutarate and pyruvate dehydrogenase, which are dependent on thiamine coenzymes, are present at lower levels in alcoholic patients with Wernicke–Korsakoff syndrome. In addition, alcohol-induced liver damage also reduces the storage and phosphorylation of thiamine, thus contributing to its deficiency [21]. Considering that this life stage is at which alcohol consumption starts, the measurement of thiamine levels ought to be considered.
In Wernicke–Korsakoff syndrome in alcoholic patients, thiamine supplements are typically administered as a dose of 500–1200 mg per day intravenously or via intramuscular injection over 2–3 doses for five days, then an oral dose of 300 mg per day for 1–2 weeks, and finally 100 mg for maintenance. Hence, it is recommended that alcoholic patients should be supplemented with thiamine at a dose of 100–300 mg per day [22].
Abruptly stopping alcohol intake with thiamine deficiency could increase glutamate function and release, which could cause neurotoxicity due to glutamate hyperactivity. Thus, it is suggested that alcohol withdrawal should be accompanied by thiamine supplementation to detoxify the brain [21]. Adult depression, which might be a consequence of alcohol consumption, was also reported to reduce thiamine bioavailability [17].
An exciting application of thiamine is lessening the symptoms of premenstrual syndrome (PMS), which occurs in 85–90% of women of reproductive age. The symptoms can be behavioral (mood swings, depression, anxiety, and tension) or physical (abdominal cramps, fatigue, flushing, and dizziness) with different degrees of severity. PMS is hypothesized to be caused by an alteration in dopamine and serotonin levels, aldosterone activity, and essential fatty acids. Thiamine is also used to treat nausea, vomiting, muscle cramps, and anxiety, which are all symptoms similar to PMS. Thiamine is involved in carbohydrates and amino acids metabolism, as well as neurotransmitters biosynthesis that could, in part, lessen PMS symptoms.
In the same context, thiamine was investigated in menorrhagia, which occurs when bleeding lasts for more than seven days or if the blood volume is over 80 mL and affects mental and social health and might lead to anemia. A double-blind clinical trial was conducted in Iran in 2016–2017 on female students aged 18–26. The study showed that thiamine could treat menorrhagia with minimal side effects, manifested as a reduction in the duration of menstrual bleeding. While the mechanism is not fully known, thiamine represents a safe and inexpensive way to manage menstruation symptoms and improve female adults’ productivity [23].
Elderly: Furthermore, impaired cerebral glucose metabolism indicates Alzheimer’s disease (AD) is likely to occur in the elderly stages. Thiamine deficiency causes beriberi, WE, and Korsakoff Syndrome, exhibiting symptoms of ataxia, mental confusion, and later amnesia, similar to AD. AD patients exhibit reduced α-ketoglutarate and pyruvate dehydrogenase enzyme activity and levels linked to thiamine deficiency. It was also concluded that lower TDP levels could be a predisposing factor for AD that is also gender-related [24]. In contrast, another study failed to show any thiamine deficiency in a group of elderly patients. However, this might not be indicative as low thiamine metabolism was not investigated, nor was there any evidence of supplement consumption among subjects [25].
2.2. Riboflavin
The recommended daily intake, its primary action, and natural sources of vitamin B2 are presented in Table 2.

Table 2.
Recommended daily intake (RDI), main action, and natural sources of vitamin B2 (riboflavin) in different life stages.
Pregnancy: Riboflavin deficiency appeared as a possible risk factor for preeclampsia. Insufficient levels of riboflavin-derived cofactors Flavin mononucleotide (FMN) and Flavin adenine dinucleotide (FAD) could contribute to the established pathophysiologic changes in preeclampsia, including mitochondrial dysfunction, enhanced oxidative stress, and disturbances in nitric oxide release [26]. In lactation, the average intake of mothers is 0.39 mg/L, with 0.01–0.55 mg secreted in breast milk. Such intake is lower than the RDA, which is 1.6 mg/L. Lactating mothers should thus increase riboflavin consumption to avoid its depletion. Unlike thiamine, riboflavin levels in breast milk remain consistent over time [27].
Infancy: Indications have been reported for riboflavin being important in the early postnatal development of the brain. Riboflavin can be destroyed by UV light exposure; thus, UV therapy in infants with hyperbilirubinemia could result in riboflavin deficiency. Infant nutrition could be improved by adding weaning food fortified with riboflavin to reach up to 0.4 mg/day [16].
Childhood and adolescence: Complementary and alternative medicine is recently being used to treat children and adolescent diseases, i.e., migraine, pain, and attention deficit hyperactivity disorder (ADHD). Alternative medicine to treat migraines includes riboflavin, magnesium, coenzyme Q10, and others. Since riboflavin plays a role in mitochondrial energy production as a coenzyme in the flavoprotein redox reactions, it could reduce migraine attacks regarding mitochondrial malfunction. Nevertheless, clinical trials failed to demonstrate a significant difference in migraine severity or frequency when comparing riboflavin and placebo. An observational test administered at a dose of 20–400 mg riboflavin per day to children and teenagers (7–18 years) for six months who suffered from migraines showed a reduction in the frequency and intensity after 3–4 months. Still, it showed a non-significant difference between the placebo and riboflavin groups [28].
Adulthood: Riboflavin should also be considered in adult migraines, similar to childhood, which is similarly associated with mitochondrial dysfunction. Only 27 mg of the riboflavin, at most, is absorbed per dose with a ca. one h half-life. An administration of 50–400 mg/day of riboflavin concluded that only some adult populations would be able to benefit from the treatment due to genetic variability [29]. Similar to thiamine, riboflavin intake in women was also associated with a decreased incidence of PMS [30].
The malfunction of one-carbon metabolism, which involves reactions donating methyl groups, is one factor that can promote breast cancer’s carcinogenesis. Riboflavin is a cofactor for some enzymes involved in these reactions, so it might play a role in reducing the risk of breast cancer. However, a meta-analysis that analyzed ten studies from 1966 to 2016 with a total of 12,268 cases aged between 20 and 80 years failed to demonstrate the ability of riboflavin to decrease breast cancer risk. The analysis was limited by the number of detailed studies [31].
Elderly: Riboflavin could also reduce the risk of Type 2 diabetes (T2DM) in the elderly as a common degenerative disease. T2DM is associated with oxidative reactions that lead to insulin resistance. The exact mechanism of riboflavin is not fully elucidated, but its preventive role could be attributed to its antioxidant activity and the ability to reduce iron overload. A study examining 19,168 healthy Japanese men and women for five years concluded that a 44% reduced risk of T2DM was associated with riboflavin intake of 1.8 mg/day, but only in women [32].
2.3. Niacin
Vitamin B3 has diverse functions and dietary sources, as depicted in Table 3.

Table 3.
Recommended daily intake (RDI), main actions, and natural sources of vitamin B3 (niacin) in different life stages.
Pregnancy: Pregnant women are more susceptible to micronutrient deficiency; their RDA is higher than ordinary adults, increasing from 14 mg niacin equivalents (NE) to 18 mg NE, ca. 25–30% extra dose intake. By studying the whole human exome, variation was found in the de novo kynurenine pathway (HAAO gene, encoding 3-hydroxyanthranilic acid 3,4-dioxygenase, and KYNU gene, encoding kynureni-nase). An evident relationship between niacin deficiency and multiple congenital malformations in the fetus was observed. Both participate in the NAD synthesis, resulting in NAD deficiency leading to VACTERL malformations (vertebral anomalies, anorectal malformations, cardiovascular anomalies, tracheoesophageal fistula, esophageal atresia, renal and/or radial anomalies, and limb defects). Preclinical studies on rats showed that mutations disappeared in embryos upon niacin supplementation with mothers [33,34], which shed light on the value of gestational niacin intake and the necessity to measure NAD levels in the body [35].
Lactation: Exclusive breastfeeding is recommended for the first six months after birth. Therefore, any maternal micronutrient fluctuations may affect growth and development at future stages. Unlike minerals, maternal intake and stores affect vitamin levels in breastmilk. Any deficiency in the mother’s niacin levels consequently affects nicotinamide levels in breastmilk [36], which is the primary form of niacin in breastmilk.
Infancy: Niacin is essential for normal infancy development and growth. Its content in human milk is approximately 1.5 mg/L, whereas its precursor tryptophan content reaches 210 mg/L. Hence, the total content is ca. 5 mg niacin equivalents/L secreted daily in human milk [37]. Similar to niacin, the adequate intake of pantothenate is 1.7 mg/day in infants, which is crucial for average growth and intellectual functions [38].
Adulthood: Adequate niacin levels can also prevent the occurrence of pellagra-associated dermatitis. Unexpectedly, supplemental niacin intake did not necessarily decrease the risk or prevent atopic dermatitis in women to the extent that increased additional niacin intake was positively associated with atopic dermatitis in some cases [39]. However, topical or orally administered nicotinamide was associated with restoring epidermal barrier integrity, which is usually impaired in atopic dermatitis patients [40]. Further studies addressing this topic in different geographical areas and medical histories are eagerly required.
Increased niacin intake with vitamin B6 and folate in young adulthood positively affected cognitive functions in later life. Cognitive functions were improved with niacin intake of more than 17.53 mg/1000 kcal. Such significance diminished with higher education groups of people, suggesting that race, gender, and educational level are contributing factors [41]. Another study comprising 127 adults of men and women showed that higher niacin intake was positively related to arterial flow-mediated dilation that might result in reduced LDL levels and associated oxidative stress [42].
In addition, it is recommended that niacin RDA for athletes aged 19 to 25 years is 22.8 mg for women and 30.36 mg for men with the maintenance of their recommended caloric intake [43].
2.4. Pantothenic Acid
Despite its importance, the abundance of pantothenic acid dietary sources and the rarity of its deficiency cases have led to limited research on its importance in the different life cycle stages. Its significance and nutritional sources are listed in Table 4.

Table 4.
Adequate daily intake (AI), main actions, and natural sources of vitamin B5 (pantothenic acid) in different life stages.
Pregnancy: Pregnant females can maintain the average blood level via increased calorie intake or more carefully selecting foods rich in pantothenic acid. On the other hand, increased pantothenic acid intake of more than 5.6 mg/day and high biotin and riboflavin intake above 22.5 μg/day and 2.42 mg/day, respectively, are associated with genome instability, profoundly manifested during pregnancy, and could lead to teratogenicity [44].
Childhood and adulthood: Although vitamin B5 deficiency is rare, a lack of this vitamin can adversely affect a wide range of metabolic functions, such as energy production and reduction in the synthesis of lipids. Symptoms vary from a loss of appetite, growth impairment, dermatitis, weakness, and ataxia to paralysis, adrenal hypertrophy, ulcers, and hepatic steatosis, which are usually misdiagnosed as a deficiency in other vitamins, delaying proper treatment. [45].
Elderly: Higher levels of pantothenic acid were also suggested to be associated with increasing cerebral amyloid-β peptide burden in cognitive impairment patients, significantly worsening cases of Alzheimer’s disease [46]. Still, more comprehensive scientific research needs to be directed towards such controversy by analyzing larger subject groups over extended periods to clarify the mechanism underlying such adverse effects.
In contrast, by examining 908 subjects aged more than 40 years over five years, pantothenic acid was found to be inversely related to the levels of the C-reactive protein, which is an indicator of low-grade inflammation and plays a critical role in the development and progression of atherosclerosis [47].
2.5. Pyridoxine
Table 5 illustrates the importance of vitamin B6, its RDA in different stages, and its dietary sources.

Table 5.
Recommended daily intake (RDI), main actions, and natural sources of vitamin B6 (pyridoxine) in different life stages.
During pregnancy, the placenta produces alkaline phosphatase (ALP), which hydrolyzes the active form of vitamin B6 (PLP) into pyridoxal resulting in decreased levels of vitamin B6 [48]. Consequently, a higher intake of vitamin B6 in pregnant women (population reference intake of 1.8 mg/kg) is recommended to maintain adequate serum PLP levels [49]. It was also suggested that daily administration of 5.5–7.6 mg of pyridoxine would be sufficient to optimize pyridoxine levels of a pregnant female [50]. Non-pregnant women taking oral contraceptive agents (OCAs) share the same high demands for vitamin B. During the OCAs course, estrogen stimulates the aminotransferase enzyme, which causes abnormalities in vitamin B6 metabolism leading to its deficiency [51,52].
The significance of Vitamin B6 for a pregnant female is extended from pregnancy stabilization during the first trimester to mood elevation after birth, including nausea and anemia improvement. Monitoring pyridoxine levels during the first trimester is essential to decrease nausea and vomiting, maintain pregnancy, and prevent miscarriages. Typically, pregnancy is stabilized by the action of a placental enzyme called diamine oxidase, whose activity is controlled by vitamin B6. Consequently, pregnant women with pyridoxine deficiency may experience spontaneous abortion and stillbirth [53], which warrants a vitamin B6 supplement prescription for females with frequent miscarriages. Moreover, the low level of methylene tetrahydrofolate reductase enzyme that converts homocysteine (Hcy) into methionine was reported in these females, leading to high levels of Hcy, which results in spontaneous abortion [54]. Secondly, a deficiency in vitamin B6 has been linked to hyperemesis gravidarum, nausea, and vomiting [55]. Vitamin B6 supplements in doses of up to 510 mg/day effectively improve such symptoms without increasing the risk of fetal malformation [56,57]. More studies are still needed to unveil the molecular and biochemical mechanism of how vitamin B6 improves these symptoms.
One of the most frequent complications among pregnant women is iron-deficiency anemia. However, some anemic pregnant women are non-responsive to iron supplementation. Surprisingly, anemia in those patients was shifted after vitamin B6 administration. Vitamin B6 is not only essential for heme and porphyrin synthesis but also for proper iron utilization by red blood cells [48]. Thus, vitamin B6 inadequacy can be linked to anemia in general and iron-deficiency anemia specifically. Therefore, an appropriate diagnosis of anemic pregnant women requires monitoring vitamin B6 and iron levels. Pyridoxine supplementation in a dose of 80 mg/day before birth at gestational week 28 and 40 mg/day after birth can be used as mood elevators to overcome postpartum depression [58].
The maternal intake of vitamin B6 determines the fetal status of pyridoxine. Therefore, it has been recommended to administer more than 4 mg/day of pyridoxine during pregnancy to maintain adequate PLP levels that ensure normal fetal development [59].
Lactation: Vitamin B6 also plays a crucial role in average infant growth in height and weight [60]. Therefore, pyridoxine RDA for lactating mothers is the same as for pregnant females. Moreover, mothers with deficient vitamin B6 levels are advised to provide their newly born with vitamin B6 supplements [61]. Puerperal lactation and hyperprolactinemia are some problems lactating mothers face due to excessive prolactin release [62]. Since L-dopa stimulates prolactin release, it is predicted that vitamin B6 may be effective in prolactin reduction by converting L-dopa to dopamine [62]. However, more research is needed to confirm this hypothesis.
Infancy: The glutamic acid decarboxylase enzyme maintains the balance between the glutamate and the inhibitory GABA excitatory neurotransmitter glutamate. Some infants are born with an autosomal recessive defect in this enzyme, which will shift the balance toward excitatory glutamate. This state of excitation is seen as non-responsive polymorphic seizures. Pyridoxine administration at a dose of 10–300 mg/day showed significant improvements, whereas seizure recurrence was observed after pyridoxine termination. Hence, lifelong treatment with vitamin B6 supplements is essential for those infants [63].
Childhood and adolescence: Pyridoxine is required for thymidine biosynthesis and host immunocompetence; therefore, it has a role in carcinogenesis and tumor growth [64]. Vitamin B6 regulates the synthesis and metabolism of 5-hydroxy tryptamine (5HT), serotonin receptors, and catecholamines. Thus, it is widely used to treat the symptoms of behavioral disorders during childhood. For instance, it improves abnormal behavioral conditions associated with autism, hyperkinetic syndrome, and schizophrenia [65,66]. Moreover, it is an effective adjuvant to anti-epileptic drugs, such as levetiracetam, to prevent agitation, irritability, and depression [67].
Furthermore, vitamin B6 was reported to exert beneficial effects on stress accompanying the adolescence phase owing to its role in facilitating magnesium (Mg) uptake. Stress is associated with low Mg levels; however, providing magnesium alone was ineffective in achieving calmness and relaxation. As vitamin B6 facilitates Mg uptake, pyridoxine is given in adjunct with magnesium at a ratio of 10 Mg: 1 Vitamin B6. Moreover, pyridoxine reduces corticosteroid release peripherally and affects the central biosynthesis of various neurotransmitters related to depression and anxiety, meaning it is suggested as an anti-stress agent at a dose of 100–300 mg/day [68].
Adulthood: Vitamin-B6-deficient adults may develop microcytic hypochromic anemia, termed pyridoxine-responsive anemia and remediable by pyridoxine only [69]. Lymphopenia is a disease where T-cells percentage is significantly reduced, leading to poor immune responses. In adults, vitamin B6 deficiency affects immune response negatively by decreasing T-helper levels. Pyridoxine intake at a dose more significant than the recommended for this age group, reaching 50 mg/day, is adequate and highly recommended in such cases [70].
Similar to in infants, vitamin B6 insufficiency decreased GABA levels and increased nerve excitability. During adulthood, convulsions caused by vitamin B6 deficiency are either due to poor intake, liver disease, pregnancy, or certain medications, with seizures found to be immediately remedied by adequate pyridoxine intake. Adults with tuberculosis and under isonicotinic acid hydrazine (INH) treatment may develop pyridoxine-dependent seizures attributed to the effect of INH metabolite in inhibiting the pyridoxine phosphokinase enzyme, leading to reduced PLP levels [71].
In contrast to pyridoxine’s effect on childhood leukemia, it is incredibly beneficial against colorectal cancer in adult males. This is justified by the action of PLP in inhibiting RNA polymerase, RNA reverse transcriptase, and DNA polymerase, which are overly expressed during increased cellular proliferation and oncogenic transformation [72].
The carnitine palmitoyl transferase enzyme is essential in controlling the availability of long-chain fatty acids for mitochondrial oxidation. It is synthesized endogenously in the liver and kidneys in the presence of vitamin B6. Consequently, carnitine biosynthesis is decreased with vitamin B6 deficiency concurrent with fatty acid accumulation and lipid profile alteration. Therefore, vitamin B6 is given to men with hypertriglyceridemia to reduce plasma cholesterol levels [73].
For both males and females, marginal vitamin B6 restriction decreases the plasma concentration of long-chain PUFA, n-3, and n-6, and increases the chance of developing CVD [74].
Elderly: Poor nutritional status was associated with low B6 levels, albeit B6 deficiency also occurred in subjects without malnutrition. Old age, inactivity, low serum albumin, alanine aminotransferase levels, and high homocysteine levels were associated with pyridoxine deficiency. Irritable bowel syndrome was also correlated with vitamin B6 deficiency [75].
2.6. Biotin
Biotin has necessary human functions, which are shown along with its RDA and dietary sources in Table 6.

Table 6.
Adequate daily intake (AI), main actions, and natural sources of vitamin B7 (Biotin) in different life stages.
Infancy: During infancy, vitamin B7 is critical in maintaining healthy hair, skin, and nails and preventing severe brain abnormalities. Biotin insufficiency in infancy is linked to alopecia and dermatitis around body orifices. Symptoms are reversed with a daily dose of 1 mg biotin. Since no recurrence was observed when biotin was stopped gradually, patients can gradually decrease the dose to 0.5 mg, 0.25 mg, and 0.1 mg over seven months [76,77,78,79]. Biotin is advised not to be abruptly stopped as it might cause sudden infant death syndrome (SIDS). The relationship is based on clinical observations that hepatic biotin level in infants who died from SIDS was lower than in infants of similar age who died of explicable causes [80,81]. However, these clinical studies are still insufficient, and further investigations are indispensable to reveal the exact causes of SIDS and unveil the precise attribution of biotin deficiency in these cases.
Childhood: Biotin supplementation at a dose of 2.5 mg/day for 180 days was efficient in both shiny and opaque types of trachyonychia. Trachyonychia, also called dystrophy, is an abnormal nail plate roughness that occurs during childhood and has been linked to biotin deficiency [82].
Adulthood: Biotinidase deficiency is one of the causes underlying biotin deficiency in adults. Since it is an inherited disorder, neonates should be screened early to avoid its delayed onset in adulthood, manifesting as myelopathy and irreversible neurological damage [83].
Elderly: Additionally, biotin deficiency is connected to specific diseases such as diabetes mellitus, liver and skin disorders, immunological and neurological abnormalities, and epilepsy. Moreover, it plays a role in bone mineral homeostasis, which is critical in such elderly life stages, and allergic and autoimmune disorders via biotinyl IgG [84].
2.7. Folic Acid
Vitamin B9 has indispensable significance in human health in different life stages, which is presented in Table 7.

Table 7.
Recommended daily intake (RDI), main actions, and natural sources of vitamin B9 (folic acid) in different life stages.
Pregnancy and lactation: Folic acid is a well-known supplement in pregnancy because of its prominent role in the fetus’ normal neural and physical development. Folate deficiency in the gestational period resulted in severe fetal adverse effects, including congenital neural tube defects, cardiac and urinary tract defects, and even cancer [85], besides affecting birth weight [86]. The development of neural tube defects from altered levels of neurotransmitters and limited myelination leads to the long-term impairment of cognitive function, learning, and memory deficits and brain atrophy detected in infants [86]. Its deficiency also might cause metabolic effects such as insulin resistance, glomerular sclerosis, neuropathy in the extremities, and megaloblastic anemia in the mothers [87].
The folate daily requirement during pregnancy and lactation is higher than normal, reaching 600–800 µg daily to meet the higher demands of the growing fetus and infant. During lactation, women risk folate deficiency due to increased demands to accommodate milk folate levels [88]. Moreover, maternal folate levels are critical factors for the proper development of the offspring.
The hyperhomocysteinemia accompanying folate deficiency also imposes health risks on pregnant women. It might induce apoptosis and DNA damage in placental vascular cells and maternal endothelial malfunction leading to severe complications [89]. It was evident that hyperhomocysteinemia could increase the risk of pregnancy complications ca. 18-fold in the second trimester [90].
On the other hand, excess folic acid can lead to the T allele of methylene tetrahydrofolate in infants. Infants with this type of allele suffer from sudden abnormal neurological symptoms during adult life, including bipolar disorder, depression, and schizophrenia [86].
In neonates, infants, children, and adolescents, inborn folate transport and metabolism errors are often associated with several clinical manifestations. These include developmental delays, cognitive deterioration, motor and gait abnormalities, behavioral or psychiatric symptoms, seizures, signs of demyelination or failure of myelination, and vascular changes seen on magnetic resonance imaging or postmortem examination. Less commonly, subacute combined degeneration and peripheral neuropathy might also occur [91].
In addition, infants and children of women who suffered from folate deficiency during pregnancy were found to experience asthma in later stages of life [55]. However, the underlying mechanism is still unclear. In addition, folic acid deficiency might cause megaloblastic anemia and infant neural tube defects [87].
Adulthood and Elderly: Concerning folic acid, smoking changes folate storage and metabolism because the one-carbon metabolism is influenced by the redox balance, which is readily altered by smoking [89].
2.8. Cobalamin
Table 8 comprises the dietary sources, RDA, and main actions of vitamin B12.

Table 8.
Adequate daily intake (AI), main actions, and natural sources of vitamin B12 (cobalamin) in different life stages.
Pregnancy: At the start of pregnancy, women must be supplemented with folic acid and vitamin B12 to meet the increased requirements of increased DNA, RNA, and protein synthesis. The recommended daily allowance of vitamin B12 is 2.6 mg/d for pregnant women and 2.8 mg/d for lactating women [92].
As previously mentioned, folate and cobalamin status of mothers during gestation highly affects the infant’s quality regarding both vitamins later in future life stages [85]. Additionally, it was observed that pregnant women with poor folate and cobalamin status suffer from high body mass indexes [92].
Low cobalamin levels and increased risk of low birth weight (LBW) are differentially related to the trimester during which the mother encountered vitamin B12 deficiency, i.e., the first-trimester lack can increase the risk of LBW up to 8 times [90]. The deficiency of vitamin B12 has also been related to other pregnancy complications such as recurrent miscarriages, preterm delivery, and intrauterine growth restriction [85].
Lactation: Breastfed infants’ cobalamin levels depended on their mothers’ status. In contrast, formula-fed infants were less at risk of deficiency as they were supplemented with all micronutrients meeting AI, which is 0.4–0.5 μg/day of cobalamin in the first year of life [93]. Breast milk vitamin B12 content varies according to maternal diet and cobalamin status; thus, nursing mothers following a vegan or macrobiotic diet should receive vitamin B12 supplements [92]. Vitamin B12 is limited in a vegan diet and should always be supplemented.
As with any other nutrient in breast milk, an infant experiences nutrient deficiency at six months, and breast milk nutrient content can no longer satisfy the baby’s nutritional daily requirements. Therefore, as weaning starts, vitamin B12-rich food such as meat and poultry should be included in the infant’s diet [92].
Infancy and childhood: Vitamin B12 deficiency in infants and children is manifested in the form of neurological, insufficient physical growth (failure to thrive), and hematological disorders, with most manifestations being found treatable except those affecting the nervous system, which might be irreversible. Such symptoms must be followed up closely, particularly in infants experiencing pathological malabsorption.
Some regimens involve the intramuscular injection of vitamin B12 (at a dose of 1 mg) and continue according to the response. The improvement of symptoms varies, with recovery from anemia found to be even faster with treatment with folic acid and iron supplements, including cobalamin [92].
In general, early childhood malnutrition has been related to poor cognitive function, school performance, and IQ scores in the short and long term. Deficiencies in various vitamins, such as vitamin B12, thiamine, and niacin, have been associated with cognitive impairment [94]. It should be noted that the recommended adequate intake of pantothenic acid is 1.7 to 5.0 mg/day for children, as it plays an essential role in normal development and growth [38]. Regarding vitamin B12, the child will continue to suffer from deficiency symptoms if it is not diagnosed early in infancy. As a result, school-aged children with cobalamin deficiency might suffer from altered motor development, cognitive disorders, and speech and language skills [92].
Adulthood: In addition, the clinical manifestation of cobalamin deficiency in adults resembles that in adulthood, which is highly heterogeneous, ranging from fatigue, common sensory neuropathy, neuropsychiatric symptoms, atrophic glossitis (Hunter’s glossitis), isolated macrocytosis and neutrophil hypersegmentation, to severe disorders, including combined sclerosis of the spinal cord, hemolytic anemia, and even pancytopenia [95].
Elderly: Vitamin B12 deficiency and/or age-related impairments in its function are increasingly recognized as contributing to age-related cognitive decline, subtle deficits, and frank dementia [96]. Regarding cobalamin, its deficiency results in disrupted cellular metabolism in all life cycles, in addition to age-related disease and functional decline, including cognition, cardiovascular disease, and bone health. Last but not least, it was postulated that a link exists between folic acid deficiency in older adults with homocysteine, aging, depression, dementia, and vascular disease [91].
3. Conclusions
Micronutrient intake is a crucial issue that should be considered. Despite the small amounts needed in DRI, they have inevitable metabolic functions and are involved in many enzymatic reactions as cofactors, maintaining health and preventing diseases. Thus, any imbalance, either deficiency or over-consumption, may cause a wide array of reversible and irreversible symptoms that may sometimes lead to death. Fortified foods can be a solution to prevent their deficiency. The RDA of micronutrients differs along the life cycle based on age, gender, ethnicity, physical activity level, etc., and RDAs generally increase by age to fulfill the growth and energy requirements. RDAs during pregnancy and lactation are even higher to sustain the milk’s increased demands and vitamin secretion. Thiamine, riboflavin, niacin, pyridoxine, and folic acid are crucial for maternal and fetus health. During infancy and childhood, B vitamins are integrated into physical and psychological development, which have a pivotal impact on one’s overall health in adolescence and adulthood. A higher intake of B vitamins in the elderly also prevents aging problems, especially inflammation-related. However, all supplementations should be carefully monitored to avoid overdoses and hypervitaminosis. Hence, more research should be invested to study each vitamin individually, concerning nutritional disparities in each life stage, with extensive attention paid to cultural differences and lifestyles.
Author Contributions
Conceptualization, M.A.A., H.A.H., M.A.K. and M.A.F.; methodology, H.I.G.; software, M.S.; validation, M.A.A., H.A.H., M.A.K. and M.A.F.; formal analysis, H.I.G.; investigation, M.A.F.; resources, M.S.; data curation, M.A.A., H.A.H., M.A.K. and M.A.F.; writing—original draft preparation, H.I.G., M.A.A., H.A.H. and M.A.K.; writing—review and editing, M.S., M.A.K., H.A.H. and M.A.F.; visualization, H.I.G. and M.S.; supervision, M.A.F.; project administration, M.A.A. and H.I.G.; funding acquisition, H.I.G. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The King Khalid University Deanship of Scientific Research, which provided funding for this project via the Large Groups Project with grant number R.G.P 2/200/43.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be demanded upon request.
Acknowledgments
We thank King Khalid University’s Deanship of Scientific Research for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5HT | Serotonin |
| Ach | Acetylcholine |
| AD | Alzheimer’s disease |
| AI | Adequate intake |
| ALP | Alkaline phosphatase |
| CoA | Coenzyme A |
| DFE | Dietary Folate Equivalents |
| FAD | Flavin adenine dinucleotide |
| FMN | Flavin mononucleotide |
| Hcy | Homocysteine |
| HG | Hyperemesis gravidarum |
| HLCS | holocarboxylase synthetase (HLCS) |
| KS | Korsakoff syndrome |
| LBW | Low birth weight |
| Mg | Magnesium |
| NAD | Nicotinamide adenine dinucleotide |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NE | Niacin equivalents |
| NTG | normal-tension glaucoma |
| OCAs | Oral contraceptive agents |
| PDH | Pyruvate dehydrogenase |
| PL | Pyridoxal |
| PM | Pyridoxamine |
| PMS | Premenstrual syndrome |
| PN | Pyridoxine |
| PNG | Pyridoxine-59-b-D-glucoside |
| RBC | Red blood cell |
| RDA | Recommended dietary allowance |
| RDI | Recommended dietary intake |
| SIDS | Sudden infant death syndrome |
| TDP | Thiamine diphosphate |
| TPP | Thiamine pyrophosphate |
| WE | Wernicke’s encephalopathy |
| α-KGDH | α-ketoglutarate dehydrogenase |
References
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy-A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Smith, A.G.; Croft, M.T.; Moulin, M.; Webb, M.E. Plants need their vitamins too. Curr. Opin. Plant Biol. 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Hans, K.B.; Jana, T. Micronutrients in the life cycle: Requirements and sufficient supply. NFS J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Herrmann, W.; Obeid, R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch. Arztebl. Int. 2008, 105, 680–685. [Google Scholar] [CrossRef] [PubMed]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Singh, A.; Trumpff, C.; Genkinger, J.; Davis, A.; Spann, M.; Werner, E.; Monk, C. Micronutrient dietary intake in Latina pregnant adolescents and its association with level of depression, stress, and social support. Nutrients 2017, 9, 1212. [Google Scholar] [CrossRef]
- Robinson, S.; Fall, C. Infant nutrition and later health: A review of current evidence. Nutrients 2012, 4, 859–874. [Google Scholar] [CrossRef]
- Agostoni, C.; Baselli, L.; Mazzoni, M.B. Early nutrition patterns and diseases of adulthood: A plausible link? Eur. J. Intern. Med. 2013, 24, 5–10. [Google Scholar] [CrossRef]
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef]
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Oudman, E.; Wijnia, J.W.; Oey, M.; van Dam, M.; Painter, R.C.; Postma, A. Wernicke’s encephalopathy in hyperemesis gravidarum: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 84–93. [Google Scholar] [CrossRef]
- Allen, L.H. B vitamins in breast milk: Relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 2012, 3, 362–369. [Google Scholar] [CrossRef]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef]
- Nazir, M.; Lone, R.; Charoo, B.A. Infantile thiamine deficiency: New insights into an old disease. Indian Pediatr. 2019, 56, 673–681. [Google Scholar] [CrossRef]
- Bhat, J.I.; Ahmed, Q.I.; Ahangar, A.A.; Charoo, B.A.; Sheikh, M.A.; Syed, W.A. Wernicke’s encephalopathy in exclusive breastfed infants. World J. Pediatr. 2017, 13, 485–488. [Google Scholar] [CrossRef]
- Lonsdale, D.; Shamberger, R.J.; Audhya, T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: A pilot study. Neuro Endocrinol. Lett. 2002, 23, 303–308. [Google Scholar]
- Thomson, A.D.; Guerrini, I.; Marshall, E.J. The Evolution and Treatment of Korsakoff’s Syndrome Out of Sight, Out of Mind? Neuropsychol. Rev. 2012, 22, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Polegato, B.F.; Pereira, A.G.; Azevedo, P.S.; Costa, N.A.; Zornoff, L.A.M.; Paiva, S.A.R.; Minicucci, M.F. Role of thiamin in health and disease. Nutr. Clin. Pract. 2019, 34, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Latifiyan, Z.; Torkashvand, R.; Saatchi, M. The effect of vitamin B1 on heavy menstrual bleeding. Prog. Nutr. 2019, 21, 843–848. [Google Scholar]
- Wang, C.; Fei, G.; Pan, X.; Sang, S.; Wang, L.; Zhong, C.; Jin, L. High thiamine diphosphate level as a protective factor for Alzheimer’s disease. Neurol. Res. 2018, 40, 658–665. [Google Scholar] [CrossRef]
- Pourhassan, M.; Biesalski, H.K.; Angersbach, B.; Lueg, G.; Klimek, C.; Wirth, R. Prevalence of thiamine deficiency in older hospitalized patients. Clin. Interv. Aging 2018, 13, 2247–2250. [Google Scholar] [CrossRef]
- Wacker, J.; Frühauf, J.; Schulz, M.; Chiwora, F.M.; Volz, J.; Becker, K. Riboflavin deficiency and preeclampsia. Obstet. Gynecol. 2000, 96, 38–44. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Adair, L.S.; Bentley, M.E.; Flax, V.L.; Jamieson, D.J.; Ellington, S.R.; Tegha, G.; Chasela, C.S.; Kamwendo, D.; et al. Thiamin and Riboflavin in Human Milk: Effects of Lipid-Based Nutrient Supplementation and Stage of Lactation on Vitamer Secretion and Contributions to Total Vitamin Content. PLoS ONE 2016, 11, e0149479. [Google Scholar]
- Sangermani, R.; Boncimino, A. The use of nutraceutics in children‘s and adolescent’s headache. Neurol. Sci. 2017, 38, 121–124. [Google Scholar] [CrossRef]
- Thompson, D.F.; Saluja, H.S. Prophylaxis of migraine headaches with riboflavin: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 394–403. [Google Scholar] [CrossRef]
- Chocano-Bedoya, P.O.; Manson, J.E.; Hankinson, S.E.; Willett, W.C.; Johnson, S.R.; Chasan-Taber, L.; Ronnenberg, A.G.; Bigelow, C.; Bertone-Johnson, E.R. Dietary B vitamin intake and incident premenstrual syndrome. Am. J. Clin. Nutr. 2011, 93, 1080–1086. [Google Scholar] [CrossRef]
- Yu, L.; Tan, Y.; Zhu, L. Dietary vitamin B2 intake and breast cancer risk: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 721–729. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Muraki, I.; Tamakoshi, A. Among the water-soluble vitamins, dietary intakes of vitamins C, B2 and folate are associated with the reduced risk of diabetes in Japanese women but not men. Br. J. Nutr. 2019, 121, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Enriquez, A.; Rapadas, M.; Martin, E.M.M.A.; Wang, R.; Moreau, J.; Lim, C.K.; Szot, J.O.; Ip, E.; Hughes, J.N.; et al. NAD deficiency, congenital malformations, and niacin supplementation. N. Engl. J. Med. 2017, 377, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Penke, M.; Kiess, W. Paediatric endocrinology: Can niacin supplementation protect against congenital malformations? Nat. Rev. Endocrinol. 2017, 13, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dang, S.; Cheng, Y.; Qiu, H.; Mi, B.; Jiang, Y.; Qu, P.; Zeng, L.; Wang, Q.; Li, Q.; et al. Dietary intakes and dietary patterns among pregnant women in Northwest China. Public Health Nutr. 2017, 20, 282–293. [Google Scholar] [CrossRef]
- Daniels, L.; Gibson, R.S.; Diana, A.; Haszard, J.J.; Rahmannia, S.; Luftimas, D.E.; Hampel, D.; Shahab-Ferdows, S.; Reid, M.; Melo, L.; et al. Micronutrient intakes of lactating mothers and their association with breast milk concentrations and micronutrient adequacy of exclusively breastfed Indonesian infants. Am. J. Clin. Nutr. 2019, 110, 391–400. [Google Scholar] [CrossRef]
- Committee on Nutrition. Composition of Human Milk: Normative Data. In Pediatric Nutrition Handbook, 2nd ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 1985; pp. 363–368. [Google Scholar]
- Litwack, G. Vitamins and Nutrition. In Human Biochemistry; Academic Press: Cambridge, MA, USA, 2018; pp. 645–680. [Google Scholar]
- Drucker, A.M.; Li, W.Q.; Park, M.K.; Li, T.; Qureshi, A.A.; Cho, E. Niacin intake and incident adult-onset atopic dermatitis in women. J. Allergy Clin. Immunol. 2017, 139, 2020–2022.e2. [Google Scholar] [CrossRef][Green Version]
- Ito, M.; Morita, T.; Okazaki, S.; Koto, M.; Ichikawa, Y.; Takayama, R.; Hoashi, T.; Saeki, H.; Kanda, N. Dietary habits in adult Japanese patients with atopic dermatitis. J. Dermatol. 2019, 46, 515–521. [Google Scholar] [CrossRef]
- Qin, B.; Xun, P.; Jacobs, D.R.; Zhu, N.; Daviglus, M.L.; Reis, J.P.; Steffen, L.M.; Van Horn, L.; Sidney, S.; He, K. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Clin. Nutr. 2017, 106, 1032–1040. [Google Scholar] [CrossRef]
- Kaplon, R.E.; Gano, L.B.; Seals, D.R. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J. Appl. Physiol. 2014, 116, 156–163. [Google Scholar] [CrossRef]
- Majewski, M.; Kozłowska, A.; Thoene, M.; Lebiedzińska, A. Variations of niacin content with regard to carbohydrates in energy-rich diets of elite European athletes and their relation with dietary RDA. J. Elem. 2016, 21, 745–755. [Google Scholar] [CrossRef]
- Fenech, M.; Baghurst, P.; Luderer, W.; Turner, J.; Record, S.; Ceppi, M.; Bonassi, S. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, beta-carotene and high intake of pantothenic acid, biotin and riboflavin are significantly associated with increased genome instability—Results from a dietary intake and micronucleus index survey in South Australia. Carcinogenesis 2005, 26, 991–999. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; McClung, J.P. Pantothenic Acid. In The Vitamins; Academic Press: Cambridge, MA, USA, 2017; pp. 387–398. [Google Scholar]
- Lee, J.H.; Ahn, S.Y.; Lee, H.A.; Won, K.S.; Chang, H.W.; Oh, J.S.; Kim, H.W. Dietary intake of pantothenic acid is associated with cerebral amyloid burden in patients with cognitive impairment. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.K.; Choi, B.Y. The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 806–816. [Google Scholar] [CrossRef]
- Hisano, M.; Suzuki, R.; Sago, H.; Murashima, A.; Yamaguchi, K. Vitamin B6 deficiency and anemia in pregnancy. Eur. J. Clin. Nutr. 2010, 64, 221–223. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Dietary Reference Values for vitamin B6. EFSA J. 2016, 14, e04485. [Google Scholar] [CrossRef]
- Barnard, H.C.; Dekock, J.J.; Vermaak, W.J.H.; Potgieter, G.M. A new perspective in the assessment of vitamin B-6 nutritional status during pregnancy in humans. J. Nutr. 1987, 117, 1303–1306. [Google Scholar] [CrossRef]
- Roepke, J.L.; Kirksey, A. Vitamin B6 nutriture during pregnancy and lactation. I. Vitamin B6 intake, levels of the vitamin in biological fluids, and condition of the infant at birth. Am. J. Clin. Nutr. 1979, 32, 2249–2256. [Google Scholar] [CrossRef]
- Roepke, J.L.; Kirksey, A. Vitamin B6 nutriture during pregnancy and lactation. II. The effect of long-term use of oral contraceptives. Am. J. Clin. Nutr. 1979, 32, 2257–2264. [Google Scholar] [CrossRef]
- Martner-Hewes, P.M.; Hunt, I.F.; Murphy, N.J.; Swendseid, M.E.; Settlage, R.H. Vitamin B-6 nutriture and plasma diamine oxidase activity in pregnant Hispanic teenagers. Am. J. Clin. Nutr. 1986, 44, 907–913. [Google Scholar] [CrossRef]
- Serapinas, D.; Boreikaite, E.; Bartkeviciute, A.; Bandzeviciene, R.; Silkunas, M.; Bartkeviciene, D. The importance of folate, vitamins B6 and B12 for the lowering of homocysteine concentrations for patients with recurrent pregnancy loss and MTHFR mutations. Reprod. Toxicol. 2017, 72, 159–163. [Google Scholar] [CrossRef]
- Reinken, L.; Gant, H. Vitamin B6 nutrition in women with hyperemesis gravidarum during the first trimester of pregnancy. Clin. Chim. Acta 1974, 55, 101–102. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Interventions with Vitamins B6, B12 and C in Pregnancy. Paediatr. Perinat. Epidemiol. 2012, 26, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Shrim, A.; Boskovic, R.; Maltepe, C.; Navios, Y.; Garcia-Bournissen, F.; Koren, G. Pregnancy outcome following use of large doses of vitamin B6 in the first trimester. J. Obstet. Gynaecol. 2006, 26, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Khodadad, M.; Kheirabadi, G.; Bahadoran, P. Efficacy of vitamin B6 on pregnancy outcomes: A randomized clinical trial. J. Pharm. Res. Int. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Lumeng, L.; Cleary, R.E.; Wagner, R.; Yu, P.L.; Li, T.K. Adequacy of vitamin B6 supplementation during pregnancy: A prospective study. Am. J. Clin. Nutr. 1976, 29, 1376–1383. [Google Scholar] [CrossRef]
- Heiskanen, K.; Siimes, M.A.; Salmenperä, L.; Perheentupa, J. Low vitamin B6 status associated with slow growth in healthy breast-fed infants. Pediatr. Res. 1995, 38, 740–746. [Google Scholar] [CrossRef]
- Kang-Yoon, S.A.; Kirksey, A.; Giacoia, G.; West, K. Vitamin B-6 status of breast-fed neonates: Influence of pyridoxine supplementation on mothers and neonates. Am. J. Clin. Nutr. 1992, 56, 548–558. [Google Scholar] [CrossRef]
- Husami, N.; Idriss, W.; Jewelewicz, R.; Ferin, M.; Vande Wiele, R.L. Lack of acute effects of pyridoxine on prolactin secretion and lactation. Fertil. Steril. 1978, 30, 393–397. [Google Scholar] [CrossRef]
- Schulze-Bonhage, A.; Kurthen, M.; Walger, P.; Elger, C.E. Pharmacorefractory status epilepticus due to low vitamin B6 levels during pregnancy. Epilepsia 2004, 45, 81–84. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, B.; Millman, S.E.; Chen, C.; Li, X.; Morris, J.P., IV; Mayle, A.; Ho, Y.J.; Loizou, E.; Liu, H.; et al. Vitamin B6 addiction in acute myeloid leukemia. Cancer Cell 2020, 37, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Benke, P.J.; Duchowny, M.; McKnight, D. Biotin and Acetazolamide for Treatment of an Unusual Child With Autism Plus Lack of Nail and Hair Growth. Pediatric Neurol. 2018, 79, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Martineau, J.; Barthelemy, C.; Garreau, B.; Lelord, G. Vitamin B6, magnesium, and combined B6-Mg: Therapeutic effects in childhood autism. Biol. Psychiatry 1985, 20, 467–478. [Google Scholar] [CrossRef]
- Marino, S.; Vitaliti, G.; Marino, S.D.; Pavone, P.; Provvidenti, S.; Romano, C.; Falsaperla, R. Pyridoxine add-On treatment for the control of behavioral adverse effects induced by levetiracetam in children: A case-control prospective study. Ann. Pharmacother. 2018, 52, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; Dubray, C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.W.; Whittington, R.M.; Weisman, R.; Horrigan, D.L. Pyridoxine responsive anemia in the human adult. Proc. Soc. Exp. Biol. Med. 1956, 91, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Ribayamercado, J.D.; Russell, R.M.; Sahyoun, N.; Morrow, F.D.; Gershoff, S.N. Vitamin B-6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am. J. Clin. Nutr. 1991, 53, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, Y.; Shin, H.; Kang, K.; Park, J.M.; Kim, B.K.; Kwon, O.; Lee, J.J. Seizures related to vitamin B6 deficiency in adults. J. Epilepsy Res. 2015, 5, 23–24. [Google Scholar] [CrossRef]
- Lee, J.E.; Li, H.; Giovannucci, E.; Lee, I.M.; Selhub, J.; Stampfer, M.; Ma, J. Prospective study of plasma vitamin B6 and risk of colorectal cancer in men. Cancer Epidemiol Biomark. Prev. 2009, 18, 1197–1202. [Google Scholar] [CrossRef]
- Hlais, S.; Abou Reslan, D.R.; Sarieddine, H.K.; Nasreddine, L.; Taan, G.; Azar, S.; Obeid, O.A. Effect of lysine, vitamin B(6), and carnitine supplementation on the lipid profile of male patients with hypertriglyceridemia: A 12-week, open-label, randomized, placebo-controlled trial. Clin. Ther. 2012, 34, 1674–1682. [Google Scholar] [CrossRef]
- Zhao, M.; Lamers, Y.; Ralat, M.A.; Coats, B.S.; Stacpoole, P.W.; Chi, Y.Y.; Muller, K.E.; Bain, J.R.; Newgard, C.B. Marginal vitamin B6 deficiency affects fatty acid profiles in healthy men and women. FASEB J. 2011, 25, 586.3. [Google Scholar] [CrossRef]
- Kjeldby, I.K.; Fosnes, G.S.; Ligaarden, S.C.; Farup, P.G. Vitamin B6 deficiency and diseases in elderly people-a study in nursing homes. BMC Geriatr. 2013, 13, 13. [Google Scholar] [CrossRef]
- Avcin, M. Dermatitis seborrheica and biotin deficiency in infants. Zdrav. Vestn. 1952, 21, 15–21. [Google Scholar] [PubMed]
- Fujimoto, W.; Inaoki, M.; Fukui, T.; Inoue, Y.; Kuhara, T. Biotin deficiency in an infant fed with amino acid formula. J. Dermatol. 2005, 32, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Noda, E.; Koyama, Y.; Shirai, T.; Horino, A.; Juri, T.; Koike, M. Biotin deficiency in an infant fed with amino acid formula and hypoallergenic rice. Acta Paediatr. 1996, 85, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Mamada, Y.; Murata, T.; Taniguchi, A.; Hasegawa, Y.; Suzuki, T.; Kohda, K.; Nasuno, K.; Watanabe, T.; Yamaguchi, S.; Ishiguro, A. Biotin deficiency in amino acid formula nutrition for an infant with milk protein allergy. Arerugi 2008, 57, 552–557. [Google Scholar] [PubMed]
- Heard, G.S.; Hood, R.L.; Johnson, A.R. Hepatic biotin and the sudden infant death syndrome. Med. J. Aust. 1983, 2, 305–306. [Google Scholar] [CrossRef]
- Johnson, A.R.; Hood, R.L.; Emery, J.L. Biotin and the sudden infant death syndrome. Nature 1980, 285, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Mohrenschlager, M.; Schmidt, T.; Ring, J.; Abeck, D. Recalcitrant trachyonychia of childhood—Response to daily oral biotin supplementation: Report of two cases. J. Dermatol. Treat. 2000, 11, 113–115. [Google Scholar] [CrossRef]
- Ferreira, P.; Chan, A.; Wolf, B. Irreversibility of Symptoms with Biotin Therapy in an Adult with Profound Biotinidase Deficiency. In JIMD Reports; Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Eds.; Springer: Berlin, Germany, 2017; Volume 36, pp. 117–120. [Google Scholar]
- Watanabe, T.; Yasumura, S.; Shibata, H.; Fukui, T. Biotin status and its correlation with other biochemical parameters in the elderly people of Japan. J. Am. Coll. Nutr. 1998, 17, 48–53. [Google Scholar] [CrossRef]
- Hure, A.J.; Collins, C.E.; Smith, R. A longitudinal study of maternal folate and vitamin B12 status in pregnancy and postpartum, with the same infant markers at 6 months of age. Matern. Child Health J. 2012, 16, 792–801. [Google Scholar] [CrossRef]
- Vinaykumar, N.; Kumar, A.; Quadros, L.S.; Prasanna, L.C. Determining the effect of folate diets during pregnancy and lactation on neurobehavioural changes in the adult life of offspring. J. Taibah Univ. Med. Sci. 2019, 14, 523–530. [Google Scholar] [CrossRef]
- Hay, G.; Johnston, C.; Whitelaw, A.; Trygg, K.; Refsum, H. Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. Am. J. Clin. Nutr. 2008, 88, 105–114. [Google Scholar] [CrossRef]
- Stamm, R.A.; Houghton, L.A. Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients 2013, 5, 3920–3947. [Google Scholar] [CrossRef]
- Furness, D.; Fenech, M.; Dekker, G.; Khong, T.Y.; Roberts, C.; Hague, W. Folate, vitamin B12, vitamin B6 and homocysteine: Impact on pregnancy outcome. Matern. Child Nutr. 2013, 9, 155–166. [Google Scholar] [CrossRef]
- Mishra, J.; Tomar, A.; Puri, M.; Jain, A.; Saraswathy, K.N. Trends of folate, vitamin B 12, and homocysteine levels in different trimesters of pregnancy and pregnancy outcomes. Am. J. Hum. Biol. 2020, 32, e23388. [Google Scholar] [CrossRef]
- Reynolds, E.H. Folic acid, ageing, depression, and dementia. BMJ 2002, 324, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Murphy, M.; Solé-Navais, P.; Yajnik, C. Cobalamin status from pregnancy to early childhood: Lessons from global experience. Adv. Nutr. 2017, 8, 971–979. [Google Scholar] [CrossRef]
- Bjørke-Monsen, A.L.; Ueland, P.M. Cobalamin status in children. J. Inherit. Metab. Dis. 2011, 34, 111–119. [Google Scholar] [CrossRef]
- Fanjiang, G.; Kleinman, R.E. Nutrition and performance in children. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 342–347. [Google Scholar] [CrossRef]
- Dali-Youcef, N.; Andrès, E. An update on cobalamin deficiency in adults. QJM 2009, 102, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).