Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements
Abstract
1. Introduction
2. Scientific Mechanisms
2.1. Mechanisms Related to Aging and Disease: Antioxidant Defense, Oxidative Stress Reduction, and Anti-Inflammatory Properties
2.2. The Central Role of the Mitochondria
2.3. Gut-Synthesized Melatonin the Gut Microbiome
Gut Health, Dietary Polyphenols Melatonin
2.4. Kynurenine Pathway, Energy Regulation Stress Response
3. Clinical Uses
3.1. Central Nervous System
3.1.1. Circadian Rhythm Modulation
Circadian Rhythm Sleep-Wake Disorders
Jet Lag
Sleep Dysfunction
- Insomnia: Immediate release 1–3 mg, 30 min before bed; slow-release can be used for sleep maintenance problems
- Regulating sleep in blind individuals who often experience non-24 h sleep-wake rhythm disorder;
- Replicating the normal endogenous pattern;
- Delayed sleep phase.
3.1.2. Eye Health
3.1.3. Cognitive Conditions (Dementia)
3.1.4. Migraines and Headaches
3.1.5. Tinnitus
3.1.6. Attention-Deficit Hyperactivity Disorder (ADHD) and Autism
3.2. Cardiometabolic Health
3.3. Reproductive Health
3.3.1. Pregnancy and Fertility
3.3.2. Endometriosis
3.3.3. Polycystic Ovarian Syndrome (PCOS)
3.4. Gastrointestinal Health
3.5. (Auto)Immunity
3.5.1. Oxidative Stress and Inflammatory States
3.5.2. Cancer Prevention and Treatment
3.6. Bone Health
4. Therapeutic Considerations
4.1. Dietary Sources of Melatonin
4.1.1. Plant Sources
4.1.2. Animal Sources
4.2. Dietary Supplements
4.2.1. Chemically Synthesized Melatonin
- 5-methoxy-3-indolylacetonitrile
- 5-methoxy-3-(2-nitroethyl)-indole
- 5-methoxytryptamine
- Phthalimide (1,3-dihydro-1,3-dioxoisonidole)
- 1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid;
- 3-(phenylamino)-alanine;
- 1,1′-ethylidene bis-(tryptophan) (‘peak E’, one of the contaminants related to EMS);
- 2-(3-indolylmethyl)-tryptophan;
- Formaldehyde-melatonin;
- Formaldehyde-melatonin condensation products;
- Hydroxymelatonin isomers;
- 5-hydroxy-tryptamine derivatives;
- 5-methoxy-tryptamine derivatives;
- N-acetyl-and diacetyl-indole derivatives;
- 1,3-diphthalimidopropane;
- Hydroxy-bromo-propylphthalimide;
- Chloropropylphthalimde.
4.2.2. Phytomelatonin
| Feature | Phytomelatonin * | Synthetic Melatonin |
|---|---|---|
| Origin | Plants | Chemicals |
| Processing | Customized cultivation technique of selecting the ideal location, soil, climate and optimal method/time to harvest based on the plant’s cycles to optimize melatonin levels | Chemical synthesis |
| Constituents | Bioidentical melatonin plus other plant actives; no excipients, fillers, or binding agents | Bioidentical melatonin and possibly contaminants from the chemical synthesis; depending on the dietary supplement, it may contain excipients, fillers, or binding agents |
| Environmentally safe? | Yes | No, uses toxic solvents and generates pollution |
| Other nutritionally active compounds included | (Essential) Fatty acids, amino acids, vitamins (vitamin K, riboflavin (vitamin B2), choline, vitamin E, thiamin (vitamin B1), pyridoxine (vitamin B6), biotin), minerals (trace amounts of calcium, magnesium, zinc, iron, manganese, selenium, copper, potassium, sodium, phosphorus, chloride, iodine), phytonutrients (beta-carotene, xanthophyll, zeaxanthin, lutein, chlorophyll, violaxanthin); Concentration of these adjunctive compounds depend on growing and seasonal changes. | None |
| Anti-inflammatory activity | Yes, more effective in inhibiting COX-2 in a cellular assay compared with synthetic melatonin [34] | Yes, although not more effective than phytomelatonin * [34] |
| Antiradical scavenging activity | Yes, it possesses significantly stronger free radical scavenging capacity as compared to synthetic melatonin using a cellular assay to assess Free Radical Scavenging Percentage (DPPH%) [34]. | Yes, it has antiradical scavenging activity, although less than phytomelatonin * [34]. |
| Oxygen Radical Absorbance Capacity (ORAC) (see Figure 8) | 17,200–18,500 [270] | 1932, 4492 [271] 4830 [272] |
4.3. Dosing
4.4. Timing
4.5. Bioavailability
4.6. Contraindications and Combinations
4.6.1. Contraindications
4.6.2. Combinations
Vitamin C
Vitamin B12
Myo-Inositol, Folic Acid Vitamin D
Glutathione
4.7. Lifestyle Aspects
Blue-Light-Blocking Glasses
4.8. A Comprehensive Clinical Approach to Melatonin
Laboratory Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wunsch, N.-G. Sales of Melatonin 2020. 2021. Available online: https://www.statista.com/statistics/1267421/sales-of-melatonin-in-the-united-states/ (accessed on 31 July 2022).
- Celestin, M.N.; Musteata, F.M. Impact of Changes in Free Concentrations and Drug-Protein Binding on Drug Dosing Regimens in Special Populations and Disease States. J. Pharm. Sci. 2021, 110, 3331–3344. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P.; Rubio-Sastre, P.; Madrid, J.A.; Saxena, R.; Scheer, F.A. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 2015, 64, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? Febs. J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 2011, 17, 3888–3898. [Google Scholar] [CrossRef]
- Mahmood, D. Pleiotropic Effects of Melatonin. Drug Res. 2019, 69, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A. Developing circadian rhythmicity. Basic and clinical aspects. Pediatr. Clin. N. Am. 1997, 44, 467–487. [Google Scholar] [CrossRef]
- Caba-Flores, M.D.; Ramos-Ligonio, A.; Camacho-Morales, A.; Martínez-Valenzuela, C.; Viveros-Contreras, R.; Caba, M. Breast Milk and the Importance of Chrononutrition. Front. Nutr. 2022, 9, 867507. [Google Scholar] [CrossRef]
- Crowley, S.J.; Acebo, C.; Carskadon, M.A. Human puberty: Salivary melatonin profiles in constant conditions. Dev. Psychobiol. 2012, 54, 468–473. [Google Scholar] [CrossRef]
- Grivas, T.B.; Savvidou, O.D. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis 2007, 2, 6. [Google Scholar] [CrossRef]
- Karasek, M.; Winczyk, K. Melatonin in humans. J. Physiol. Pharmacol. 2006, 57 (Suppl. 5), 19–39. [Google Scholar]
- Maas, M.B.; Lizza, B.D.; Abbott, S.M.; Liotta, E.M.; Gendy, M.; Eed, J.; Naidech, A.M.; Reid, K.J.; Zee, P.C. Factors Disrupting Melatonin Secretion Rhythms During Critical Illness. Crit. Care Med. 2020, 48, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Uchiyama, M.; Suzuki, H.; Shibui, K.; Kim, K.; Tan, X.; Tagaya, H.; Okawa, M.; Inoue, S. Effects of nocturnal bright light on saliva melatonin, core body temperature and sleep propensity rhythms in human subjects. Neurosci. Res. 2002, 42, 115–122. [Google Scholar] [CrossRef]
- Muñóz-Hoyos, A.; Fernández-García, J.M.; Molina-Carballo, A.; Macías, M.; Escames, G.; Ruiz-Cosano, C.; Acuña-Castroviejo, D. Effect of clonidine on plasma ACTH, cortisol and melatonin in children. J. Pineal Res. 2000, 29, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, G.; Robeva, R.; Konakchieva, R. Membrane Melatonin Receptors Activated Cell Signaling in Physiology and Disease. Int. J. Mol. Sci. 2021, 23, 471. [Google Scholar] [CrossRef]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Labani, N.; Cecon, E.; Jockers, R. Melatonin Target Proteins: Too Many or Not Enough? Front. Endocrinol. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Hu, C.; Sun, W.; Xu, Y.; Gu, Y.; Wu, L.; Peng, Q.; Reiter, R.J.; Liu, L. Identification of a novel melatonin-binding nuclear receptor: Vitamin D receptor. J. Pineal Res. 2020, 68, e12618. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Sharbatoghli, M.; Rezazadeh Valojerdi, M.; Bahadori, M.H.; Salman Yazdi, R.; Ghaleno, L.R. The Relationship between Seminal Melatonin with Sperm Parameters, DNA Fragmentation and Nuclear Maturity in Intra-Cytoplasmic Sperm Injection Candidates. Cell J. 2015, 17, 547–553. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Loh, D.; Reiter, R.J. Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders. Antioxidants 2021, 10, 1483. [Google Scholar] [CrossRef]
- Mocayar Marón, F.J.; Ferder, L.; Reiter, R.J.; Manucha, W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J. Steroid Biochem. Mol. Biol. 2020, 199, 105595. [Google Scholar] [CrossRef]
- Watad, A.; Azrielant, S.; Bragazzi, N.L.; Sharif, K.; David, P.; Katz, I.; Aljadeff, G.; Quaresma, M.; Tanay, G.; Adawi, M.; et al. Seasonality and autoimmune diseases: The contribution of the four seasons to the mosaic of autoimmunity. J. Autoimmun. 2017, 82, 13–30. [Google Scholar] [CrossRef]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef]
- D’Angelo, G.; Chimenz, R.; Reiter, R.J.; Gitto, E. Use of Melatonin in Oxidative Stress Related Neonatal Diseases. Antioxidants 2020, 9, 477. [Google Scholar] [CrossRef]
- Ighodaro, O.M. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Szwajgier, D.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Głowniak, K.; Meissner, H.O. Is Phytomelatonin Complex Better Than Synthetic Melatonin? The Assessment of the Antiradical and Anti-Inflammatory Properties. Molecules 2021, 26, 6087. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–66. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, T.; Cao, M.; Yuan, C.; Reiter, R.J.; Zhao, Z.; Zhao, Y.; Chen, L.; Fan, W.; Wang, X.; et al. Gut Microbiota Dysbiosis Induced by Decreasing Endogenous Melatonin Mediates the Pathogenesis of Alzheimer’s Disease and Obesity. Front. Immunol. 2022, 13, 900132. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Boutriq, S.; Plaza-Andrades, I.; Peralta-Linero, J.; Alba, E.; González-González, A.; Queipo-Ortuño, M.I. A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent. Cancers 2021, 13, 3141. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C.; Franceschetti, L.; Gianò, M.; Favero, G. A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role. Int. J. Mol. Sci. 2022, 23, 3758. [Google Scholar] [CrossRef]
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut melatonin: A potent candidate in the diversified journey of melatonin research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef]
- Fowler, S.; Hoedt, E.C.; Talley, N.J.; Keely, S.; Burns, G.L. Circadian Rhythms and Melatonin Metabolism in Patients With Disorders of Gut-Brain Interactions. Front. Neurosci. 2022, 16, 825246. [Google Scholar] [CrossRef]
- Majka, J.; Wierdak, M.; Brzozowska, I.; Magierowski, M.; Szlachcic, A.; Wojcik, D.; Kwiecien, S.; Magierowska, K.; Zagajewski, J.; Brzozowski, T. Melatonin in Prevention of the Sequence from Reflux Esophagitis to Barrett’s Esophagus and Esophageal Adenocarcinoma: Experimental and Clinical Perspectives. Int. J. Mol. Sci. 2018, 19, 2033. [Google Scholar] [CrossRef] [PubMed]
- Kanova, M.; Kohout, P. Tryptophan: A Unique Role in the Critically Ill. Int. J. Mol. Sci. 2021, 22, 11714. [Google Scholar] [CrossRef] [PubMed]
- Bantounou, M.; Plascevic, J.; Galley, H.F. Melatonin and Related Compounds: Antioxidant and Anti-Inflammatory Actions. Antioxidants 2022, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin prevents the dysbiosis of intestinal microbiota in sleep-restricted mice by improving oxidative stress and inhibiting inflammation. Saudi J. Gastroenterol. 2022, 28, 209–217. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, S.H.; Park, J.W.; Kho, Y.; Seok, P.R.; Shin, J.H.; Choi, Y.J.; Jun, J.H.; Jung, H.C.; Kim, E.K. Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation. Intest Res. 2020, 18, 325–336. [Google Scholar] [CrossRef]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef]
- Tascioglu Aliyev, A.; Panieri, E.; Stepanić, V.; Gurer-Orhan, H.; Saso, L. Involvement of NRF2 in Breast Cancer and Possible Therapeutical Role of Polyphenols and Melatonin. Molecules 2021, 26, 1853. [Google Scholar] [CrossRef]
- Labban, S.; Alghamdi, B.S.; Alshehri, F.S.; Kurdi, M. Effects of melatonin and resveratrol on recognition memory and passive avoidance performance in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2021, 402, 113100. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Gómez-Lado, N.; Lázare-Iglesias, H.; Rey-Bretal, D.; Lamela-Gómez, I.; Otero-Espinar, F.J.; Blanco-Méndez, J.; Antúnez-López, J.R.; Pombo-Pasín, M.; Aguiar, P.; et al. Evaluation of the therapeutic activity of melatonin and resveratrol in Inflammatory Bowel Disease: A longitudinal PET/CT study in an animal model. Int. J. Pharm. 2019, 572, 118713. [Google Scholar] [CrossRef]
- Marhuenda, J.; Medina, S.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Oger, C.; Galano, J.M.; Durand, T.; Solana, A.; et al. Effect of the dietary intake of melatonin- and hydroxytyrosol-rich wines by healthy female volunteers on the systemic lipidomic-related oxylipins. Food Funct. 2017, 8, 3745–3757. [Google Scholar] [CrossRef]
- Elbe, H.; Esrefoglu, M.; Vardi, N.; Taslidere, E.; Ozerol, E.; Tanbek, K. Melatonin, quercetin and resveratrol attenuates oxidative hepatocellular injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2015, 34, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Y.; Wu, X.; Zhao, G.; Zhang, K.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin and (-)-Epigallocatechin-3-Gallate: Partners in Fighting Cancer. Cells 2019, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Chattree, V.; Singh, K.; Singh, K.; Goel, A.; Maity, A.; Lone, A. A comprehensive review on modulation of SIRT1 signaling pathways in the immune system of COVID-19 patients by phytotherapeutic melatonin and epigallocatechin-3-gallate. J. Food Biochem. 2022, e14259. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Neurotoxins: Free radical mechanisms and melatonin protection. Curr. Neuropharmacol. 2010, 8, 194–210. [Google Scholar] [CrossRef]
- Brinkmann, V.; Ale-Agha, N.; Haendeler, J.; Ventura, N. The Aryl Hydrocarbon Receptor (AhR) in the Aging Process: Another Puzzling Role for This Highly Conserved Transcription Factor. Front. Physiol. 2019, 10, 1561. [Google Scholar] [CrossRef]
- Fila, M.; Chojnacki, J.; Pawlowska, E.; Szczepanska, J.; Chojnacki, C.; Blasiak, J. Kynurenine Pathway of Tryptophan Metabolism in Migraine and Functional Gastrointestinal Disorders. Int. J. Mol. Sci. 2021, 22, 10134. [Google Scholar] [CrossRef]
- Escames, G.; López, A.; García, J.A.; García, L.; Acuña-Castroviejo, D.; García, J.J.; López, L.C. The role of mitochondria in brain aging and the effects of melatonin. Curr. Neuropharmacol. 2010, 8, 182–193. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; de Mange, J.; Phillips, W.T.; Tan, D.X.; Bitar, R.D. Melatonin in ventricular and subarachnoid cerebrospinal fluid: Its function in the neural glymphatic network and biological significance for neurocognitive health. Biochem. Biophys. Res. Commun. 2022, 605, 70–81. [Google Scholar] [CrossRef]
- Olcese, J.M.; Cao, C.; Mori, T.; Mamcarz, M.B.; Maxwell, A.; Runfeldt, M.J.; Wang, L.; Zhang, C.; Lin, X.; Zhang, G.; et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J. Pineal Res. 2009, 47, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Brown, G.M.; Cardinali, D.P. Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimers Dis. 2011, 2011, 326320. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.; Foster, R.G.; Jagannath, A. Photic Entrainment of the Circadian System. Int. J. Mol. Sci. 2022, 23, 729. [Google Scholar] [CrossRef]
- Burgess, H.J.; Revell, V.L.; Molina, T.A.; Eastman, C.I. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J. Clin. Endocrinol. Metab. 2010, 95, 3325–3331. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. Disorders. In International. Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Auger, R.R.; Burgess, H.J.; Emens, J.S.; Deriy, L.V.; Thomas, S.M.; Sharkey, K.M. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2015, 11, 1199–1236. [Google Scholar] [CrossRef]
- Iguichi, H.; Kato, K.I.; Ibayashi, H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J. Clin. Endocrinol. Metab. 1982, 55, 27–29. [Google Scholar] [CrossRef]
- Knutsson, A. Health disorders of shift workers. Occup. Med. 2003, 53, 103–108. [Google Scholar] [CrossRef]
- Zee, P.C.; Goldstein, C.A. Treatment of shift work disorder and jet lag. Curr. Treat. Options. Neurol. 2010, 12, 396–411. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Lee-Chiong, T.; Alessi, C.; Friedman, L.; Aurora, R.N.; Boehlecke, B.; Brown, T.; Chesson, A.L., Jr.; Kapur, V.; Maganti, R.; et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007, 30, 1445–1459. [Google Scholar] [CrossRef]
- Sletten, T.L.; Magee, M.; Murray, J.M.; Gordon, C.J.; Lovato, N.; Kennaway, D.J.; Gwini, S.M.; Bartlett, D.J.; Lockley, S.W.; Lack, L.C.; et al. Efficacy of melatonin with behavioural sleep-wake scheduling for delayed sleep-wake phase disorder: A double-blind, randomised clinical trial. PLoS Med. 2018, 15, e1002587. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Jet lag: Therapeutic use of melatonin and possible application of melatonin analogs. Travel. Med. Infect. Dis. 2008, 6, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Herxheimer, A.; Petrie, K.J. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst. Rev. 2002, 2, Cd001520. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, D.C.; Jansen van Rensburg, A.; Fowler, P.M.; Bender, A.M.; Stevens, D.; Sullivan, K.O.; Fullagar, H.H.K.; Alonso, J.M.; Biggins, M.; Claassen-Smithers, A.; et al. Managing Travel Fatigue and Jet Lag in Athletes: A Review and Consensus Statement. Sports Med. 2021, 51, 2029–2050. [Google Scholar] [CrossRef] [PubMed]
- National Center for Complementary and Integrative Health. Sleep Disorders: In Depth. 2015. Available online: https://www.nccih.nih.gov/health/sleep-disorders-in-depth (accessed on 31 July 2022).
- Haack, M.; Simpson, N.; Sethna, N.; Kaur, S.; Mullington, J. Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology 2020, 45, 205–216. [Google Scholar] [CrossRef]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2017, 42, 129–155. [Google Scholar] [CrossRef]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [CrossRef]
- Kline, C.E.; Hall, M.H.; Buysse, D.J.; Earnest, C.P.; Church, T.S. Poor Sleep Quality is Associated with Insulin Resistance in Postmenopausal Women With and Without Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 183–189. [Google Scholar] [CrossRef]
- Sondrup, N.; Termannsen, A.D.; Eriksen, J.N.; Hjorth, M.F.; Færch, K.; Klingenberg, L.; Quist, J.S. Effects of sleep manipulation on markers of insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2022, 62, 101594. [Google Scholar] [CrossRef]
- Rahman, H.H.; Niemann, D.; Yusuf, K.K. Association of urinary arsenic and sleep disorder in the US population: NHANES 2015-2016. Environ. Sci. Pollut. Res. Int. 2022, 29, 5496–5504. [Google Scholar] [CrossRef]
- Shiue, I. Urinary arsenic, pesticides, heavy metals, phthalates, polyaromatic hydrocarbons, and polyfluoroalkyl compounds are associated with sleep troubles in adults: USA NHANES, 2005-2006. Environ. Sci. Pollut. Res. Int. 2017, 24, 3108–3116. [Google Scholar] [CrossRef]
- Morgenthaler, T.; Kramer, M.; Alessi, C.; Friedman, L.; Boehlecke, B.; Brown, T.; Coleman, J.; Kapur, V.; Lee-Chiong, T.; Owens, J.; et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An american academy of sleep medicine report. Sleep 2006, 29, 1415–1419. [Google Scholar] [PubMed]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.N.; Vanderheyden, W.M. The Molecular Relationship between Stress and Insomnia. Adv. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Lu, L.; Knutson, K.L.; Carnethon, M.R.; Fly, A.D.; Luo, J.; Haas, D.M.; Shikany, J.M.; Kahe, K. Association of magnesium intake with sleep duration and sleep quality: Findings from the CARDIA study. Sleep 2022, 45, zsab276. [Google Scholar] [CrossRef]
- Ikonte, C.J.; Mun, J.G.; Reider, C.A.; Grant, R.W.; Mitmesser, S.H. Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005-2016. Nutrients 2019, 11, 2335. [Google Scholar] [CrossRef]
- Reid, K.; Van den Heuvel, C.; Dawson, D. Day-time melatonin administration: Effects on core temperature and sleep onset latency. J. Sleep Res. 1996, 5, 150–154. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. Focus 2018, 16, 113–118. [Google Scholar] [CrossRef]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Duffy, J.F.; Wang, W.; Ronda, J.M.; Czeisler, C.A. High dose melatonin increases sleep duration during nighttime and daytime sleep episodes in older adults. J. Pineal Res. 2022, 73, e12801. [Google Scholar] [CrossRef] [PubMed]
- Kunz, D.; Stotz, S.; Bes, F. Treatment of isolated REM sleep behavior disorder using melatonin as a chronobiotic. J. Pineal Res. 2021, 71, e12759. [Google Scholar] [CrossRef]
- Aubin, S.; Kupers, R.; Ptito, M.; Jennum, P. Melatonin and cortisol profiles in the absence of light perception. Behav. Brain Res. 2017, 317, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ostrin, L.A. Ocular and systemic melatonin and the influence of light exposure. Clin. Exp. Optom. 2019, 102, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.L.; Fleitas, M.F.G.; Dieguez, H.; Iaquinandi, A.; Sande, P.H.; Dorfman, D.; Rosenstein, R.E. Melatonin as a Therapeutic Resource for Inflammatory Visual Diseases. Curr. Neuropharmacol. 2017, 15, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Martín-Gil, A.; Carpena-Torres, C.; Pastrana, C.; Carracedo, G. Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma. Biomolecules 2021, 11, 340. [Google Scholar] [CrossRef]
- Gubin, D.; Weinert, D. Melatonin, circadian rhythms and glaucoma: Current perspective. Neural Regen. Res. 2022, 17, 1759–1760. [Google Scholar] [CrossRef]
- Rastmanesh, R. Potential of melatonin to treat or prevent age-related macular degeneration through stimulation of telomerase activity. Med. Hypotheses 2011, 76, 79–85. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Hemati, K.; Reiter, R.J.; Hosseinzadeh, A. Mitochondrial dysfunction in age-related macular degeneration: Melatonin as a potential treatment. Expert Opin. Ther. Targets 2020, 24, 359–378. [Google Scholar] [CrossRef]
- Lin, L.; Huang, Q.X.; Yang, S.S.; Chu, J.; Wang, J.Z.; Tian, Q. Melatonin in Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 14575–14593. [Google Scholar] [CrossRef]
- Roy, J.; Tsui, K.C.; Ng, J.; Fung, M.L.; Lim, L.W. Regulation of Melatonin and Neurotransmission in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 6841. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wan, J.; Liu, A.; Sun, J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020, 132, 110887. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; von Gizycki, H.; Zizi, F. Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J. Pineal Res. 1998, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Furio, A.M.; Brusco, L.I.; Cardinali, D.P. Possible therapeutic value of melatonin in mild cognitive impairment: A retrospective study. J. Pineal Res. 2007, 43, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Tractenberg, R.E.; Singer, C.M.; Cummings, J.L.; Thal, L.J. The Sleep Disorders Inventory: An instrument for studies of sleep disturbance in persons with Alzheimer’s disease. J. Sleep Res. 2003, 12, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Esteban, S.; Garau, C.; Aparicio, S.; Moranta, D.; Barceló, P.; Fiol, M.A.; Rial, R. Chronic melatonin treatment and its precursor L-tryptophan improve the monoaminergic neurotransmission and related behavior in the aged rat brain. J. Pineal Res. 2010, 48, 170–177. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Martini Ferreira, A.; Ribeiro, R.T.; Zukerman, E.; Cipolla-Neto, J.; Peres, M.F. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1127–1132. [Google Scholar] [CrossRef]
- Gelfand, A.A.; Goadsby, P.J. The Role of Melatonin in the Treatment of Primary Headache Disorders. Headache 2016, 56, 1257–1266. [Google Scholar] [CrossRef]
- Danilov, A.B.; Danilov, A.B.; Kurushina, O.V.; Shestel, E.A.; Zhivolupov, S.A.; Latysheva, N.V. Safety and Efficacy of Melatonin in Chronic Tension-Type Headache: A Post-Marketing Real-World Surveillance Program. Pain Ther. 2020, 9, 741–750. [Google Scholar] [CrossRef]
- Hurtuk, A.; Dome, C.; Holloman, C.H.; Wolfe, K.; Welling, D.B.; Dodson, E.E.; Jacob, A. Melatonin: Can it stop the ringing? Ann. Otol. Rhinol. Laryngol. 2011, 120, 433–440. [Google Scholar] [CrossRef]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Smits, M.; Boss, M.; Miedema, I.; van Geijlswijk, I. Efficacy and safety of supplemental melatonin for delayed sleep-wake phase disorder in children: An overview. Sleep Med. X 2020, 2, 100022. [Google Scholar] [CrossRef] [PubMed]
- Avcil, S.; Uysal, P.; Yenisey, Ç.; Abas, B.I. Elevated Melatonin Levels in Children With Attention Deficit Hyperactivity Disorder: Relationship to Oxidative and Nitrosative Stress. J. Atten. Disord. 2021, 25, 693–703. [Google Scholar] [CrossRef]
- Melke, J.; Goubran Botros, H.; Chaste, P.; Betancur, C.; Nygren, G.; Anckarsäter, H.; Rastam, M.; Ståhlberg, O.; Gillberg, I.C.; Delorme, R.; et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 2008, 13, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Kothare, S.; DeBassio, W. The Assessment and Treatment of Sleep Abnormalities in Children and Adolescents with Autism Spectrum Disorder: A Review. J. Can. Acad. Child Adolesc. Psychiatry 2021, 30, 25–35. [Google Scholar] [PubMed]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 2011, 50, 261–266. [Google Scholar] [CrossRef]
- Lee, E.K.; Poon, P.; Yu, C.P.; Lee, V.W.; Chung, V.C.; Wong, S.Y. Controlled-release oral melatonin supplementation for Hypertension and nocturnal Hypertension: A systematic review and meta-analysis. J. Clin. Hypertens. 2022, 24, 529–535. [Google Scholar] [CrossRef]
- Simko, F.; Baka, T.; Paulis, L.; Reiter, R.J. Elevated heart rate and nondipping heart rate as potential targets for melatonin: A review. J. Pineal Res. 2016, 61, 127–137. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Ojike, N.I.; Akinseye, O.A.; Kendzerska, T.; Buttoo, K.; Dhandapany, P.S.; Brown, G.M.; Cardinali, D.P. Melatonin and Human Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 122–132. [Google Scholar] [CrossRef]
- Nduhirabandi, F.; Maarman, G.J. Melatonin in Heart Failure: A Promising Therapeutic Strategy? Molecules 2018, 23, 1819. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Klein, T.; Geenen, L.W.; Tu, L.; Tian, S.; van den Bosch, A.E.; de Rijke, Y.B.; Reiss, I.K.M.; Boersma, E.; Duncker, D.J.; et al. Lower Plasma Melatonin Levels Predict Worse Long-Term Survival in Pulmonary Arterial Hypertension. J. Clin. Med. 2020, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.G.; Heshmat-Ghahdarijani, K.; Khosrawi, S.; Garakyaraghi, M.; Shafie, D.; Roohafza, H.; Mansourian, M.; Azizi, E.; Gheisari, Y.; Sadeghi, M. Effect of melatonin supplementation on endothelial function in heart failure with reduced ejection fraction: A randomized, double-blinded clinical trial. Clin. Cardiol. 2021, 44, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, E.S.; Kampmann, U.; Pedersen, M.G.B.; Christensen, L.L.; Jessen, N.; Møller, N.; Støy, J. Three months of melatonin treatment reduces insulin sensitivity in patients with type 2 diabetes-A randomized placebo-controlled crossover trial. J. Pineal Res. 2022, 73, e12809. [Google Scholar] [CrossRef]
- Kampmann, U.; Lauritzen, E.S.; Grarup, N.; Jessen, N.; Hansen, T.; Møller, N.; Støy, J. Acute metabolic effects of melatonin-A randomized crossover study in healthy young men. J. Pineal Res. 2021, 70, e12706. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 2018, 37, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Lopez-Minguez, J.; Dashti, H.S.; Vetter, C.; Hernández-Martínez, A.M.; Pérez-Ayala, M.; Baraza, J.C.; Wang, W.; Florez, J.C.; Scheer, F.; et al. Interplay of Dinner Timing and MTNR1B Type 2 Diabetes Risk Variant on Glucose Tolerance and Insulin Secretion: A Randomized Crossover Trial. Diabetes Care 2022, 45, 512–519. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Soares, J.M., Jr.; Gallo, C.C.; Furtado, A.; Cavaco, J.E.; Gonçalves, I.; Santos, C.R.A.; Quintela, T. The Crosstalk between Melatonin and Sex Steroid Hormones. Neuroendocrinology 2022, 112, 115–129. [Google Scholar] [CrossRef]
- Wilkinson, D.; Shepherd, E.; Wallace, E.M. Melatonin for women in pregnancy for neuroprotection of the fetus. Cochrane Database Syst. Rev. 2016, 3, Cd010527. [Google Scholar] [CrossRef]
- Aversa, S.; Pellegrino, S.; Barberi, I.; Reiter, R.J.; Gitto, E. Potential utility of melatonin as an antioxidant during pregnancy and in the perinatal period. J. Matern. Fetal Neonatal. Med. 2012, 25, 207–221. [Google Scholar] [CrossRef]
- Zeng, K.; Gao, Y.; Wan, J.; Tong, M.; Lee, A.C.; Zhao, M.; Chen, Q. The reduction in circulating levels of melatonin may be associated with the development of preeclampsia. J. Hum. Hypertens. 2016, 30, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.O.; Evsyukova, I.I.; Mironova, E.S.; Polyakova, V.O.; Kvetnoy, I.M.; Nasyrov, R.A. Maternal Melatonin Deficiency Leads to Endocrine Pathologies in Children in Early Ontogenesis. Int. J. Mol. Sci. 2021, 22, 2058. [Google Scholar] [CrossRef] [PubMed]
- Soleimani Rad, S.; Abbasalizadeh, S.; Ghorbani Haghjo, A.; Sadagheyani, M.; Montaseri, A.; Soleimani Rad, J. Evaluation of the melatonin and oxidative stress markers level in serum of fertile and infertile women. Iran. J. Reprod. Med. 2015, 13, 439–444. [Google Scholar] [PubMed]
- Soleimani Rad, S.; Abbasalizadeh, S.; Ghorbani Haghjo, A.; Sadagheyani, M.; Montaseri, A.; Soleimani Rad, J. Serum Levels of Melatonin and Oxidative Stress Markers and Correlation between Them in Infertile Men. J. Caring Sci. 2013, 2, 287–294. [Google Scholar] [CrossRef][Green Version]
- Kratz, E.M.; Piwowar, A.; Zeman, M.; Stebelová, K.; Thalhammer, T. Decreased melatonin levels and increased levels of advanced oxidation protein products in the seminal plasma are related to male infertility. Reprod. Fertil. Dev. 2016, 28, 507–515. [Google Scholar] [CrossRef]
- Kratz, E.M.; Piwowar, A. Melatonin, advanced oxidation protein products and total antioxidant capacity as seminal parameters of prooxidant-antioxidant balance and their connection with expression of metalloproteinases in context of male fertility. J. Physiol. Pharmacol. 2017, 68, 659–668. [Google Scholar]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef]
- Berbets, A.M.; Davydenko, I.S.; Barbe, A.M.; Konkov, D.H.; Albota, O.M.; Yuzko, O.M. Melatonin 1A and 1B Receptors’ Expression Decreases in the Placenta of Women with Fetal Growth Restriction. Reprod. Sci. 2021, 28, 197–206. [Google Scholar] [CrossRef]
- Fernando, S.; Rombauts, L. Melatonin: Shedding light on infertility?—A review of the recent literature. J. Ovarian. Res. 2014, 7, 98. [Google Scholar] [CrossRef]
- Bao, Z.; Li, G.; Wang, R.; Xue, S.; Zeng, Y.; Deng, S. Melatonin Improves Quality of Repeated-Poor and Frozen-Thawed Embryos in Human, a Prospective Clinical Trial. Front. Endocrinol. 2022, 13, 853999. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, K.; Zhang, C.; Chen, B.; Zou, H.; Zou, W.; Xue, R.; Ji, D.; Yu, Z.; Rao, B.; et al. Effect of melatonin on the clinical outcome of patients with repeated cycles after failed cycles of in vitro fertilization and intracytoplasmic sperm injection. Zygote 2022, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, C.; Çelik, S.; Hatirnaz, S.; Çelik, H.; Avcı, B.; Tinelli, A. The Role of Delivery Route on Colostrum Melatonin and Serum Il-6 Levels: A Prospective Controlled Study. Z. Für Geburtshilfe Neonatol. 2021, 225, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Trifu, S.; Vladuti, A.; Popescu, A. The neuroendocrinological aspects of pregnancy and postpartum depression. Acta Endocrinol. 2019, 15, 410–415. [Google Scholar] [CrossRef]
- Namlı Kalem, M.; Kalem, Z.; Yuce, T.; Bakırarar, B.; Söylemez, F. Comparison of Melatonin Levels in the Colostrum between Vaginal Delivery and Cesarean Delivery. Am. J. Perinatol. 2018, 35, 481–485. [Google Scholar] [CrossRef]
- Kiabi, F.H.; Emadi, S.A.; Jamkhaneh, A.E.; Aezzi, G.; Ahmadi, N.S. Effects of preoperative melatonin on postoperative pain following cesarean section: A randomized clinical trial. Ann. Med. Surg. 2021, 66, 102345. [Google Scholar] [CrossRef]
- Schwertner, A.; Conceição Dos Santos, C.C.; Costa, G.D.; Deitos, A.; de Souza, A.; de Souza, I.C.; Torres, I.L.; da Cunha Filho, J.S.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase II, randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef]
- Söderman, L.; Edlund, M.; Böttiger, Y.; Marions, L. Adjuvant use of melatonin for pain management in dysmenorrhea—A randomized double-blinded, placebo-controlled trial. Eur. J. Clin. Pharmacol. 2022, 78, 191–196. [Google Scholar] [CrossRef]
- Chuffa, L.G.A.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2019, 21, 300. [Google Scholar] [CrossRef]
- Anderson, G. Endometriosis Pathoetiology and Pathophysiology: Roles of Vitamin A, Estrogen, Immunity, Adipocytes, Gut Microbiome and Melatonergic Pathway on Mitochondria Regulation. Biomol. Concepts 2019, 10, 133–149. [Google Scholar] [CrossRef]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Tamura, H.; Cruz, M.H.; Fuentes-Broto, L. Clinical relevance of melatonin in ovarian and placental physiology: A review. Gynecol. Endocrinol. 2014, 30, 83–89. [Google Scholar] [CrossRef]
- Simon, S.L.; McWhirter, L.; Diniz Behn, C.; Bubar, K.M.; Kaar, J.L.; Pyle, L.; Rahat, H.; Garcia-Reyes, Y.; Carreau, A.M.; Wright, K.P.; et al. Morning Circadian Misalignment Is Associated With Insulin Resistance in Girls With Obesity and Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 3525–3534. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Zhang, C. Women with polycystic ovary syndrome (PCOS) have reduced melatonin concentrations in their follicles and have mild sleep disturbances. BMC Womens Health 2022, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Xu, J.; Shi, H.; Li, W.; Li, Q.; Sun, Y.P. Association between melatonin receptor gene polymorphisms and polycystic ovarian syndrome: A systematic review and meta-analysis. Biosci. Rep. 2020, 40, BSR20200824. [Google Scholar] [CrossRef]
- Tagliaferri, V.; Romualdi, D.; Scarinci, E.; Cicco, S.; Florio, C.D.; Immediata, V.; Tropea, A.; Santarsiero, C.M.; Lanzone, A.; Apa, R. Melatonin Treatment May Be Able to Restore Menstrual Cyclicity in Women With PCOS: A Pilot Study. Reprod. Sci. 2018, 25, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Karandish, M.; Asghari Jafarabadi, M.; Heidari, L.; Nikbakht, R.; Babaahmadi Rezaei, H.; Mousavi, R. Metabolic and hormonal effects of melatonin and/or magnesium supplementation in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Nutr. Metab. 2021, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Arushanian, É.B.; Karakov, K.G.; Él’bek’ian, K.S. Therapeutic potential of melatonin in oral cavity diseases. Eksp. Klin. Farmakol. 2012, 75, 48–52. [Google Scholar]
- Cengiz, M.; Cengiz, S.; Wang, H.L. Melatonin and oral cavity. Int. J. Dent. 2012, 2012, 491872. [Google Scholar] [CrossRef]
- Celinski, K.; Konturek, P.C.; Konturek, S.J.; Slomka, M.; Cichoz-Lach, H.; Brzozowski, T.; Bielanski, W. Effects of melatonin and tryptophan on healing of gastric and duodenal ulcers with Helicobacter pylori infection in humans. J. Physiol. Pharmacol. 2011, 62, 521–526. [Google Scholar] [CrossRef]
- Mozaffari, S.; Abdollahi, M. Melatonin, a promising supplement in inflammatory bowel disease: A comprehensive review of evidences. Curr. Pharm. Des. 2011, 17, 4372–4378. [Google Scholar] [CrossRef]
- Kandil, T.S.; Mousa, A.A.; El-Gendy, A.A.; Abbas, A.M. The potential therapeutic effect of melatonin in Gastro-Esophageal Reflux Disease. BMC Gastroenterol. 2010, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Siah, K.T.; Wong, R.K.; Ho, K.Y. Melatonin for the treatment of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jurado, A.; Escribano, B.M.; Caballero-Villarraso, J.; Galván, A.; Agüera, E.; Santamaría, A.; Túnez, I. Melatonin and multiple sclerosis: Antioxidant, anti-inflammatory and immunomodulator mechanism of action. Inflammopharmacology 2022, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Farez, M.F.; Mascanfroni, I.D.; Méndez-Huergo, S.P.; Yeste, A.; Murugaiyan, G.; Garo, L.P.; Balbuena Aguirre, M.E.; Patel, B.; Ysrraelit, M.C.; Zhu, C.; et al. Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell 2015, 162, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Sowa, M.; Pierzchala, K.; Sowa, P.; Polaniak, R.; Kukla, M.; Hartel, M. Influence of melatonin supplementation on serum antioxidative properties and impact of the quality of life in multiple sclerosis patients. J. Physiol. Pharmacol. 2014, 65, 543–550. [Google Scholar]
- Sánchez-López, A.L.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramírez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.C.; Ramírez-Jirano, L.J.; Velázquez-Brizuela, I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch. Med. Res. 2018, 49, 391–398. [Google Scholar] [CrossRef]
- Yosefifard, M.; Vaezi, G.; Malekirad, A.A.; Faraji, F.; Hojati, V. A Randomized Control Trial Study to Determine the Effect of Melatonin on Serum Levels of IL-1β and TNF-α in Patients with Multiple Sclerosis. Iran. J. Allergy Asthma Immunol. 2019, 18, 649–654. [Google Scholar] [CrossRef]
- Anderson, G.; Rodriguez, M.; Reiter, R.J. Multiple Sclerosis: Melatonin, Orexin, and Ceramide Interact with Platelet Activation Coagulation Factors and Gut-Microbiome-Derived Butyrate in the Circadian Dysregulation of Mitochondria in Glia and Immune Cells. Int. J. Mol. Sci. 2019, 20, 5500. [Google Scholar] [CrossRef]
- Danailova, Y.; Velikova, T.; Nikolaev, G.; Mitova, Z.; Shinkov, A.; Gagov, H.; Konakchieva, R. Nutritional Management of Thyroiditis of Hashimoto. Int. J. Mol. Sci. 2022, 23, 5144. [Google Scholar] [CrossRef]
- Giovane, R.A.; Di Giovanni-Kinsley, S.; Keeton, E. Micronutrients for potential therapeutic use against COVID-19; a review. Clin. Nutr. ESPEN 2021, 46, 9–13. [Google Scholar] [CrossRef]
- Molina-Carballo, A.; Palacios-López, R.; Jerez-Calero, A.; Augustín-Morales, M.C.; Agil, A.; Muñoz-Hoyos, A.; Muñoz-Gallego, A. Protective Effect of Melatonin Administration against SARS-CoV-2 Infection: A Systematic Review. Curr. Issues Mol. Biol. 2021, 44, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients 2021, 13, 1261. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Khorshidi, M.; Emami, M.; Janmohammadi, P.; Kord-Varkaneh, H.; Mousavi, S.M.; Mohammed, S.H.; Saedisomeolia, A.; Alizadeh, S. Melatonin supplementation and pro-inflammatory mediators: A systematic review and meta-analysis of clinical trials. Eur. J. Nutr. 2020, 59, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Díaz-Castro, J.; Kajarabille, N.; García, C.; Guisado, I.M.; De Teresa, C.; Guisado, R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 2011, 51, 373–380. [Google Scholar] [CrossRef]
- Mączka, W.; Grabarczyk, M.; Wińska, K. Can Antioxidants Reduce the Toxicity of Bisphenol? Antioxidants 2022, 11, 413. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Cattaneo, G.; Tancini, G.; Esposti, G.; Esposti, D.; Fraschini, F. Clinical results with the pineal hormone melatonin in advanced cancer resistant to standard antitumor therapies. Oncology 1991, 48, 448–450. [Google Scholar] [CrossRef]
- Lissoni, P.; Messina, G.; Lissoni, A.; Franco, R. The psychoneuroendocrine-immunotherapy of cancer: Historical evolution and clinical results. J. Res. Med. Sci. 2017, 22, 45. [Google Scholar] [CrossRef]
- Lissoni, P.; Rovelli, F.; Vigorè, L.; Messina, G.; Lissoni, A.; Porro, G.; Di Fede, G. How to Monitor the Neuroimmune Biological Response in Patients Affected by Immune Alteration-Related Systemic Diseases. Methods Mol. Biol. 2018, 1781, 171–191. [Google Scholar] [CrossRef]
- Elsabagh, H.H.; Moussa, E.; Mahmoud, S.A.; Elsaka, R.O.; Abdelrahman, H. Efficacy of Melatonin in prevention of radiation-induced oral mucositis: A randomized clinical trial. Oral. Dis. 2020, 26, 566–572. [Google Scholar] [CrossRef]
- Johnston, D.L.; Zupanec, S.; Nicksy, D.; Morgenstern, D.; Narendran, A.; Deyell, R.J.; Samson, Y.; Wu, B.; Baruchel, S. Phase I dose-finding study for melatonin in pediatric oncology patients with relapsed solid tumors. Pediatr. Blood Cancer 2019, 66, e27676. [Google Scholar] [CrossRef]
- Lund Rasmussen, C.; Klee Olsen, M.; Thit Johnsen, A.; Petersen, M.A.; Lindholm, H.; Andersen, L.; Villadsen, B.; Groenvold, M.; Pedersen, L. Effects of melatonin on physical fatigue and other symptoms in patients with advanced cancer receiving palliative care: A double-blind placebo-controlled crossover trial. Cancer 2015, 121, 3727–3736. [Google Scholar] [CrossRef] [PubMed]
- Sookprasert, A.; Johns, N.P.; Phunmanee, A.; Pongthai, P.; Cheawchanwattana, A.; Johns, J.; Konsil, J.; Plaimee, P.; Porasuphatana, S.; Jitpimolmard, S. Melatonin in patients with cancer receiving chemotherapy: A randomized, double-blind, placebo-controlled trial. Anticancer Res 2014, 34, 7327–7337. [Google Scholar] [PubMed]
- Messina, G.; Lissoni, P.; Marchiori, P.; Bartolacelli, E.; Brivio, F.; Magotti, L. Enhancement of the efficacy of cancer chemotherapy by the pineal hormone melatonin and its relation with the psychospiritual status of cancer patients. J. Res. Med. Sci. 2010, 15, 225–228. [Google Scholar] [PubMed]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2021, 11, e01960. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.T.; Heist, E.K.; McGeoy, S.S.; Hanada, K.; Lesser, I.M. Neuroendocrine aspects of primary endogenous depression. XI. Serum melatonin measures in patients and matched control subjects. Arch. Gen. Psychiatry 1992, 49, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Messina, G.; Rovelli, F. Cancer as the main aging factor for humans: The fundamental role of 5-methoxy-tryptamine in reversal of cancer-induced aging processes in metabolic and immune reactions by non-melatonin pineal hormones. Curr. Aging Sci. 2012, 5, 231–235. [Google Scholar] [CrossRef]
- Hasan, M.; Browne, E.; Guarinoni, L.; Darveau, T.; Hilton, K.; Witt-Enderby, P.A. Novel Melatonin, Estrogen, and Progesterone Hormone Therapy Demonstrates Anti-Cancer Actions in MCF-7 and MDA-MB-231 Breast Cancer Cells. Breast Cancer 2020, 14, 1178223420924634. [Google Scholar] [CrossRef]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef]
- Cherrie, J.W. Shedding Light on the Association between Night Work and Breast Cancer. Ann. Work Expo. Health 2019, 63, 608–611. [Google Scholar] [CrossRef]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef]
- Haim, A.; Zubidat, A.E. Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140121. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.A.; Carvalho, R.F.; Justulin, L.A.; Cury, S.S.; Seiva, F.R.F.; Jardim-Perassi, B.V.; Zuccari, D.; Reiter, R.J. A meta-analysis of microRNA networks regulated by melatonin in cancer: Portrait of potential candidates for breast cancer treatment. J. Pineal Res. 2020, 69, e12693. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, G.; Liu, X.; Li, Z. Assessment of the Therapeutic Potential of Melatonin for the Treatment of Osteoporosis Through a Narrative Review of Its Signaling and Preclinical and Clinical Studies. Front. Pharmacol. 2022, 13, 866625. [Google Scholar] [CrossRef] [PubMed]
- Kotlarczyk, M.P.; Lassila, H.C.; O’Neil, C.K.; D’Amico, F.; Enderby, L.T.; Witt-Enderby, P.A.; Balk, J.L. Melatonin osteoporosis prevention study (MOPS): A randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J. Pineal Res. 2012, 52, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: A randomized controlled trial. J. Pineal Res. 2015, 59, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X. Melatonin: An antioxidant in edible plants. Ann. N. Y. Acad. Sci. 2002, 957, 341–344. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
- Xie, X.; Ding, D.; Bai, D.; Zhu, Y.; Sun, W.; Sun, Y.; Zhang, D. Melatonin biosynthesis pathways in nature and its production in engineered microorganisms. Synth. Syst. Biotechnol. 2022, 7, 544–553. [Google Scholar] [CrossRef]
- Yang, R.; Pu, D.; Tan, R.; Wu, J. Association of methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms (C677T and A1298C) with thyroid dysfunction: A meta-analysis and trial sequential analysis. Arch. Endocrinol. Metab. 2022, 66, 551–581. [Google Scholar] [CrossRef]
- Mabhida, S.E.; Muhamed, B.; Sharma, J.R.; Apalata, T.; Nomatshila, S.; Mabasa, L.; Benjeddou, M.; Masilela, C.; Ziqubu, K.; Shabalala, S.; et al. Methylenetetrahydrofolate Reductase Polymorphism (rs1801133) and the Risk of Hypertension among African Populations: A Narrative Synthesis of Literature. Genes 2022, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Huang, S.; Yang, Y.; He, X.; Fei, L.; Xing, Y. Association Between MTHFR Polymorphisms and the Risk of Essential Hypertension: An Updated Meta-analysis. Front. Genet. 2021, 12, 698590. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Błasiak, J.; Fichna, J.; Chojnacki, J.; Popławski, T. Evaluation of Melatonin Secretion and Metabolism Exponents in Patients with Ulcerative and Lymphocytic Colitis. Molecules 2018, 23, 272. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Yamakawa, M.; Nakashima, Y.; Koda, S.; Uji, T.; Onuma, S.; Oba, S.; Maruyama, Y.; Hattori, A. Associations Between Dietary Melatonin Intake and Total and Cause-Specific Mortality Among Japanese Adults in the Takayama Study. Am. J. Epidemiol. 2021, 190, 2639–2646. [Google Scholar] [CrossRef]
- Aguilera, Y.; Herrera, T.; Benítez, V.; Arribas, S.M.; López de Pablo, A.L.; Esteban, R.M.; Martín-Cabrejas, M.A. Estimation of scavenging capacity of melatonin and other antioxidants: Contribution and evaluation in germinated seeds. Food Chem. 2015, 170, 203–211. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The Potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef]
- Tan, D.X.; Zanghi, B.M.; Manchester, L.C.; Reiter, R.J. Melatonin identified in meats and other food stuffs: Potentially nutritional impact. J. Pineal Res. 2014, 57, 213–218. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An unexpected molecule with amazing performances in plants. J. Exp. Bot. 2022, erac009. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Melatonin in Mediterranean diet, a new perspective. J. Sci. Food Agric. 2015, 95, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Samara, M.T.; Huhn, M.; Chiocchia, V.; Schneider-Thoma, J.; Wiegand, M.; Salanti, G.; Leucht, S. Efficacy, acceptability, and tolerability of all available treatments for insomnia in the elderly: A systematic review and network meta-analysis. Acta. Psychiatr. Scand. 2020, 142, 6–17. [Google Scholar] [CrossRef]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot Study of the Tart Cherry Juice for the Treatment of Insomnia and Investigation of Mechanisms. Am. J. Ther. 2018, 25, e194–e201. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, W.R.; Carr, M.; Gorman, C.; Perlis, M.L. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: A pilot study. J. Med. Food 2010, 13, 579–583. [Google Scholar] [CrossRef]
- Hillman, A.R.; Trickett, O.; Brodsky, C.; Chrismas, B. Montmorency tart cherry supplementation does not impact sleep, body composition, cellular health, or blood pressure in healthy adults. Nutr. Health 2022, 2601060221111230. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Badria, F.A. Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Tan, D.X.; Manchester, L.C.; Reiter, R.J. Purslane: A plant source of omega-3 fatty acids and melatonin. J. Pineal Res. 2005, 39, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Adv. Bot. 2014, 2014, 815769. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Yılmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Riga, P.; Medina, S.; García-Flores, L.A.; Gil-Izquierdo, Á. Melatonin content of pepper and tomato fruits: Effects of cultivar and solar radiation. Food Chem. 2014, 156, 347–352. [Google Scholar] [CrossRef]
- Reinholds, I.; Pugajeva, I.; Radenkovs, V.; Rjabova, J.; Bartkevics, V. Development and Validation of New Ultra-High-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap Mass Spectrometry Method for Determination of Melatonin in Fruits. J. Chromatogr. Sci. 2016, 54, 977–984. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Liang, D.; Lin, L.; Deng, H.; Wang, J.; et al. Melatonin Accumulation in Sweet Cherry and Its Influence on Fruit Quality and Antioxidant Properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.T.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuffs: Measurement using a MEPS-HPLC-F method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid-liquid extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Dufoo-Hurtado, E.; Olvera-Bautista, R.; Wall-Medrano, A.; Loarca-Piña, G.; Campos-Vega, R. In vitro gastrointestinal digestion and simulated colonic fermentation of pistachio nuts determine the bioaccessibility and biosynthesis of chronobiotics. Food Funct. 2021, 12, 4921–4934. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of Melatonin from Lentil Sprouts and Its Role in the Plasmatic Antioxidant Status in Rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Herrera, T.; Liébana, R.; Rebollo-Hernanz, M.; Sanchez-Puelles, C.; Martín-Cabrejas, M.A. Impact of Melatonin Enrichment during Germination of Legumes on Bioactive Compounds and Antioxidant Activity. J. Agric. Food Chem. 2015, 63, 7967–7974. [Google Scholar] [CrossRef] [PubMed]
- Sangsopha, J.; Johns, N.P.; Johns, J.; Moongngarm, A. Dietary sources of melatonin and benefits from production of high melatonin pasteurized milk. J. Food Sci. Technol. 2020, 57, 2026–2037. [Google Scholar] [CrossRef]
- Padumanonda, T.; Johns, J.; Sangkasat, A.; Tiyaworanant, S. Determination of melatonin content in traditional Thai herbal remedies used as sleeping aids. Daru 2014, 22, 6. [Google Scholar] [CrossRef]
- Murch, S.J.; Simmons, C.B.; Saxena, P.K. Melatonin in feverfew and other medicinal plants. Lancet 1997, 350, 1598–1599. [Google Scholar] [CrossRef]
- Stege, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silva, M.F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef]
- Chen, G.; Huo, Y.; Tan, D.X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Venegas, C.; Cabrera-Vique, C.; García-Corzo, L.; Escames, G.; Acuña-Castroviejo, D.; López, L.C. Determination of coenzyme Q10, coenzyme Q9, and melatonin contents in virgin argan oils: Comparison with other edible vegetable oils. J. Agric. Food Chem. 2011, 59, 12102–12108. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pachón, M.S.; Medina, S.; Herrero-Martín, G.; Cerrillo, I.; Berná, G.; Escudero-López, B.; Ferreres, F.; Martín, F.; García-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. “Expression of Concern to Spectrofluorimetric Determination of Melatonin in Kernels of Four Different Pistacia Varieties after Ultrasound-Assisted Solid-Liquid Extraction” [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 132 (2014) 326–329]. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 217, 322. [Google Scholar] [CrossRef]
- Losso, J.N. Melatonin Contents of Raw and Roasted American Pistachios; School of Nutrition and Food Sciences, Louisiana State University: Baton Rouge, LA, USA, 2018. [Google Scholar]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan. Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef]
- Navarro-Alarcón, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; MM, A.S.; Acuña-Castroviejo, D.; Fernández-Vázquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Hardeland, R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology 2017, 104, 382–397. [Google Scholar] [CrossRef]
- Mullin, G.E.; Limektkai, B.; Wang, L.; Hanaway, P.; Marks, L.; Giovannucci, E. Dietary Supplements for COVID-19. Adv. Exp. Med. Biol. 2021, 1318, 499–515. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Simko, F.; Dominguez-Rodriguez, A.; Tesarik, J.; Neel, R.L.; Slominski, A.T.; Kleszczynski, K.; Martin-Gimenez, V.M.; Manucha, W.; et al. Melatonin: Highlighting its use as a potential treatment for SARS-CoV-2 infection. Cell Mol. Life Sci. 2022, 79, 143. [Google Scholar] [CrossRef]
- Li, J.; Somers, V.K.; Xu, H.; Lopez-Jimenez, F.; Covassin, N. Trends in Use of Melatonin Supplements Among US Adults, 1999-2018. JAMA 2022, 327, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Bronstein, A.C.; Rivers, L.J.; Pham, N.P.T.; Weber, J. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin. Toxicol. 2021, 59, 1282–1501. [Google Scholar] [CrossRef] [PubMed]
- Lelak, K.; Vohra, V.; Neuman, M.I.; Toce, M.S.; Sethuraman, U. Pediatric Melatonin Ingestions—United States, 2012–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 725–729. [Google Scholar] [CrossRef]
- Erland, L.A.; Saxena, P.K. Melatonin Natural Health Products and Supplements: Presence of Serotonin and Significant Variability of Melatonin Content. J. Clin. Sleep Med. 2017, 13, 275–281. [Google Scholar] [CrossRef]
- Sabarese, A. Lawsuits Filed Against Olly, PG, Alleging Deceptive Advertising of Melatonin Supplements. 2022. Available online: https://vitaminretailer.com/lawsuits-filed-against-olly-pg-alleging-deceptive-advertising-of-melatonin-supplements/ (accessed on 31 July 2022).
- Bovine spongiform encephalopathy: “Mad cow disease”. Nutr. Rev. 1996, 54, 208–210. [CrossRef]
- Zetner, D.; Andersen, L.P.K.; Alder, R.; Jessen, M.L.; Tolstrup, A.; Rosenberg, J. Pharmacokinetics and Safety of Intravenous, Intravesical, Rectal, Transdermal, and Vaginal Melatonin in Healthy Female Volunteers: A Cross-Over Study. Pharmacology 2021, 106, 169–176. [Google Scholar] [CrossRef]
- Zetner, D.; Andersen, L.P.; Rosenberg, J. Pharmacokinetics of Alternative Administration Routes of Melatonin: A Systematic Review. Drug Res. 2016, 66, 169–173. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon 2020, 6, e03648. [Google Scholar] [CrossRef]
- He, L.; Li, J.-L.; Zhang, J.-J.; Su, P.; Zheng, S.-L. Microwave Assisted Synthesis of Melatonin. Synthetic. Commun. 2003, 33, 741–747. [Google Scholar] [CrossRef]
- Williamson, B.L.; Tomlinson, A.J.; Naylor, S.; Gleich, G.J. Contaminants in commercial preparations of melatonin. Mayo Clin. Proc. 1997, 72, 1094–1095. [Google Scholar] [CrossRef]
- Williamson, B.L.; Tomlinson, A.J.; Mishra, P.K.; Gleich, G.J.; Naylor, S. Structural characterization of contaminants found in commercial preparations of melatonin: Similarities to case-related compounds from L-tryptophan associated with eosinophilia-myalgia syndrome. Chem. Res. Toxicol. 1998, 11, 234–240. [Google Scholar] [CrossRef]
- Allen, J.A.; Peterson, A.; Sufit, R.; Hinchcliff, M.E.; Mahoney, J.M.; Wood, T.A.; Miller, F.W.; Whitfield, M.L.; Varga, J. Post-epidemic eosinophilia-myalgia syndrome associated with L-tryptophan. Arthritis Rheum. 2011, 63, 3633–3639. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a Phytomelatonin-Rich Extract from Cultured Plants with Excellent Biochemical and Functional Properties as an Alternative to Synthetic Melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.M.; Tharmarajah, S.; Jia, Y.; Semba, R.D.; Schaumberg, D.A.; Robinson, K.A. The Effect of Lutein/Zeaxanthin Intake on Human Macular Pigment Optical Density: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 2244–2254. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.O. (Analytical Resource Laboratory, West Valley, UT, USA). Personal communication, 2021.
- Sofic, E.; Rimpapa, Z.; Kundurovic, Z.; Sapcanin, A.; Tahirovic, I.; Rustembegovic, A.; Cao, G. Antioxidant capacity of the neurohormone melatonin. J. Neural. Transm. 2005, 112, 349–358. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I. Comparative evaluation of the antioxidant activity of melatonin and related indoles. J. Food Compos. Anal. 2012, 28, 16–22. [Google Scholar] [CrossRef]
- Vural, E.M.; van Munster, B.C.; de Rooij, S.E. Optimal dosages for melatonin supplementation therapy in older adults: A systematic review of current literature. Drugs Aging 2014, 31, 441–451. [Google Scholar] [CrossRef]
- Menczel Schrire, Z.; Phillips, C.L.; Chapman, J.L.; Duffy, S.L.; Wong, G.; D’Rozario, A.L.; Comas, M.; Raisin, I.; Saini, B.; Gordon, C.J.; et al. Safety of higher doses of melatonin in adults: A systematic review and meta-analysis. J. Pineal Res. 2022, 72, e12782. [Google Scholar] [CrossRef]
- Benedict, C. Melatonin’s Potential Side Effects: It May Be in Your Genes. Mayo Clin. Proc. 2022, 97, 1401. [Google Scholar] [CrossRef]
- Bouatia-Naji, N.; Bonnefond, A.; Cavalcanti-Proença, C.; Sparsø, T.; Holmkvist, J.; Marchand, M.; Delplanque, J.; Lobbens, S.; Rocheleau, G.; Durand, E.; et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009, 41, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, R.M.; van Munster, B.C.; van Kempen, M.F.; de Rooij, S.E. Physiological melatonin levels in healthy older people: A systematic review. J. Psychosom. Res. 2016, 86, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, I.V.; Wurtman, R.J.; Regan, M.M.; Taylor, J.A.; Shi, J.P.; Leclair, O.U. Melatonin treatment for age-related insomnia. J. Clin. Endocrinol. Metab. 2001, 86, 4727–4730. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Methods of Inducing Sleep Using Melatonin. U.S. Patent US5449683A, 12 September 1995.
- Aldeghi, R.; Lissoni, P.; Barni, S.; Ardizzoia, A.; Tancini, G.; Piperno, A.; Pozzi, M.; Ricci, G.; Conti, A.; Maestroni, G.J. Low-dose interleukin-2 subcutaneous immunotherapy in association with the pineal hormone melatonin as a first-line therapy in locally advanced or metastatic hepatocellular carcinoma. Eur. J. Cancer 1994, 30, 167–170. [Google Scholar] [CrossRef]
- Lewy, A.J.; Emens, J.S.; Sack, R.L.; Hasler, B.P.; Bernert, R.A. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol. Int. 2002, 19, 649–658. [Google Scholar] [CrossRef]
- Sack, R.L.; Brandes, R.W.; Kendall, A.R.; Lewy, A.J. Entrainment of free-running circadian rhythms by melatonin in blind people. N. Engl. J. Med. 2000, 343, 1070–1077. [Google Scholar] [CrossRef]
- Suhner, A.; Schlagenhauf, P.; Johnson, R.; Tschopp, A.; Steffen, R. Comparative study to determine the optimal melatonin dosage form for the alleviation of jet lag. Chronobiol. Int. 1998, 15, 655–666. [Google Scholar] [CrossRef]
- Petrie, K.; Conaglen, J.V.; Thompson, L.; Chamberlain, K. Effect of melatonin on jet lag after long haul flights. Br. Med. J. 1989, 298, 705–707. [Google Scholar] [CrossRef]
- Petrie, K.; Dawson, A.G.; Thompson, L.; Brook, R. A double-blind trial of melatonin as a treatment for jet lag in international cabin crew. Biol. Psychiatry 1993, 33, 526–530. [Google Scholar] [CrossRef]
- Wright, S.W.; Lawrence, L.M.; Wrenn, K.D.; Haynes, M.L.; Welch, L.W.; Schlack, H.M. Randomized clinical trial of melatonin after night-shift work: Efficacy and neuropsychologic effects. Ann. Emerg. Med. 1998, 32, 334–340. [Google Scholar] [CrossRef]
- James, M.; Tremea, M.O.; Jones, J.S.; Krohmer, J.R. Can melatonin improve adaptation to night shift? Am. J. Emerg. Med. 1998, 16, 367–370. [Google Scholar] [CrossRef]
- Jorgensen, K.M.; Witting, M.D. Does exogenous melatonin improve day sleep or night alertness in emergency physicians working night shifts? Ann. Emerg. Med. 1998, 31, 699–704. [Google Scholar] [CrossRef]
- Buscemi, N.; Vandermeer, B.; Hooton, N.; Pandya, R.; Tjosvold, L.; Hartling, L.; Vohra, S.; Klassen, T.P.; Baker, G. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: Meta-analysis. Br. Med. J. 2006, 332, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Hoebert, M.; van der Heijden, K.B.; van Geijlswijk, I.M.; Smits, M.G. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J. Pineal Res. 2009, 47, 1–7. [Google Scholar] [CrossRef]
- Boafo, A.; Greenham, S.; Alenezi, S.; Robillard, R.; Pajer, K.; Tavakoli, P.; De Koninck, J. Could long-term administration of melatonin to prepubertal children affect timing of puberty? A clinician’s perspective. Nat. Sci. Sleep 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Ma, X.; Idle, J.R.; Krausz, K.W.; Gonzalez, F.J. Metabolism of melatonin by human cytochromes p450. Drug Metab. Dispos. 2005, 33, 489–494. [Google Scholar] [CrossRef]
- Braam, W.; van Geijlswijk, I.; Keijzer, H.; Smits, M.G.; Didden, R.; Curfs, L.M. Loss of response to melatonin treatment is associated with slow melatonin metabolism. J. Intellect. Disabil. Res. 2010, 54, 547–555. [Google Scholar] [CrossRef]
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [CrossRef]
- Savage, R.A.; Zafar, N.; Yohannan, S.; Miller, J.M.M. Melatonin. In StatPearls; StatPearls Publishing. Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Harpsøe, N.G.; Andersen, L.P.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef]
- Molska, A.; Nyman, A.K.G.; Sofias, A.M.; Kristiansen, K.A.; Hak, S.; Widerøe, M. In vitro and in vivo evaluation of organic solvent-free injectable melatonin nanoformulations. Eur. J. Pharm. Biopharm. 2020, 152, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Mistraletti, G.; Paroni, R.; Umbrello, M.; Moro Salihovic, B.; Coppola, S.; Froio, S.; Finati, E.; Gasco, P.; Savoca, A.; Manca, D.; et al. Different routes and formulations of melatonin in critically ill patients. A pharmacokinetic randomized study. Clin. Endocrinol. 2019, 91, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2019, 42, 65–81. [Google Scholar] [CrossRef]
- Facciolá, G.; Hidestrand, M.; von Bahr, C.; Tybring, G. Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur. J. Clin. Pharmacol. 2001, 56, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Huuhka, K.; Riutta, A.; Haataja, R.; Ylitalo, P.; Leinonen, E. The effect of CYP2C19 substrate on the metabolism of melatonin in the elderly: A randomized, double-blind, placebo-controlled study. Methods Find Exp. Clin. Pharmacol. 2006, 28, 447–450. [Google Scholar] [CrossRef]
- Foster, B.C.; Cvijovic, K.; Boon, H.S.; Tam, T.W.; Liu, R.; Murty, M.; Vu, D.; Jaeger, W.; Tsuyuki, R.T.; Barnes, J.; et al. Melatonin Interaction Resulting in Severe Sedation. J. Pharm. Pharm. Sci. 2015, 18, 124–131. [Google Scholar] [CrossRef]
- Härtter, S.; Wang, X.; Weigmann, H.; Friedberg, T.; Arand, M.; Oesch, F.; Hiemke, C. Differential effects of fluvoxamine and other antidepressants on the biotransformation of melatonin. J. Clin. Psychopharmacol. 2001, 21, 167–174. [Google Scholar] [CrossRef]
- Ursing, C.; Wikner, J.; Brismar, K.; Röjdmark, S. Caffeine raises the serum melatonin level in healthy subjects: An indication of melatonin metabolism by cytochrome P450(CYP)1A2. J. Endocrinol. Investig. 2003, 26, 403–406. [Google Scholar] [CrossRef]
- Therapeutic Research. Melatonin. Available online: https://naturalmedicines.therapeuticresearch.com/ (accessed on 31 July 2022).
- Raza, Z.; Naureen, Z. Melatonin ameliorates the drug induced nephrotoxicity: Molecular insights. Nefrologia 2020, 40, 12–25. [Google Scholar] [CrossRef]
- Shaki, F.; Ashari, S.; Ahangar, N. Melatonin can attenuate ciprofloxacin induced nephrotoxicity: Involvement of nitric oxide and TNF-α. Biomed. Pharmacother. 2016, 84, 1172–1178. [Google Scholar] [CrossRef]
- Honma, K.; Kohsaka, M.; Fukuda, N.; Morita, N.; Honma, S. Effects of vitamin B12 on plasma melatonin rhythm in humans: Increased light sensitivity phase-advances the circadian clock? Experientia 1992, 48, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Kohsaka, M.; Morita, N.; Fukuda, N.; Honma, S.; Honma, K. Vitamin B12 enhances the phase-response of circadian melatonin rhythm to a single bright light exposure in humans. Neurosci. Lett. 1996, 220, 129–132. [Google Scholar] [CrossRef]
- Mayer, G.; Kröger, M.; Meier-Ewert, K. Effects of vitamin B12 on performance and circadian rhythm in normal subjects. Neuropsychopharmacology 1996, 15, 456–464. [Google Scholar] [CrossRef]
- Rizzo, P.; Raffone, E.; Benedetto, V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 555–561. [Google Scholar]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2016, 32, 69–73. [Google Scholar] [CrossRef]
- Wdowiak, A.; Filip, M. The effect of myo-inositol, vitamin D3 and melatonin on the oocyte quality and pregnancy in in vitro fertilization: A randomized prospective controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8529–8536. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 14784. [Google Scholar] [CrossRef]
- Gu, J.C.; Wu, Y.G.; Huang, W.G.; Fan, X.J.; Chen, X.H.; Zhou, B.; Lin, Z.J.; Feng, X.L. Effect of vitamin D on oxidative stress and serum inflammatory factors in the patients with type 2 diabetes. J. Clin. Lab. Anal. 2022, 36, e24430. [Google Scholar] [CrossRef]
- Tähkämö, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Hester, L.; Dang, D.; Barker, C.J.; Heath, M.; Mesiya, S.; Tienabeso, T.; Watson, K. Evening wear of blue-blocking glasses for sleep and mood disorders: A systematic review. Chronobiol. Int. 2021, 38, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Alpert, M.; Kubulins, V.; Hansler, R.L. Use of modified spectacles and light bulbs to block blue light at night may prevent postpartum depression. Med. Hypotheses 2009, 73, 251–253. [Google Scholar] [CrossRef]
- Shechter, A.; Kim, E.W.; St-Onge, M.P.; Westwood, A.J. Blocking nocturnal blue light for insomnia: A randomized controlled trial. J. Psychiatr. Res. 2018, 96, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Kitajima, T.; Ito, Y.; Koike, S.; Nakao, Y.; Tsuchiya, A.; Hirose, M.; Iwata, N. Wearing blue light-blocking glasses in the evening advances circadian rhythms in the patients with delayed sleep phase disorder: An open-label trial. Chronobiol. Int. 2016, 33, 1037–1044. [Google Scholar] [CrossRef]
- Rzepka-Migut, B.; Paprocka, J. Melatonin-Measurement Methods and the Factors Modifying the Results. A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 1916. [Google Scholar] [CrossRef]
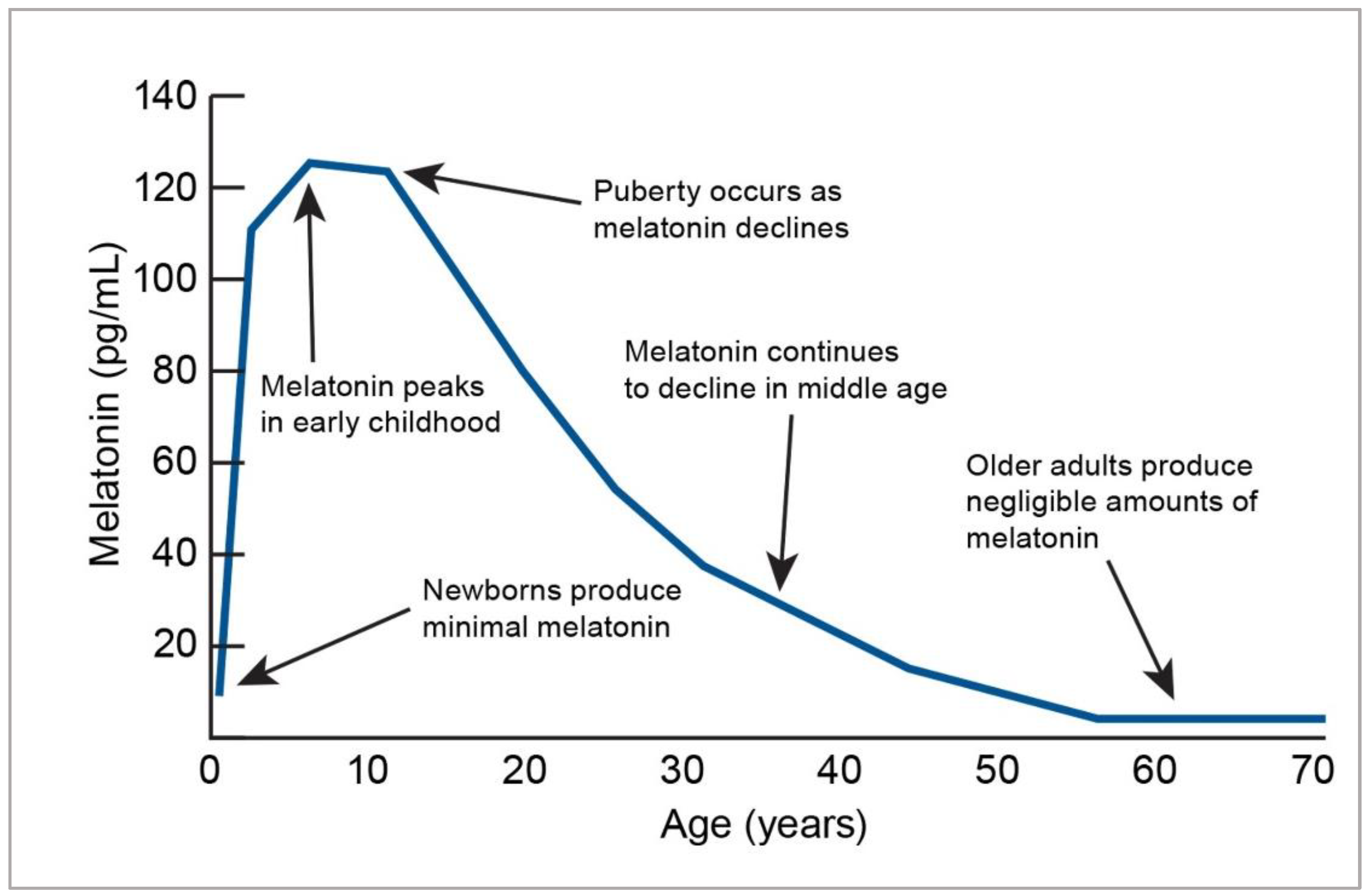
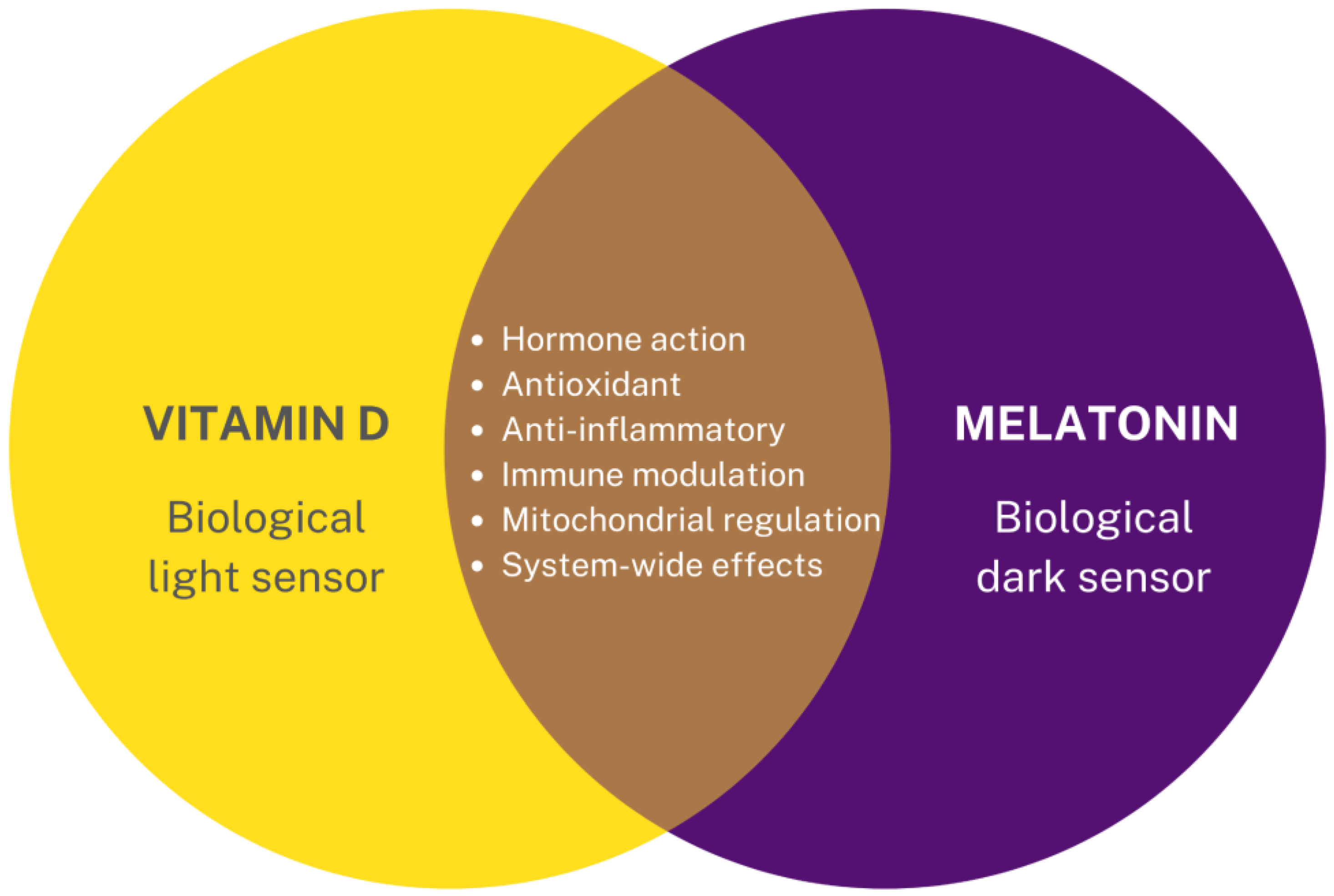
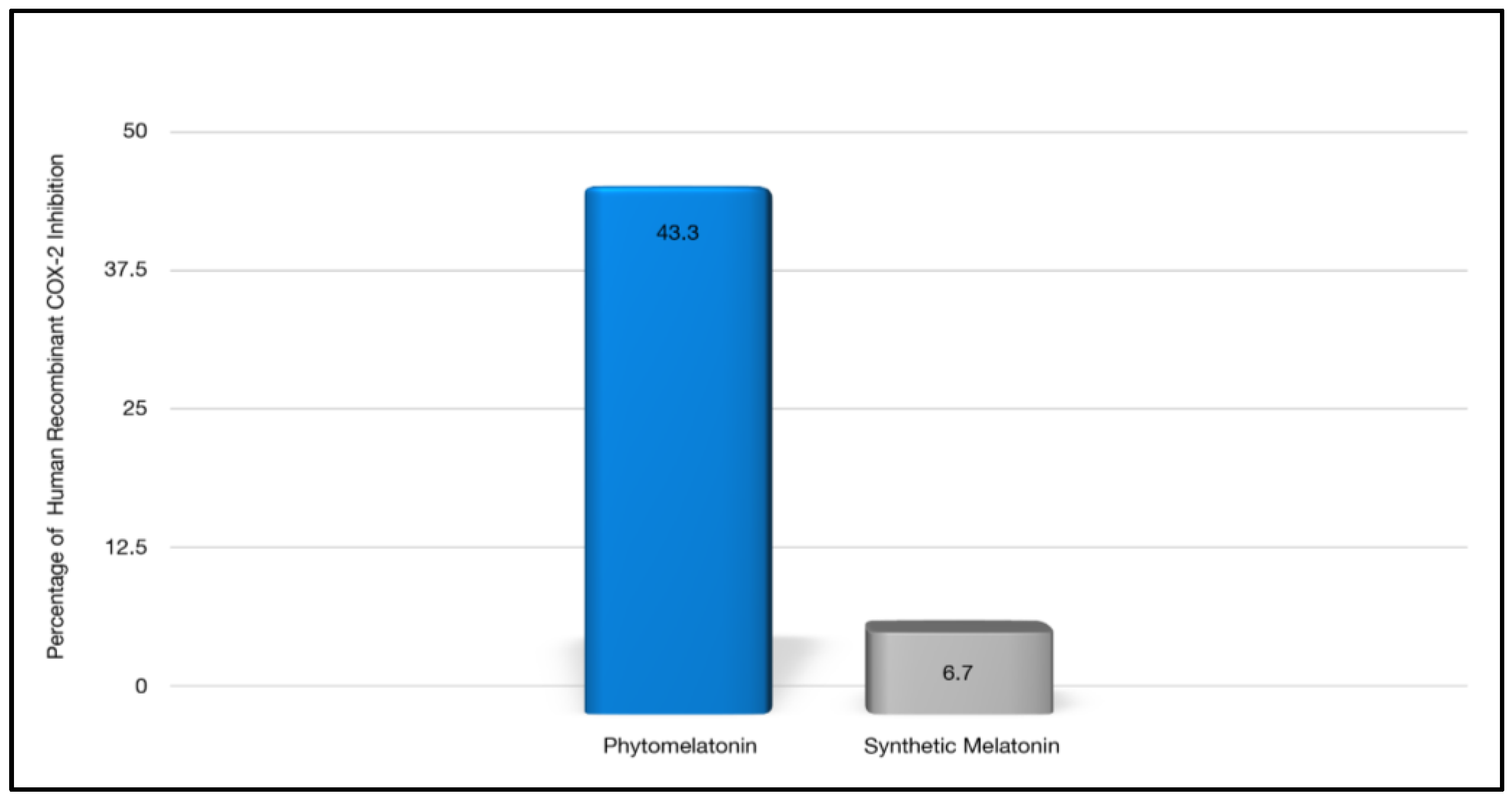


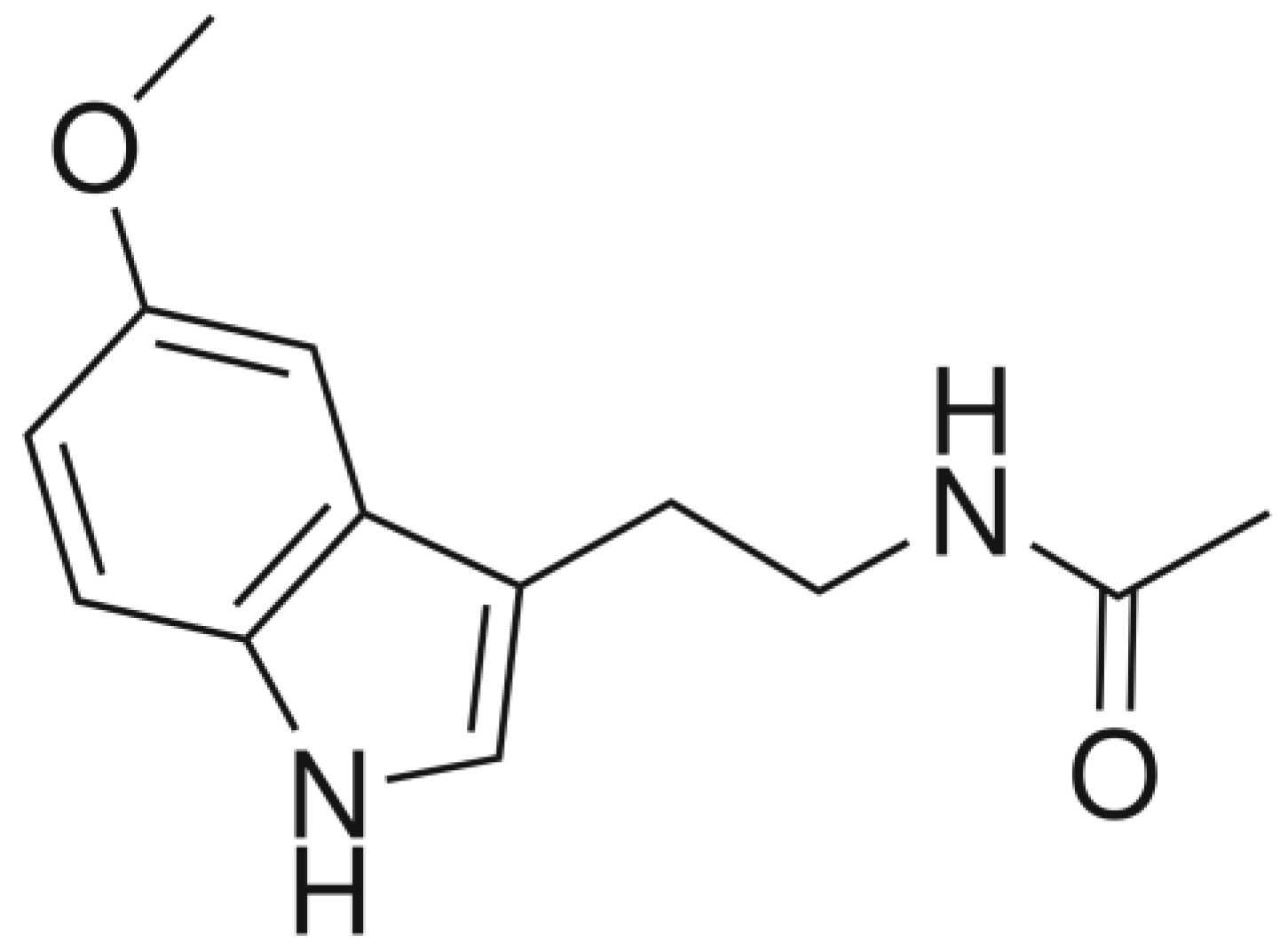
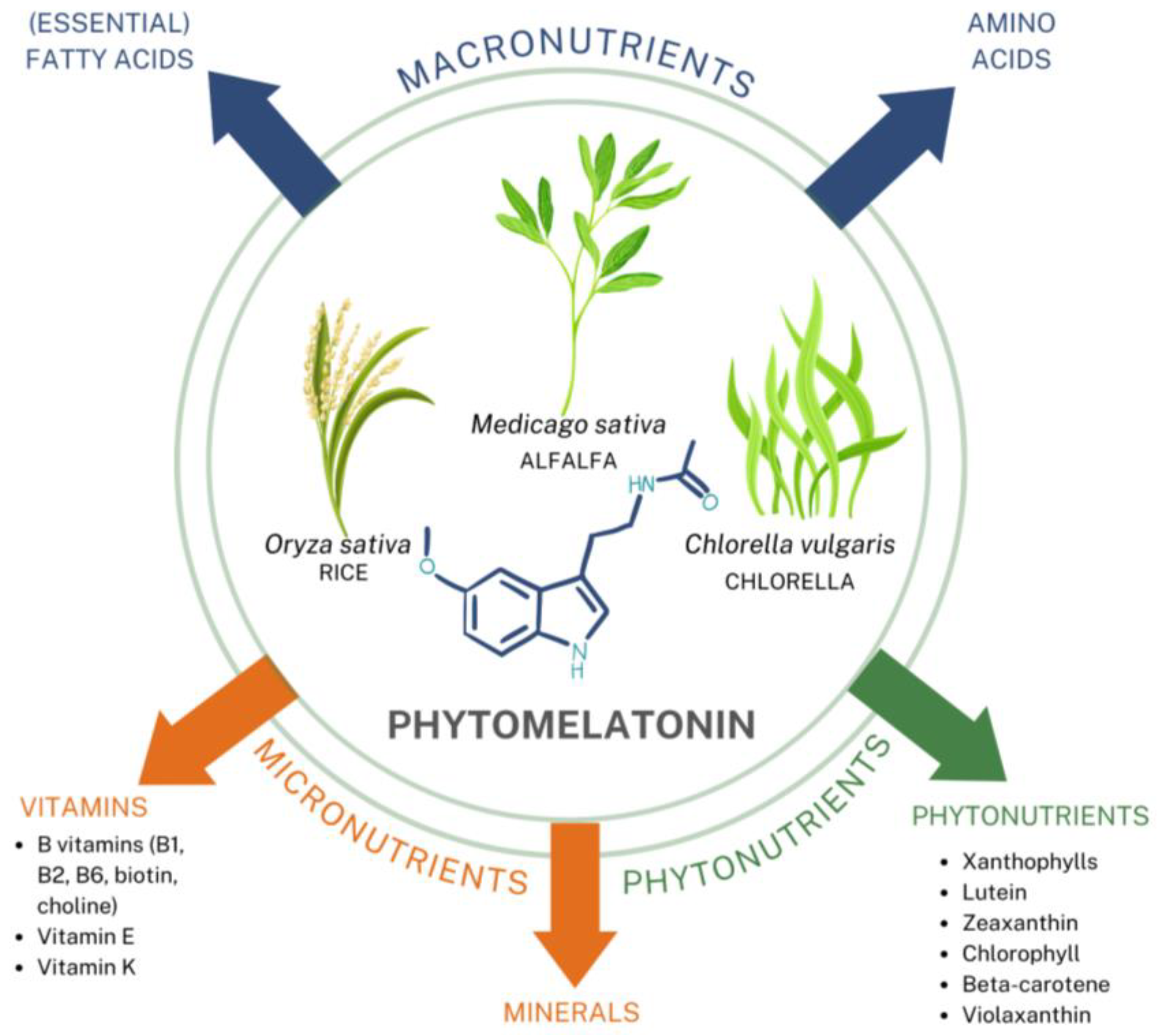

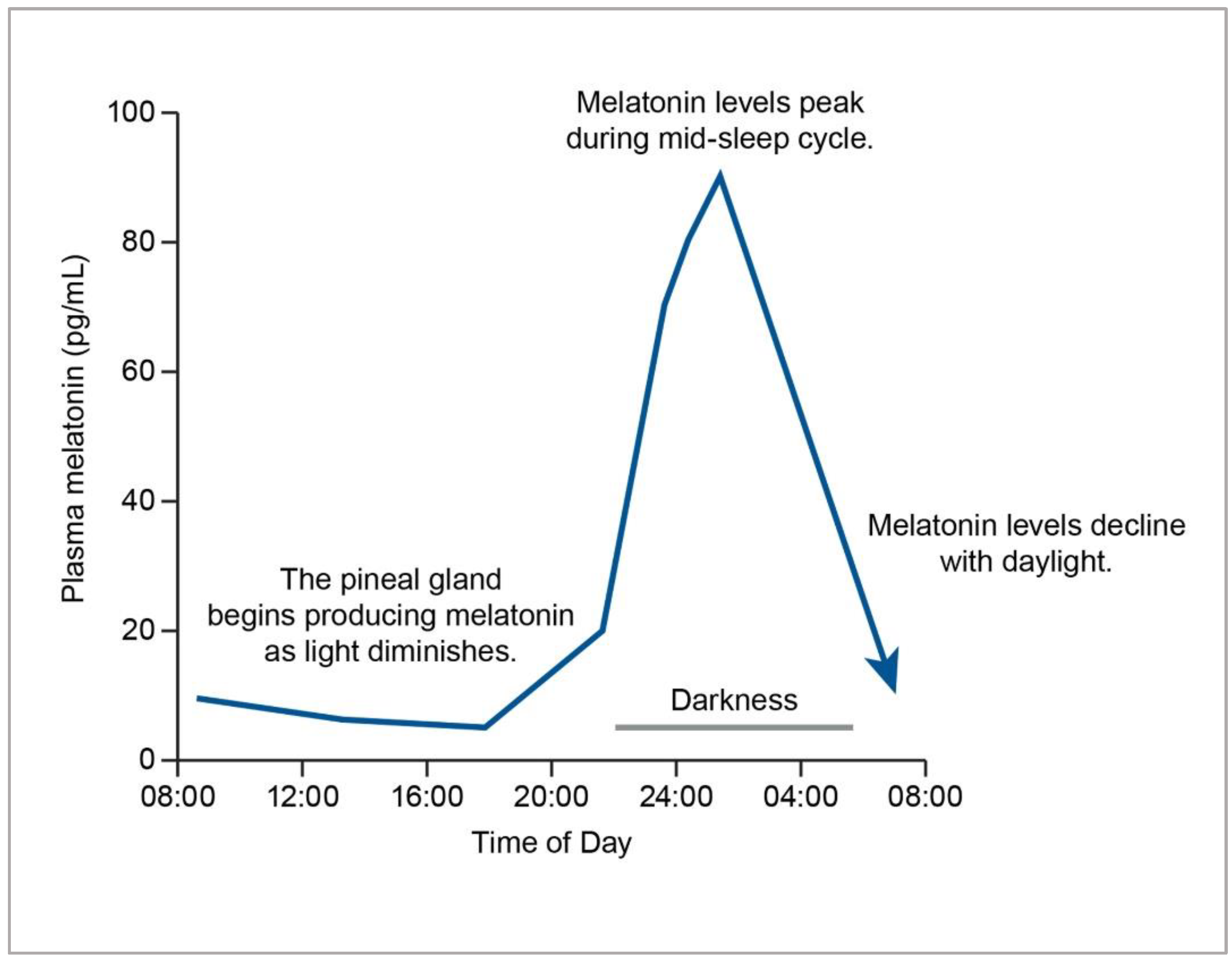
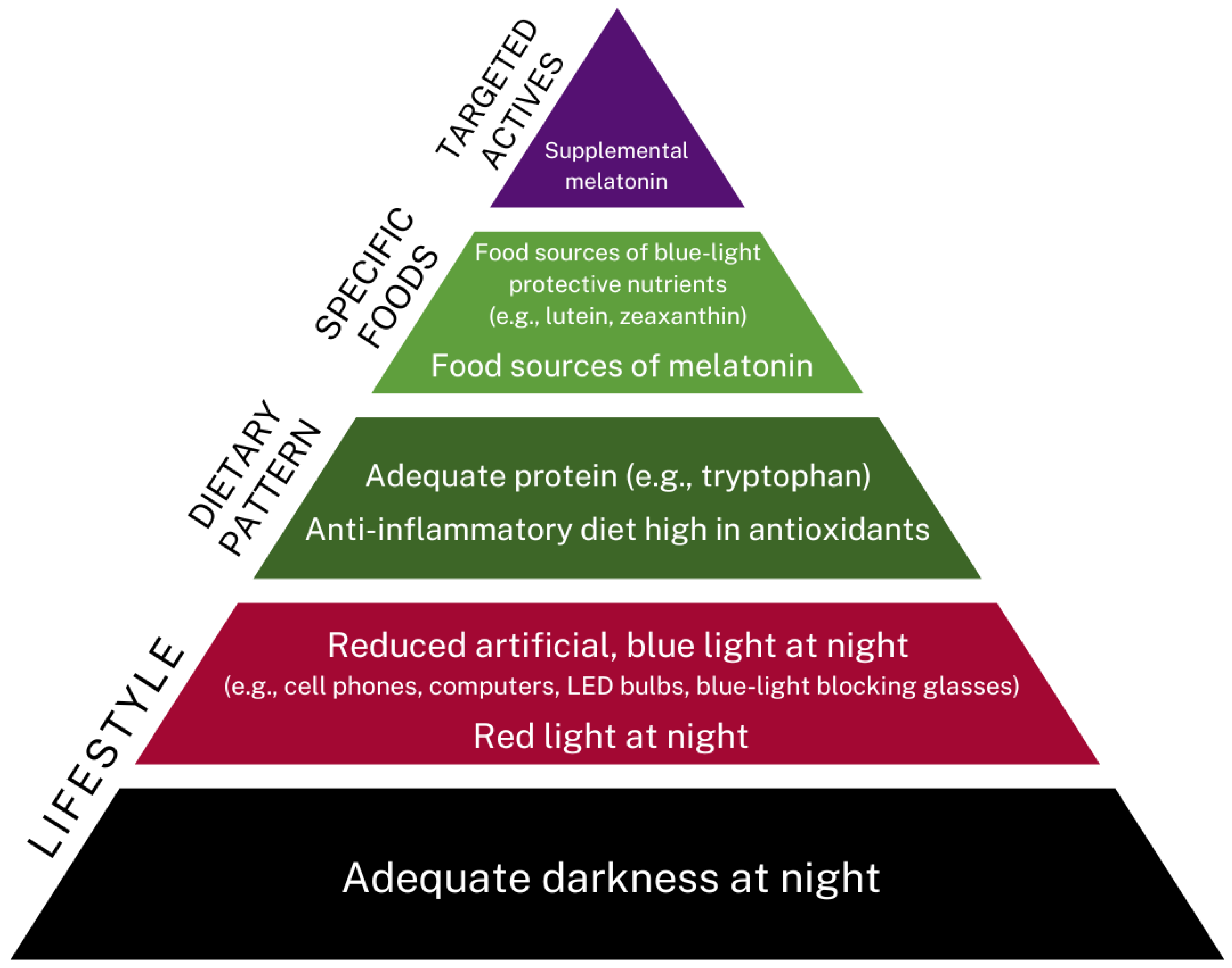
| Feature | Vitamin D | Melatonin |
|---|---|---|
| Basic functions | Considered to act as a hormone; Antioxidant; Anti-inflammatory compound; Mitochondrial regulator | Hormone; Antioxidant; Anti-inflammatory compound; Mitochondrial regulator |
| Bodily systems | All | All |
| Relationship with light | Light (UV) is needed for synthesis. | Darkness is needed for synthesis. |
| Synthesis | Synthesized in the skin, activated by liver and kidney | Synthesized in the skin and many other tissues; Produced by pineal gland and gut (enterochromaffin cells) |
| Seasonal variation | Yes | Yes [28] |
| Chemical nature | Lipid-soluble | Amphiphilic |
| Transport | Crosses blood–brain barrier | Crosses blood–brain barrier |
| Nutritional status | Greater risk of insufficiency and/or deficiency with increasing age | Greater risk of insufficiency and/or deficiency with increasing age |
| Obtained from dietary sources | Yes | Yes |
| Biological need may change depending on lifestyle | Yes | Yes |
| Body System | Possible Clinical Uses |
|---|---|
| Central Nervous System | Circadian rhythm modulation Sleep-wake disorders Sleep disturbance Cognitive conditions such as dementia Migraines and headache Tinnitus Attention-Deficit Hyperactivity Disorder (ADHD) Autism Eye disorders (e.g., glaucoma) |
| Cardiovascular System | Hypercholesterolemia Hypertension/high systolic blood pressure Metabolic syndrome Endothelial dysfunction Glycemic balance (varying effects due to differing response in MTNR1B G-risk allele carriers) |
| Reproductive System | Preeclampsia Fertility As an adjunct to care for endometriosis Polycystic Ovarian Syndrome (PCOS) |
| Gastrointestinal System | Gastroesophageal Reflux Disease (GERD) Ulcers Irritable Bowel Syndrome (IBS) |
| Immune System | Autoimmune conditions (Multiple sclerosis, Hashimoto’s thyroiditis) Coronavirus Disease (COVID-19) Oxidative stress from athletic performance stress Oxidative stress from excessive environmental toxin load Cancer; chemopreventive and as an adjunct to treatment depending on the cancer type and individual |
| Musculoskeletal System | Osteopenia |
| Category | Select Types (Listed in Alphabetical Order) | References |
|---|---|---|
| Vegetables | Several types: Asparagus, beetroot, cabbage, carrot, corn, ginger root, purslane, spinach, taro | [209,211,220,221,222,223,224,225] |
| Fruits | Several types: Apple, banana, cherries (sweet, tart), cucumber, grapes, kiwifruit, peppers, pineapple, pomegranate, strawberries, tomatoes | [220,221,222,225,226,227,228,229,230,231] |
| Nuts | Almonds, pistachios, walnuts | [224,225,226,232,233,234] |
| Seeds | Anise, celery, coriander, fennel, fenugreek, flax, green cardamom, mustard (black, white), poppy, sunflower; Raw and germinated seeds of alfalfa, broccoli, lentil, mung bean, onion, red cabbage, and radish | [209,225,235,236] |
| Grains | Barley, oat, rice, wheat | [221,222,224,225] |
| Beans Legumes | Kidney beans (sprouts), soybeans | [237,238] |
| Herbs Spices | Black pepper, feverfew, sage, St. John’s wort, select Chinese medicinal herbs | [239,240,241,242] |
| Oils | Argan oil, extra virgin olive oil, grapeseed oil, linseed oil, primrose oil, sesame oil, soybean oil, sunflower oil, walnut oil, wheat germ oil | [243] |
| Beverages | Beer, coffee, grape juice, orange juice, wine | [226,231,244,245,246] |
| Factor | Details | General Comments |
|---|---|---|
| Source | Animal (pineal gland) Chemical synthesis Phytomelatonin Microbial fermentation products (bioengineered) | Synthetic melatonin is the most common form of melatonin on the market but can result in the use of potentially unwanted solvents and substrates in addition to it being environmentally undesirable [210]. Plant-based melatonin presents challenges in concentrating to a viable dose of melatonin. Animal-based melatonin can involve the risk of viral infections. Microbial fermentation products are under development. |
| Route | Oral intake Oral, immediate release Oral, sustained, time-release Sublingual Intravenous Intramuscular Intranasal Transdermal Anal/Suppository Vaginal delivery | There are a variety of formats available, and each needs to be individualized to the person’s needs. Several newer formats are being developed for optimizing delivery, although only oral administration is considered a dietary supplement in the U.S. [260,261]. |
| Delivery | Capsule Tablet Chewable Gummies | A trending format is that of gummies, which is a sweetened gelatinous-type delivery for greater palatability. While it may be the desired delivery form for consumers, there are concerns about the stability of melatonin in such a hygroscopic matrix, the resulting sugar content, the addition of dyes or flavoring agents, and the potential for an overdose of melatonin, especially in the case of children. |
| Actives | As an isolated compound In combination with other actives In a plant matrix with other phytonutrients | Often, dietary supplements of melatonin will include other nutritional or herbal actives with the intention of synergy or improved efficacy, although, on the whole, these types of preparations have not been effectively studied for interactions. |
| Quality | Certified Good Manufacturing Practices (cGMP) Third-party testing for heavy metals, and contaminants Packaging integrity to ensure shelf-life and stability. | Not all dietary supplements have the same quality. cGMP and third-party testing can be markers of objective quality measures. Melatonin can degrade in the presence of air and light, so minimizing exposure [262] in oxygen-barrier blister packs would be preferential over open bottle format. |
| Dose | Physiological dose (0.3–1.0 mg) Supraphysiological dose for occasional use (≥3 mg) Therapeutic dose prescribed by a qualified healthcare practitioner | There is much debate about proper dose levels. Consider safety in addition to efficacy for the clinical condition it is being used for in a patient, as well as the duration of use, whether low dose, short term or high dose, long term. |
| Category | Website |
|---|---|
| Clinical dosing recommendation and contraindications | Natural Medicines Comprehensive Database: https://naturalmedicines.therapeuticresearch.com/ accessed on 27 July 2022 |
| Research sites | Melatonin database of studies including phytomelatonin: https://www.phytomelatonin.com accessed on 27 July 2022 Melatonin Research: https://www.melatonin-research.net/index.php/MR accessed on 27 July 2022 National Center of Complementary and Integrative Health: https://www.nccih.nih.gov/ accessed on 27 July 2022 |
| Professional Organizations | American Association of Naturopathic Physicians: https://naturopathic.org/ accessed on 27 July 2022 American Nutrition Association: https://www.theana.org accessed on 27 July 2022 Institute for Functional Medicine: https://www.ifm.org/ accessed on 27 July 2022 |
| Clinical Aspect | Considerations |
|---|---|
| Constitution | |
| Genes, Early Life Epigenetics | Gene variants related to receptor activity, early life exposure to melatonin through breast milk |
| Conditional Influences | |
| Acute and/or Chronic Triggers | Stressful events, bouts of poor-quality sleep, travel across time zones, jet lag, inflammatory cytokines from injury or illness, oxidative stress from toxic exposures, shift work, dysregulated appetite, dysbiosis, artificial, blue light at night, insufficient darkness at night, insufficient morning light, highly processed, inflammatory diet |
| Body Systems | |
| Bone Health | Melatonin may help in the balance of osteoblasts and osteoclasts for better bone mineral density and overall structure. |
| Brain Mood | Melatonin can influence cognition and mood. High levels of kynurenine are present in the brain in depression. |
| Cardiovascular Transport | Melatonin can be produced in multiple body parts, circulate to tissues, and cross the blood–brain barrier. |
| Detoxification | Preliminary research suggests that melatonin may be helpful with the elimination of toxins in the brain (e.g., amyloid) through the glymphatic fluid. |
| Endocrine System | Melatonin is a hormone produced by the pineal gland (and gastrointestinal tract), communicating with other hormones. It is biochemically interrelated with its precursor, serotonin, and plays a key role in circadian rhythm and sleep cycles in conjunction with other hormones (e.g., cortisol, insulin) and neurotransmitters (e.g., serotonin). Higher amounts are found in children with lower nocturnal levels in puberty. |
| Gastrointestinal Tract | Melatonin is found in the gut mucosa at levels that exceed that of the pineal gland. It is produced by enterochromaffin cells, with altering responses postprandially. Furthermore, initial studies suggest it may influence the gut microbiome. |
| Immunity | Based on historical data, melatonin is most known as a potent antioxidant and anti-inflammatory agent. Research suggests it has chemopreventive and tumor-suppressing activity. |
| Metabolism | Melatonin can protect the mitochondria from oxidative stress due to its ability to cross the mitochondrial membrane. |
| Lifestyle Factors | |
| Sleep Relaxation | Aligning day-night rhythms will help to ensure healthy melatonin levels. Ensuring sleep hygiene is practiced, particularly maintaining a dark, cool room for sleeping. Wearing blue-light-blocking glasses before bedtime may help to establish better rhythm tone, enhanced sleep, and less reduction in nocturnal melatonin. |
| Physical Activity | Exercise may help increase serotonin and melatonin and result in less shunting through the kynurenine pathway. |
| Nutrition | Melatonin is found in both animal and plant dietary sources. Foods containing tryptophan may modulate melatonin levels due to the conversion of tryptophan to melatonin. Dietary supplementation could also be implemented either acutely, such as in jet lag, or more chronically at lower doses for those who do shift work. |
| Stress Regulation Resilience | Cortisol is inversely related to melatonin. Upregulation in the kynurenine pathway can be seen in stressful events. The use of meditation, calming activities, bodywork, and creative arts may help cultivate improved stress response and, ultimately, resilience. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. https://doi.org/10.3390/nu14193934
Minich DM, Henning M, Darley C, Fahoum M, Schuler CB, Frame J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients. 2022; 14(19):3934. https://doi.org/10.3390/nu14193934
Chicago/Turabian StyleMinich, Deanna M., Melanie Henning, Catherine Darley, Mona Fahoum, Corey B. Schuler, and James Frame. 2022. "Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements" Nutrients 14, no. 19: 3934. https://doi.org/10.3390/nu14193934
APA StyleMinich, D. M., Henning, M., Darley, C., Fahoum, M., Schuler, C. B., & Frame, J. (2022). Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients, 14(19), 3934. https://doi.org/10.3390/nu14193934