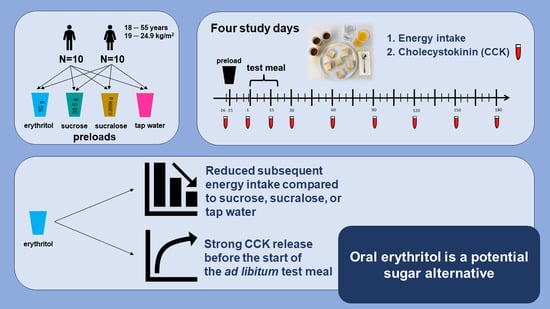
Oral Erythritol Reduces Energy Intake during a Subsequent ad libitum Test Meal: A Randomized, Controlled, Crossover Trial in Healthy Humans
Abstract
1. Introduction
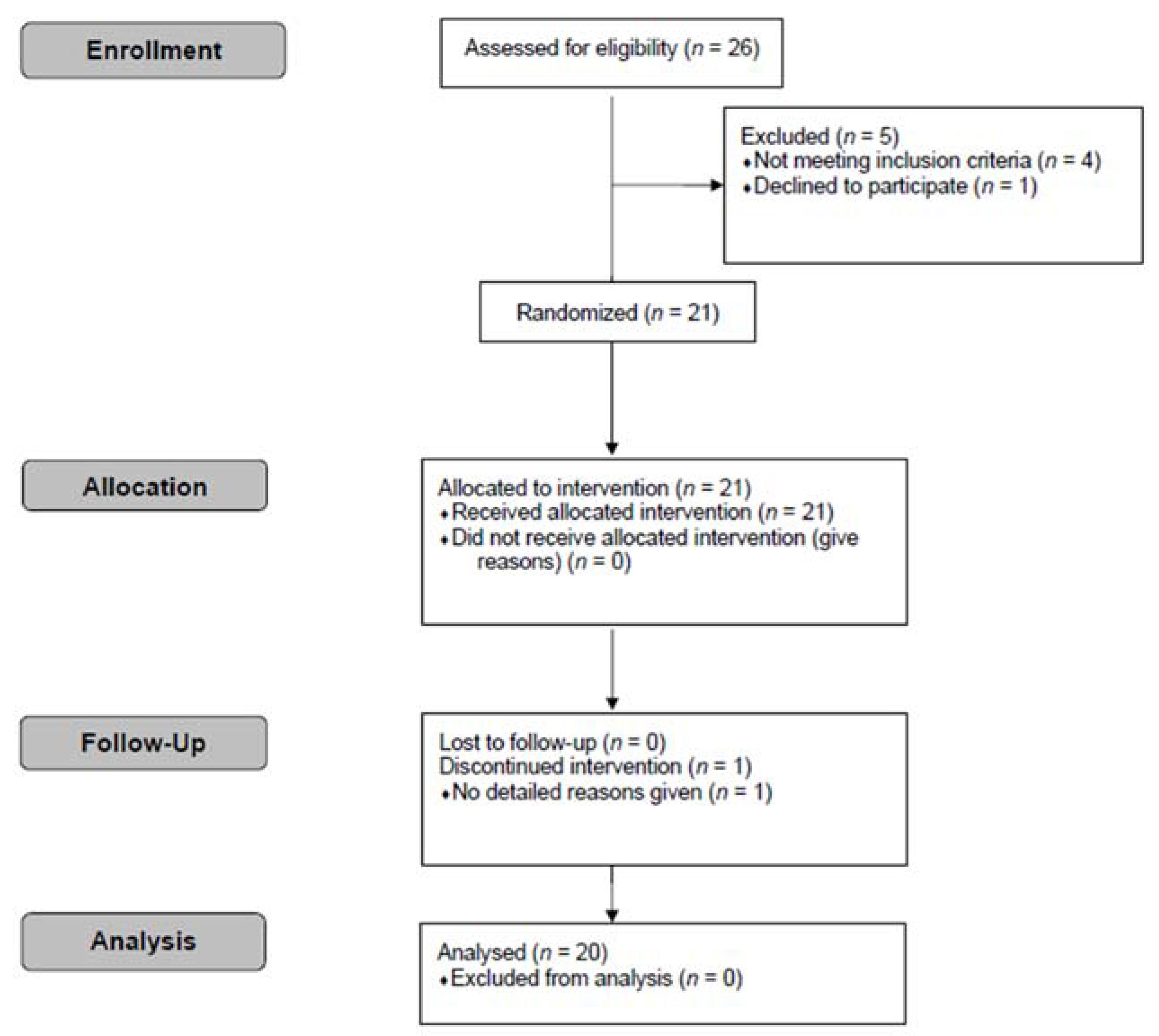
2. Participants and Methods
2.1. Participants
2.2. Ethical Approval
2.3. Study Design and Procedure
- − 50 g erythritol;
- − 33.5 g sucrose;
- − 0.0558 g sucralose;
- − 300 mL tap water.
2.4. Materials
2.5. Composition and Conduction of the Test Meal
2.6. Assessment of Energy Intake
2.7. Blood Sample Collection and Processing
2.8. Laboratory Analysis
2.9. Statistical Analysis
- −
- Comparison of energy intake between erythritol and sucrose, sucralose, or tap water to test the hypothesis that erythritol will lead to a similar subsequent energy intake as sucrose and to a lower energy intake compared to sucralose or tap water.
- −
- Comparison of post-preload administration time point −1 min versus baseline values for each substance to test the hypotheses that: (i) CCK will be released in response to erythritol and sucrose, but not in response to sucralose or tap water, (ii) the glucose and insulin concentrations will be increased in response to sucrose, but not in response to erythritol, sucralose, or tap water, and (iii) hunger/prospective food consumption will be decreased and satiety/fullness will be increased in response to erythritol and sucrose, but not in response to sucralose or tap water.
- −
- Comparison of post-preload administration time point −1 min versus baseline values between erythritol and sucrose, sucralose, or tap water to test the hypotheses that: (i) CCK in response to erythritol will be similar to sucrose, but higher compared to sucralose or tap water, (ii) glucose and insulin concentrations will be lower in response to erythritol compared to sucrose, but similar between erythritol and sucralose or tap water, and (iii) hunger/prospective food consumption and satiety/fullness, respectively, in response to erythritol will be similar to sucrose, but lower and higher, respectively, compared to sucralose or tap water.
- −
- Comparison of post-preload administration time point 15 min (during the ad libitum test meal) versus baseline values between erythritol, sucrose, sucralose, or tap water to explore CCK, glycemic control, and appetite-related sensations. No hypotheses were formulated beforehand.
- −
- Comparison of perceived sweetness and liking of the preloads, and perceived liking of the test meal between erythritol and sucrose, sucralose, or tap water to test the hypothesis that erythritol will have a similar perceived sweetness as sucrose and sucralose, but higher compared to tap water. No differences will be observed in the perceived liking of the preloads and test meal between erythritol compared to sucrose, sucralose, or tap water.
3. Results
3.1. Energy Intake and Total Energy Intake
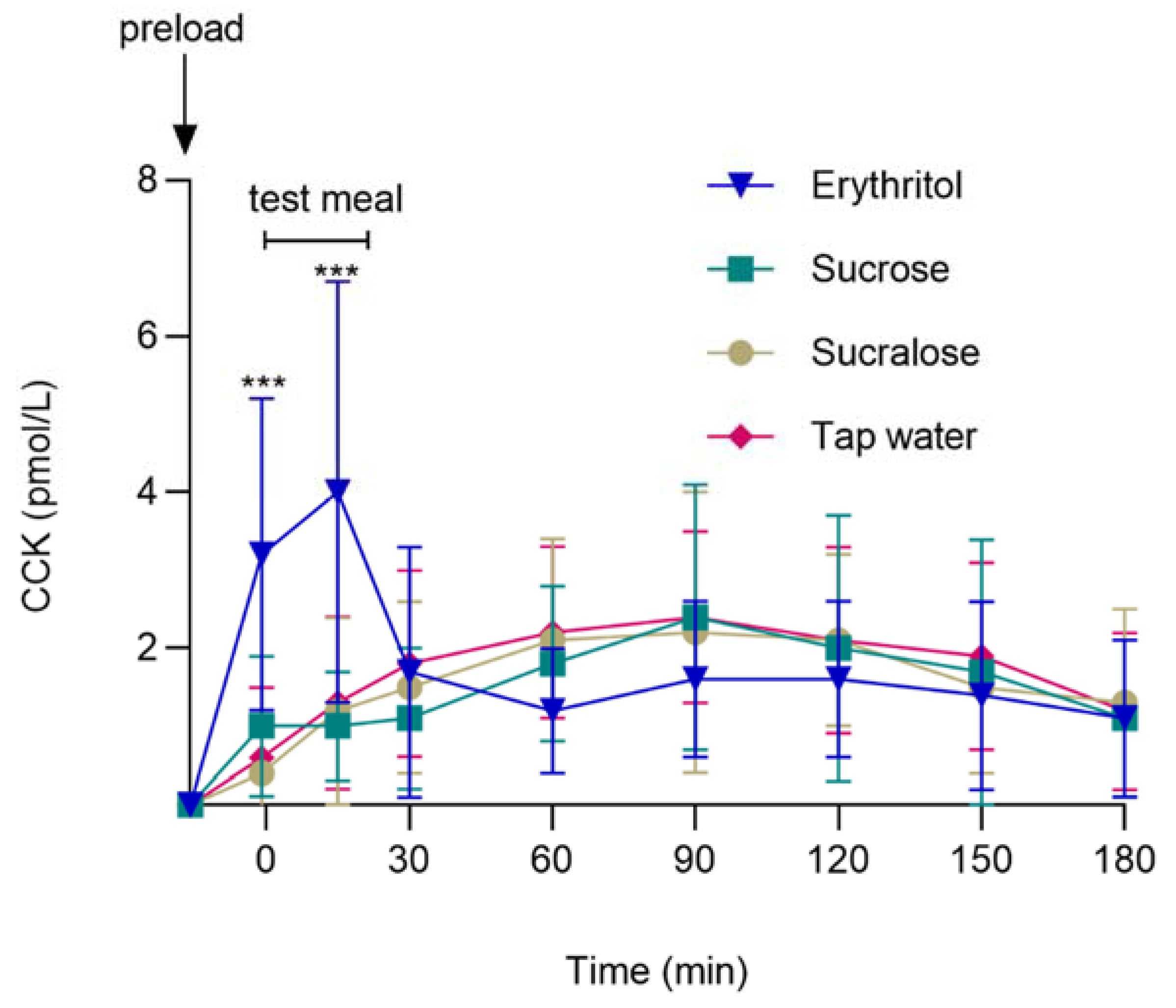
3.2. GI Satiation Hormone: Plasma CCK
3.3. Associations between CCK and Energy Intake
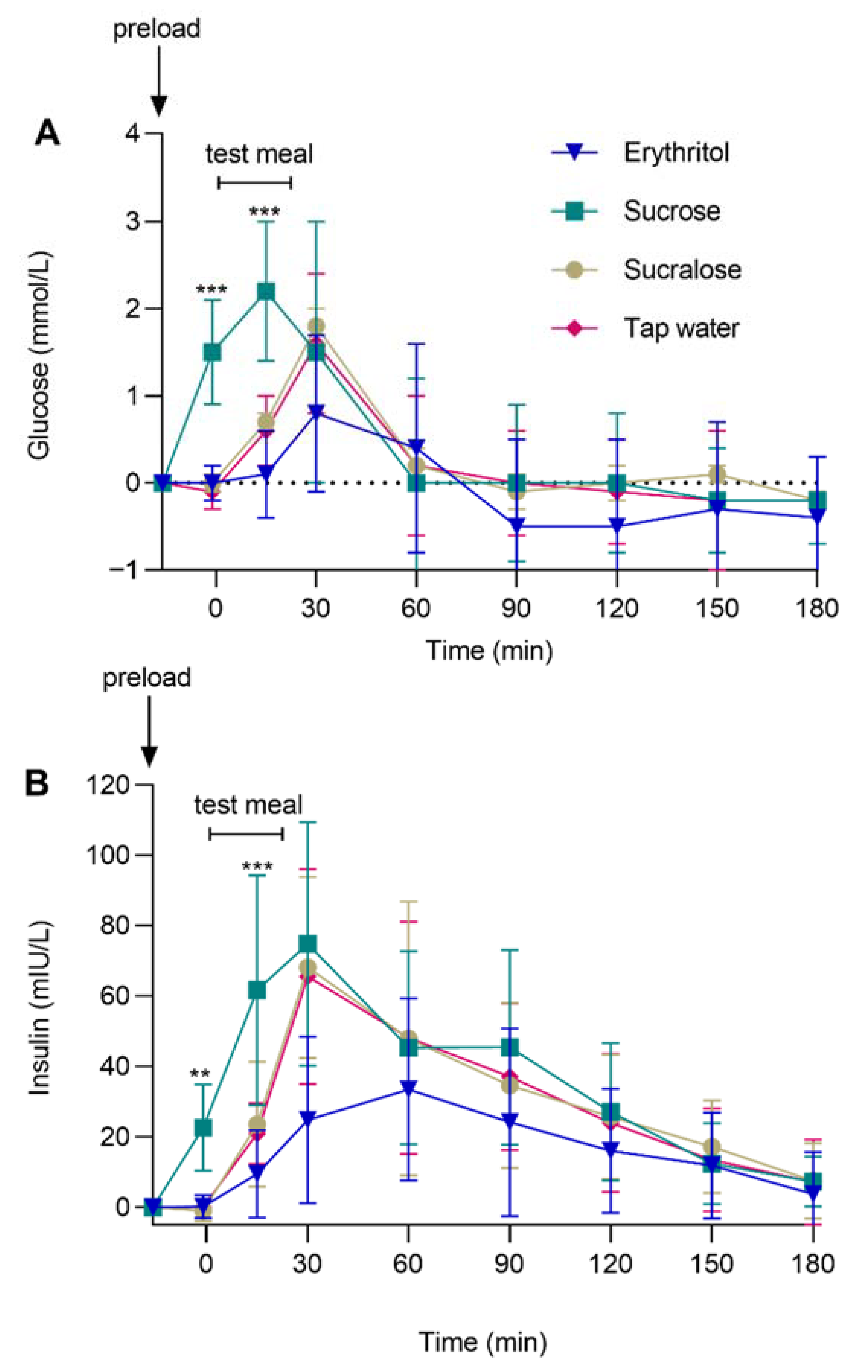
3.4. Glycemic Control: Plasma Glucose and Insulin
3.5. Appetite-Related Sensations: Hunger, Prospective Food Consumption, Satiety, and Fullness
3.5.1. Hunger
3.5.2. Prospective Food Consumption, Satiety, and Fullness
3.5.3. Perceived Sweetness and Liking of the Preloads
3.5.4. Perceived Liking of the Test Meal
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health effects of overweight and obesity in 195 countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Joost, S.; De Ridder, D.; Marques-Vidal, P.; Bacchilega, B.; Theler, J.M.; Gaspoz, J.M.; Guessous, I. Overlapping spatial clusters of sugar-sweetened beverage intake and body mass index in Geneva state, Switzerland. Nutr. Diabetes 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. Br. J. Sports Med. 2016, 50, 496–504. [Google Scholar] [CrossRef]
- WHO. Sugars Intake for Adults and Children: Guideline; WHO: Geneva, Switzerland, 2015.
- Steinert, R.E.; Frey, F.; Töpfer, A.; Drewe, J.; Beglinger, C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br. J. Nutr. 2011, 105, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Rother, K.I. Non-nutritive sweeteners and their role in the gastrointestinal tract. J. Clin. Endocrinol. Metab. 2012, 97, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Bellon, M.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Artificial sweeteners have no effect on gastric emptying, glucagon-like peptide-1, or glycemia after oral glucose in healthy humans. Diabetes Care 2013, 36, e202–e203. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, L.A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef]
- Brown, A.W.; Bohan Brown, M.M.; Onken, K.L.; Beitz, D.C. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr. Res. 2011, 31, 882–888. [Google Scholar] [CrossRef]
- Pang, M.D.; Goossens, G.H.; Blaak, E.E. The impact of artificial sweeteners on body weight control and glucose homeostasis. Front. Nutr. 2020, 7, 598340. [Google Scholar] [CrossRef]
- Huang, M.; Quddus, A.; Stinson, L.; Shikany, J.M.; Howard, B.V.; Kutob, R.M.; Lu, B.; Manson, J.E.; Eaton, C.B. Artificially sweetened beverages, sugar-sweetened beverages, plain water, and incident diabetes mellitus in postmenopausal women: The prospective Women’s Health Initiative observational study. Am. Clin. Nutr. 2017, 106, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Non-nutritive sweeteners and glycaemic control. Curr. Atheroscler. Rep. 2019, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Smith, K.R.; Crouch, A.L.; Sharma, V.; Yi, F.; Vargova, V.; LaMoia, T.E.; Dupont, L.M.; Serna, V.; Tang, F.; et al. High-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans and mice. Microbiome 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Daher, M.I.; Matta, J.M.; Abdel Nour, A.M. Non-nutritive sweeteners and type 2 diabetes: Should we ring the bell? Diabetes Res. Clin. Pract. 2019, 155, 107786. [Google Scholar] [CrossRef]
- Hunter, S.R.; Reister, E.J.; Cheon, E.; Mattes, R.D. Low calorie sweeteners differ in their physiological effects in humans. Nutrients 2019, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Dalenberg, J.R.; Patel, B.P.; Denis, R.; Veldhuizen, M.G.; Nakamura, Y.; Vinke, P.C.; Luquet, S.; Small, D.M. Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab. 2020, 31, 493–502.e497. [Google Scholar] [CrossRef]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef]
- Mace, O.J.; Affleck, J.; Patel, N.; Kellett, G.L. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J. Physiol. 2007, 582, 379–392. [Google Scholar] [CrossRef]
- Boesten, D.M.P.H.J.; den Hartog, G.J.M.; de Cock, P.; Bosscher, D.; Bonnema, A.; Bast, A. Health effects of erythritol. Nutrafoods 2015, 14, 3–9. [Google Scholar] [CrossRef]
- de Cock, P. Erythritol: A novel noncaloric sweetener ingredient. World Rev. Nutr. Diet 1999, 85, 110–116. [Google Scholar] [CrossRef]
- Bernt, W.O.; Borzelleca, J.F.; Flamm, G.; Munro, I.C. Erythritol: A review of biological and toxicological studies. Regul. Toxicol. Pharmacol. 1996, 24, S191–S197. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1053–E1061. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Drewe, J.; Verbeure, W.; le Roux, C.W.; Dellatorre-Teixeira, L.; Rehfeld, J.F.; Holst, J.J.; Hartmann, B.; Tack, J.; Peterli, R.; et al. Gastric emptying of solutions containing the natural sweetener erythritol and effects on gut hormone secretion in humans: A pilot dose-ranging study. Diabetes Obes. Metab. 2021, 23, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Teysseire, F.; Bordier, V.; Budzinska, A.; Weltens, N.; Rehfeld, J.F.; Holst, J.J.; Hartmann, B.; Beglinger, C.; Van Oudenhove, L.; Wölnerhanssen, B.K.; et al. The role of D-allulose and erythritol on the activity of the gut sweet taste receptor and gastrointestinal satiation hormone release in humans: A randomized, controlled trial. J. Nutr. 2022, 152, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Nakayama, K.; Oku, T. Serum glucose and insulin levels and erythritol balance after oral administration of erythritol in healthy subjects. Eur. J. Clin. Nutr. 1994, 48, 286–292. [Google Scholar] [PubMed]
- Overduin, J.; Collet, T.H.; Medic, N.; Henning, E.; Keogh, J.M.; Forsyth, F.; Stephenson, C.; Kanning, M.W.; Ruijschop, R.; Farooqi, I.S.; et al. Failure of sucrose replacement with the non-nutritive sweetener erythritol to alter GLP-1 or PYY release or test meal size in lean or obese people. Appetite 2016, 107, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef]
- Campbell, S.M.; Sims, C.A.; Bartoshuk, L.M.; Colquhoun, T.A.; Schwieterman, M.L.; Folta, K.M. Manipulation of sensory characteristics and volatile compounds in strawberry fruit through the use of isolated wavelengths of light. J. Food Sci. 2020, 85, 771–780. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Accurate measurement of cholecystokinin in plasma. Clin. Chem. 1998, 44, 991–1001. [Google Scholar] [CrossRef]
- Gadah, N.S.; Brunstrom, J.M.; Rogers, P.J. Cross-over studies underestimate energy compensation: The example of sucrose-versus sucralose-containing drinks. Appetite 2016, 107, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Jack, M.; Poon, T.; Noori, D.; Venditti, C.; Hamamji, S.; Musa-Veloso, K. Effects of unsweetened preloads and preloads sweetened with caloric or low-/no-calorie sweeteners on subsequent energy intakes: A systematic review and meta-analysis of controlled human intervention studies. Adv. Nutr. 2021, 12, 1481–1499. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.; Oberndorfer, T.A.; Simmons, A.N.; Paulus, M.P.; Fudge, J.L.; Yang, T.T.; Kaye, W.H. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 2008, 39, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.; Turner, M.; Naumovski, N.; Somerset, S. Role of cholecystokinin in satiation: A systematic review and meta-analysis. Br. J. Nutr. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Niedereichholz, U.; Ettler, R.; Holst, J.J.; Orskov, C.; Ritzel, R.; Schmiegel, W.H. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 1997, 273, E981–E988. [Google Scholar] [CrossRef]
- Deane, A.M.; Nguyen, N.Q.; Stevens, J.E.; Fraser, R.J.; Holloway, R.H.; Besanko, L.K.; Burgstad, C.; Jones, K.L.; Chapman, M.J.; Rayner, C.K.; et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J. Clin. Endocrinol. Metab. 2010, 95, 215–221. [Google Scholar] [CrossRef]
- Muurahainen, N.; Kissileff, H.R.; Derogatis, A.J.; Pi-Sunyer, F.X. Effects of cholecystokinin-octapeptide (CCK-8) on food intake and gastric emptying in man. Physiol. Behav. 1988, 44, 645–649. [Google Scholar] [CrossRef]
- Yunker, A.G.; Luo, S.; Jones, S.; Dorton, H.M.; Alves, J.M.; Angelo, B.; DeFendis, A.; Pickering, T.A.; Monterosso, J.R.; Page, K.A. Appetite-regulating hormones are reduced after oral sucrose vs glucose: Influence of obesity, insulin resistance, and sex. J. Clin. Endocrinol. Metab. 2021, 106, 654–664. [Google Scholar] [CrossRef]
- Yau, A.M.; McLaughlin, J.; Gilmore, W.; Maughan, R.J.; Evans, G.H. The acute effects of simple sugar ingestion on appetite, gut-derived hormone response, and metabolic markers in men. Nutrients 2017, 9, 135. [Google Scholar] [CrossRef]
- Elias, E.; Gibson, G.J.; Greenwood, L.F.; Hunt, J.N.; Tripp, J.H. The slowing of gastric emptying by monosaccharides and disaccharides in test meals. J. Physiol. 1968, 194, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yunker, A.G.; Alves, J.M.; Luo, S.; Angelo, B.; DeFendis, A.; Pickering, T.A.; Monterosso, J.R.; Page, K.A. Obesity and sex-related associations with differential effects of sucralose vs sucrose on appetite and reward processing: A randomized crossover trial. JAMA Netw. Open 2021, 4, e2126313. [Google Scholar] [CrossRef] [PubMed]
- Gerspach, A.C.; Steinert, R.E.; Schonenberger, L.; Graber-Maier, A.; Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E317–E325. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Frost, C.R.; Svendsen, B.; Holst, J.J. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes 2015, 64, 370–382. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Sun, E.W.; de Fontgalland, D.; Rabbitt, P.; Hollington, P.; Sposato, L.; Due, S.L.; Wattchow, D.A.; Rayner, C.K.; Deane, A.M.; Young, R.L.; et al. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes 2017, 66, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Vana, V.; Laerke, M.K.; Rehfeld, J.F.; Arnold, M.; Dmytriyeva, O.; Langhans, W.; Schwartz, T.W.; Hansen, H.S. Vagal afferent cholecystokinin receptor activation is required for glucagon-like peptide-1-induced satiation. Diabetes Obes. Metab. 2022, 24, 268–280. [Google Scholar] [CrossRef]
- Psichas, A.; Reimann, F.; Gribble, F.M. Gut chemosensing mechanisms. J. Clin. Investig. 2015, 125, 908–917. [Google Scholar] [CrossRef]
- Bohórquez, D.V.; Shahid, R.A.; Erdmann, A.; Kreger, A.M.; Wang, Y.; Calakos, N.; Wang, F.; Liddle, R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef]
- Buchanan, K.L.; Rupprecht, L.E.; Kaelberer, M.M.; Sahasrabudhe, A.; Klein, M.E.; Villalobos, J.A.; Liu, W.W.; Yang, A.; Gelman, J.; Park, S.; et al. The preference for sugar over sweetener depends on a gut sensor cell. Nat. Neurosci. 2022, 25, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Y.; Friel, J.K.; MacKay, D.S. The effect of the artificial sweeteners on glucose metabolism in healthy adults: A randomized, double-blinded, crossover clinical trial. Appl. Physiol. Nutr. Metab. 2020, 45, 606–612. [Google Scholar] [CrossRef]
- Bordier, V.; Teysseire, F.; Schlotterbeck, G.; Senner, F.; Beglinger, C.; Meyer-Gerspach, A.C.; Wölnerhanssen, B.K. Effect of a chronic intake of the natural sweeteners xylitol and erythritol on glucose absorption in humans with obesity. Nutrients 2021, 13, 3950. [Google Scholar] [CrossRef] [PubMed]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; Brito-Córdova, G.X.; Gómez-Díaz, R.A.; Almeda-Valdes, P. Sucralose decreases insulin sensitivity in healthy subjects: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 485–491. [Google Scholar] [CrossRef] [PubMed]

| Parameter. | Erythritol n = 20 | Sucrose n = 20 | Sucralose n = 20 | Tap Water n = 20 | p-Value (Overall) | p-Values (Post Hoc) | Effect Size |
|---|---|---|---|---|---|---|---|
| Energy intake (kcal) | 483 ± 277 | 573 ± 230 | 669 ± 297 | 655 ± 300 | p = 0.001 | A vs. B: p = 0.030 A vs. C: p < 0.001 A vs. D: p = 0.003 | dz = 0.68 dz = 1.08 dz = 0.93 |
| Total energy intake (kcal) | 483 ± 277 | 707 ± 230 | 669 ± 297 | 655 ± 300 | p < 0.001 | A vs. B: p < 0.001 A vs. C: p < 0.001 A vs. D: p = 0.003 | dz = 1.49 dz = 1.08 dz = 0.93 |
| Parameters | Time Points | Preloads | p-Values | |||
|---|---|---|---|---|---|---|
| Erythritol vs. Sucrose | Erythritol vs. Sucralose | Erythritol vs. Tap Water | Main Effect of Preload | Preload-by-Time Interaction | ||
| CCK (pmol/L) | −1 min | 0.43 ± 0.06 | 0.62 ± 0.07 | 0.52 ± 0.07 | p = 0.011 | p < 0.001 |
| pHolm < 0.001 | pHolm < 0.001 | pHolm < 0.001 | ||||
| dz = 1.51 | dz = 1.89 | dz = 1.71 | ||||
| 15 min | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | |||
| pHolm < 0.001 | pHolm < 0.001 | pHolm < 0.001 | ||||
| dz = 1.76 | dz = 1.46 | dz = 1.43 | ||||
| Glucose (mmol/L) | −1 min | −0.92 ± 0.18 | 0.06 ± 0.16 | 0.09 ± 0.18 | p = 0.003 | p < 0.001 |
| pHolm < 0.001 | pHolm = 1 | pHolm = 1 | ||||
| dz = 1.16 | ||||||
| 15 min | −0.18 ± 0.03 | −0.05 ± 0.02 | −0.04 ± 0.02 | |||
| pHolm < 0.001 | pHolm = 0.053 | pHolm = 0.085 | ||||
| dz = 1.61 | ||||||
| Insulin (mIU/L) | −1 min | −0.99 ± 0.25 | 0.33 ± 0.24 | 0.22 ± 0.23 | p < 0.001 | p < 0.001 |
| pHolm < 0.01 | pHolm = 0.344 | pHolm = 0.344 | ||||
| dz = 0.87 | ||||||
| 15 min | −0.28 ± 0.04 | −0.07 ± 0.03 | −0.07 ± 0.03 | |||
| pHolm < 0.001 | pHolm = 0.074 | pHolm = 0.074 | ||||
| dz = 1.73 | ||||||
| Hunger (cm) | −1 min | −0.66 ± 0.30 | −0.32 ± 0.26 | −0.94 ± 0.27 | p = 0.106 | p = 0.520 |
| pHolm = 0.065 | pHolm = 0.210 | pHolm = 0.003 | ||||
| dz = 0.77 | ||||||
| 15 min | −0.09 ± 0.04 | −0.04 ± 0.04 | −0.08 ± 0.04 | |||
| pHolm = 0.094 | pHolm = 0.257 | pHolm = 0.094 | ||||
| Pfc (cm) | −1 min | −0.05 ± 0.33 | −0.14 ± 0.30 | −0.38 ± 0.29 | p = 0.848 | p = 0.205 |
| pHolm = 1 | pHolm = 1 | pHolm = 0.558 | ||||
| 15 min | −0.02 ± 0.05 | −0.05 ± 0.04 | −0.02 ± 0.04 | |||
| pHolm = 1 | pHolm = 0.725 | pHolm = 1 | ||||
| Satiety (cm) | −1 min | 0.03 ± 0.43 | 0.08 ± 0.38 | 0.34 ± 0.40 | p = 0.862 | p = 0.912 |
| pHolm = 1 | pHolm = 1 | pHolm = 1 | ||||
| 15 min | 0.02 ± 0.06 | 0.05 ± 0.05 | 0.02 ± 0.06 | |||
| pHolm = 1 | pHolm = 1 | pHolm = 1 | ||||
| Fullness (cm) | −1 min | 0.19 ± 0.29 | 0.19 ± 0.29 | 0.52 ± 0.28 | p = 0.874 | p = 0.140 |
| pHolm = 1 | pHolm = 1 | pHolm = 0.190 | ||||
| 15 min | 0.03 ± 0.04 | 0.03 ± 0.04 | 0.01 ± 0.04 | |||
| pHolm = 1 | pHolm = 1 | pHolm = 1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teysseire, F.; Flad, E.; Bordier, V.; Budzinska, A.; Weltens, N.; Rehfeld, J.F.; Beglinger, C.; Van Oudenhove, L.; Wölnerhanssen, B.K.; Meyer-Gerspach, A.C. Oral Erythritol Reduces Energy Intake during a Subsequent ad libitum Test Meal: A Randomized, Controlled, Crossover Trial in Healthy Humans. Nutrients 2022, 14, 3918. https://doi.org/10.3390/nu14193918
Teysseire F, Flad E, Bordier V, Budzinska A, Weltens N, Rehfeld JF, Beglinger C, Van Oudenhove L, Wölnerhanssen BK, Meyer-Gerspach AC. Oral Erythritol Reduces Energy Intake during a Subsequent ad libitum Test Meal: A Randomized, Controlled, Crossover Trial in Healthy Humans. Nutrients. 2022; 14(19):3918. https://doi.org/10.3390/nu14193918
Chicago/Turabian StyleTeysseire, Fabienne, Emilie Flad, Valentine Bordier, Aleksandra Budzinska, Nathalie Weltens, Jens F. Rehfeld, Christoph Beglinger, Lukas Van Oudenhove, Bettina K. Wölnerhanssen, and Anne Christin Meyer-Gerspach. 2022. "Oral Erythritol Reduces Energy Intake during a Subsequent ad libitum Test Meal: A Randomized, Controlled, Crossover Trial in Healthy Humans" Nutrients 14, no. 19: 3918. https://doi.org/10.3390/nu14193918
APA StyleTeysseire, F., Flad, E., Bordier, V., Budzinska, A., Weltens, N., Rehfeld, J. F., Beglinger, C., Van Oudenhove, L., Wölnerhanssen, B. K., & Meyer-Gerspach, A. C. (2022). Oral Erythritol Reduces Energy Intake during a Subsequent ad libitum Test Meal: A Randomized, Controlled, Crossover Trial in Healthy Humans. Nutrients, 14(19), 3918. https://doi.org/10.3390/nu14193918