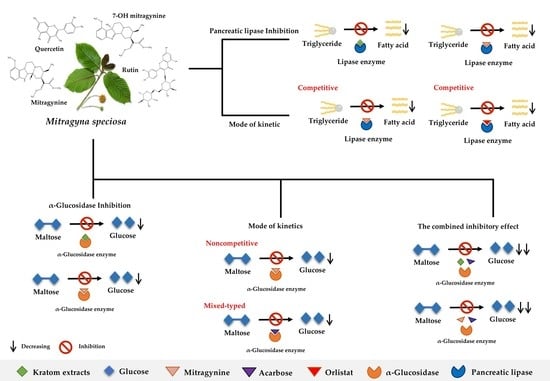
Inhibition of α-Glucosidase and Pancreatic Lipase Properties of Mitragyna speciosa (Korth.) Havil. (Kratom) Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Kratom Extracts
2.3. Determination of Total Phenolic Content, Total Flavonoid Content, and Total Alkaloid Content
2.3.1. Total Phenolic Contents (TPC)
2.3.2. Total Flavonoid Content (TFC)
2.3.3. Total Alkaloid Content (TAC)
2.4. Liquid Chromatography Analysis of Kratom Extracts
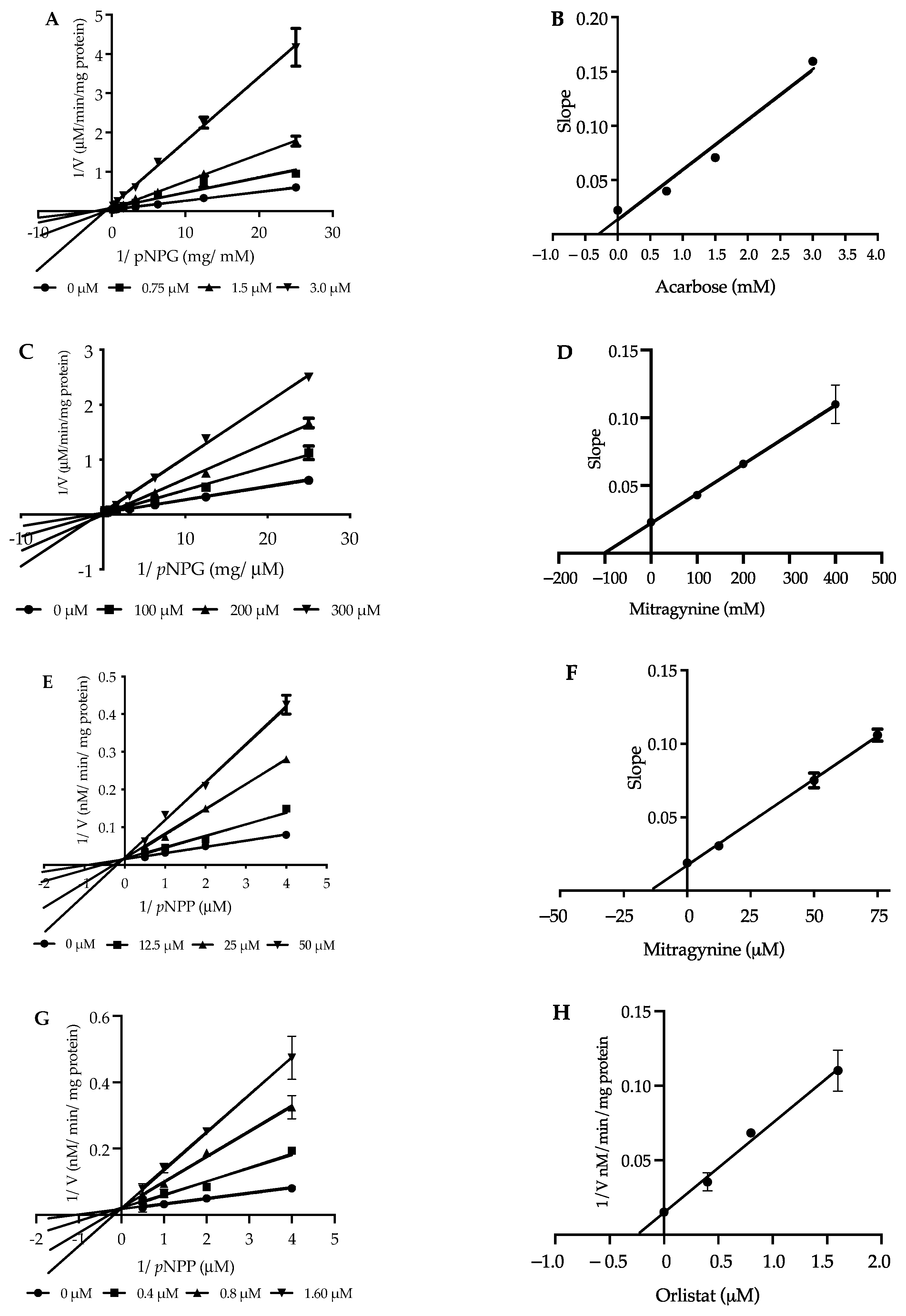
2.5. Kinetic Study of α-Glucosidase Inhibition
2.6. Kinetic Study of Pancreatic Lipase Inhibition
2.7. The Combination of Kratom Extracts and Acarbose Inhibited Enzymatic α-Glucosidase Activities
2.8. Statistical Analysis
3. Results
3.1. TPC, TFC, and TAC of All Kratom Extracts
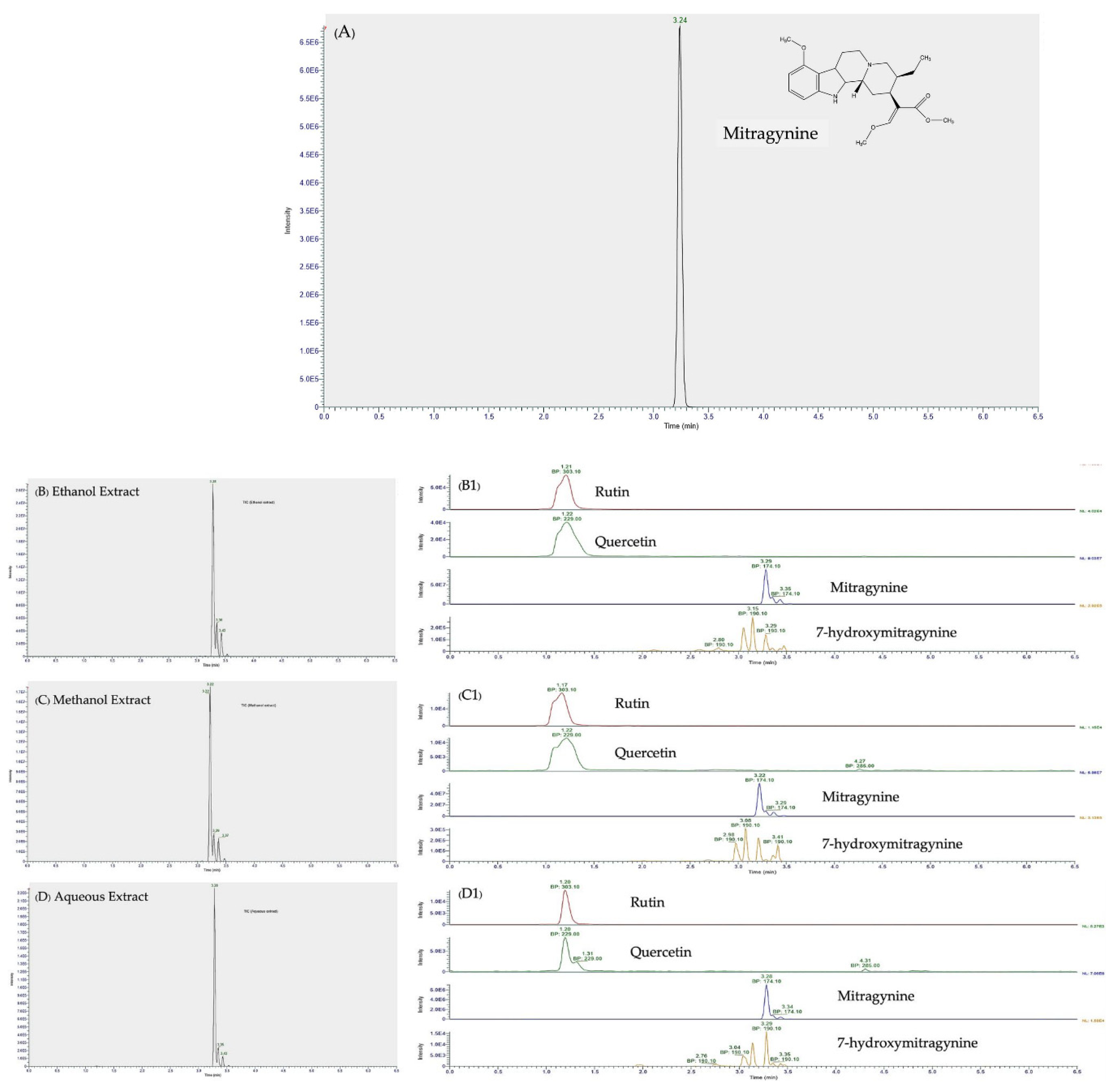
3.2. LC-MS/MS Analysis of Mitragynine, 7-hydroxymitragynine, Quercetin, and Rutin
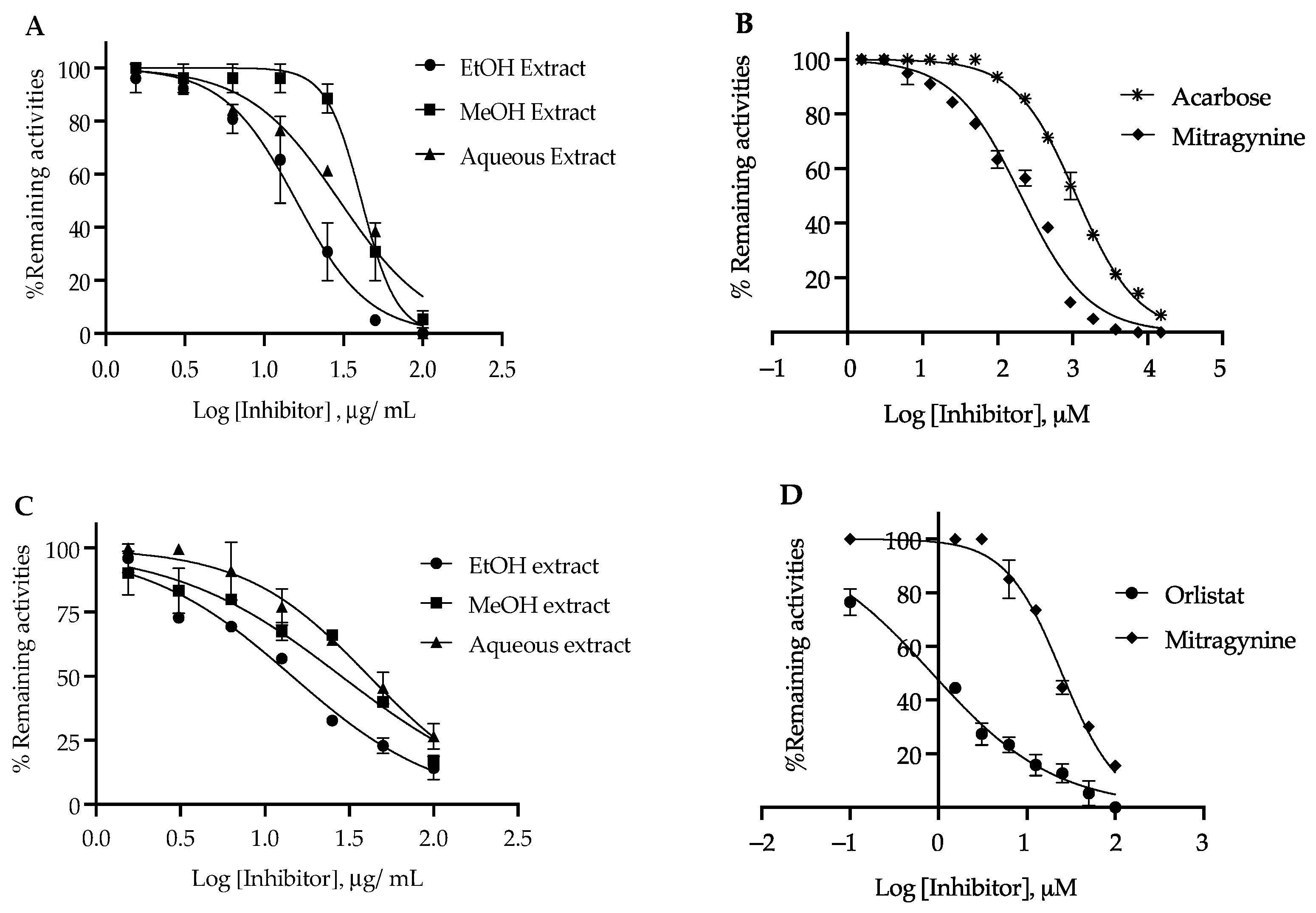
3.3. α-Glucosidase Inhibition Activity
3.4. Pancreatic Lipase Inhibition Activity
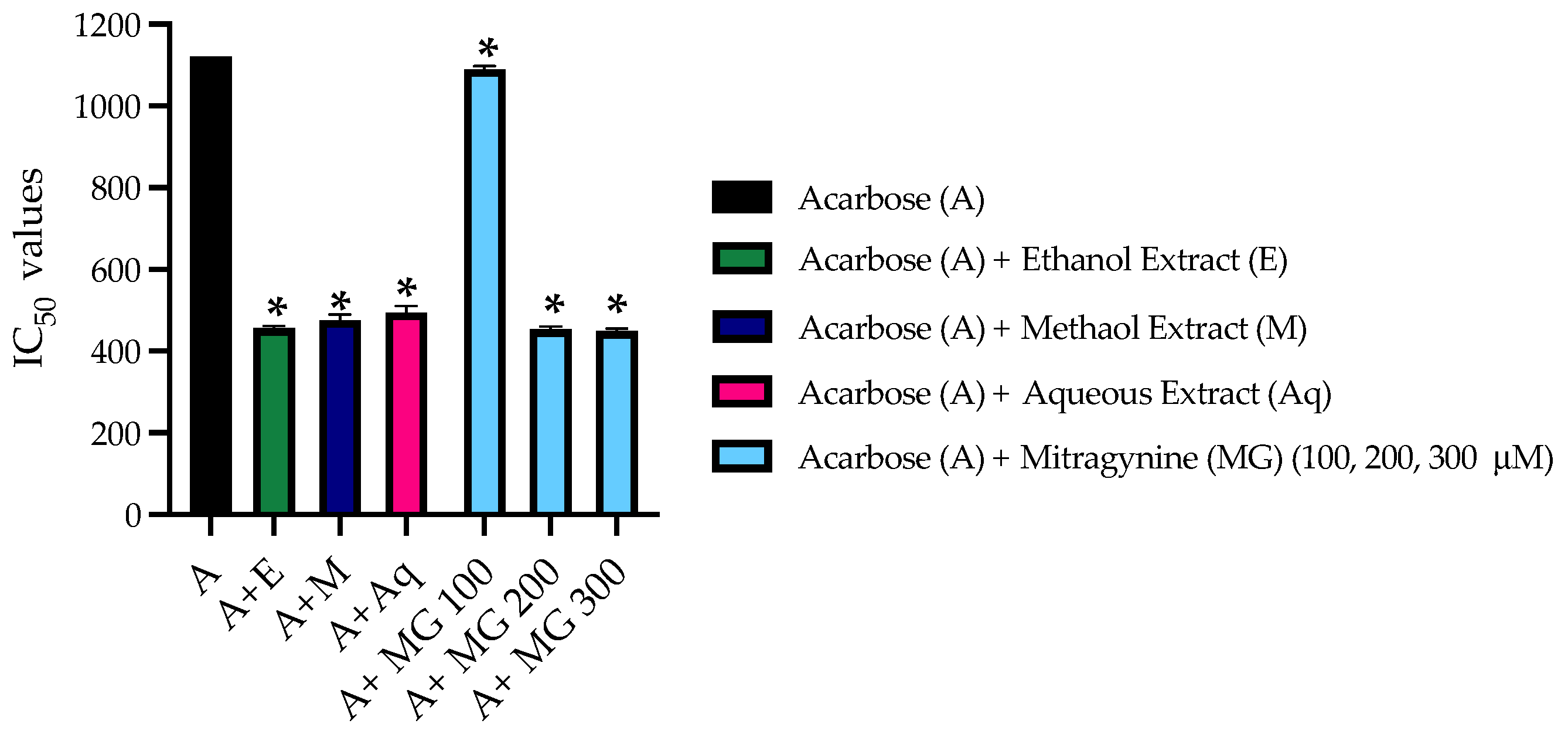
3.5. The Combined Inhibitory Effect of Kratom Extracts and Mitragynine on α-Glucosidase Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, M.; Jovanović, A.J.; Uskoković, A.; Grdović, N.; Dinić, S.; Vidović, S.; Poznanović, G.; Mujić, I.; Vidaković, M. Protective Effects of the Mushroom Lactarius deterrimus Extract on Systemic Oxidative Stress and Pancreatic Islets in Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2015, 2015, 576726. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Lankatillake, C.; Luo, S.; Flavel, M.; Lenon, G.B.; Gill, H.; Huynh, T.; Dias, A.D. Screening natural product extracts for potential enzyme inhibitors: Protocols, and the standardisation of the usage of blanks in α-amylase, α-glucosidase and lipase assays. Plant Methods 2021, 17, 3. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus—Screening for Pancreatic Lipase and α-Amylase Inhibition. Phytother. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as alpha-Amylase and alpha-Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Tomasik, P.; Horton, D. Enzymatic conversions of starch. Adv. Carbohydr. Chem. Biochem. 2012, 68, 59–436. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. Acarbose: Safe and Effective for Lowering Postprandial Hyperglycaemia and Improving Cardiovascular Outcomes. Open Heart 2015, 2, e000327. [Google Scholar] [CrossRef]
- Tushuizen, M.E.; Bunck, C.M.; Pouwels, P.J.; Bontemps, S.; Van Waesberghe, J.H.T.; Schindhelm, R.K.; Mari, A.; Heine, R.J.; Diamant, M. Pancreatic Fat Content and Beta-Cell Function in Men with and without Type 2 Diabetes. Diabetes Care 2007, 30, 2916–2921. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-Diabetic Activities of Phenolic Compounds in Muscadine Against Alpha-Glucosidase and Pancreatic Lipase. LWT—Food. Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Yang, M.H.; Chin, Y.-W.; Yoon, K.D.; Kim, J. Phenolic Compounds with Pancreatic Lipase Inhibitory Activity from Korean yam (Dioscorea opposita). J. Enzym. Inhib. Med. Chem. 2014, 29, 1–6. [Google Scholar] [CrossRef]
- Jaradat, N.; Zaid, A.N.; Hussein, F.; Maram, Z.; Alijammal, H.; Ayesh, O. Anti-Lipase Potential of the Organic and Aqueous Extracts of Ten Traditional Edible and Medicinal Plants in Palestine; a Comparison Study with Orlistat. Medicines 2017, 4, 89. [Google Scholar] [CrossRef]
- Harp, J.B. An Assessment of the Efficacy and Safety of Orlistat for the Long-Term Management of Obesity. J. Nutr. Biochem. 1998, 9, 516–521. [Google Scholar] [CrossRef]
- Shai, L.J.; Masoko, P.; Mokgotho, M.P.; Magano, S.R.; Mogale, A.M.; Boaduo, N.; Eloff, J.N. Yeast Alpha Glucosidase Inhibitory and Antioxidant Activities of Six Medicinal Plants Collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef]
- Gatto, L.J.; Oliveira de Bellei, G.R.; Rech, K.S.; Moura, P.F.; Gribner, C.; Merino, Z.; Avila, S.; Fatima Gaspari Dias, J.; Miguel, O.G.; Miguel, M.D. Inhibition of α-Glucosidase, Pancreatic Lipase, and Antioxidant Property of Myrcia hatschbachii D. Legrand containing Gallic and Ellagic Acids. Bol. Latinoam. 2021, 20, 226–243. [Google Scholar] [CrossRef]
- Itoh, K.; Matsukawa, T.; Murata, K.; Nishitani, R.; Yamagami, M.; Tomohiro, N.; Kajiyama, S.; Fumuro, M.; Iijima, M.; Shigeoka, S.; et al. Pancreatic Lipase Inhibitory Activity of Citrus unshiu Leaf Extract. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, D.; Yang, G.; Zheng, Y.; Guo, L. Screening of Anti-Lipase Components of Artemisia argyi Leaves Based on Spectrum-Effect Relationships and HPLC-MS/MS. Front. Pharmacol. 2021, 12, 675396. [Google Scholar] [CrossRef]
- Bhatia, A.; Singh, B.; Arora, R.; Arora, S. In vitro Evaluation of the α-Glucosidase Inhibitory Potential of Methanolic Extracts of Traditionally used Antidiabetic Plants. BMC Complement. Altern. Med. 2019, 19, 74. [Google Scholar] [CrossRef]
- Chelladurai, G.R.M.; Chinnachamy, C. Alpha Amylase and Alpha Glucosidase Inhibitory Effects of Aqueous Stem Extract of Salacia oblonga and Its GC-MS Analysis. Braz. J. Pharm. Sci. 2018, 54, e17151. [Google Scholar] [CrossRef]
- Elya, B.; Basah, K.; Mun’im, A.; Yuliastuti, W.; Bangun, A.; Septiana, E.K. Screening of α-Glucosidase Inhibitory Activity from Some Plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. J. Biomed. Biotechnol. 2012, 2012, 281078. [Google Scholar] [CrossRef] [PubMed]
- Dechakhamphu, A.; Wongchum, N. Screening for Anti-Pancreatic Lipase Properties of 28 Traditional Thai Medicinal Herbs. Asian Pac. J. Trop. Biomed. 2015, 5, 1042–1045. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Boffa, L.; Ghe, C.; Barge, A.; Muccioli, G.; Cravotto, G. Alkaloid Profiles and Activity in Different Mitragyna speciosa Strains. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef]
- Charoenratana, S.; Anukul, C.; Aramrattana, A. Attitudes Towards Kratom Use, Decriminalization and the Development of a Community-Based Kratom Control Mechanism in Southern Thailand. Int. J. Drug Policy 2021, 95, 103197. [Google Scholar] [CrossRef]
- Goh, Y.S.; Karunakaran, T.; Murugaiyah, V.; Santhanam, R.; Abu Bakar, M.H.; Ramanathan, S. Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) Leaves: Evaluation of Its Cytotoxicity and Antinociceptive Activity. Molecules 2021, 26, 3704. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Cornett, E.M.; Kaye, A.D. Kratom-Pharmacology, Clinical Implications, and Outlook: A Comprehensive Review. Pain Ther. 2020, 9, 55–69. [Google Scholar] [CrossRef]
- Hassan, Z.; Muzaimi, M.; Navaratnam, V.; Yusoff, N.H.M.; Suhaimi, W.F.; Vadivelu, R.; Vicknasingam, K.B.; Amato, D.; Horsten, S.V.; Ismail, N.I.W.; et al. From Kratom to Mitragynine and Its Derivatives: Physiological and Behavioural Effects Related to Use, Abuse, and Addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Ramanathan, S.; Murugaiyah, V.; Hamdan, M.R.; Mohd Said, M.I.; Lai, C.-S.; Mansor, S.M. A Simple HPLC–DAD Method for The Detection and Quantification of Psychotropic Mitragynine in Mitragyna speciosa (ketum) and Its Products for the Application in Forensic Investigation. Forensic. Sci. Int. 2013, 226, 183–187. [Google Scholar] [CrossRef]
- Leong Bin Abdullah, M.F.I.; Tan, K.L.; Isa, S.M.; Yusoff, N.S.; Yeou Chear, N.J.; Singh, D. Lipid Profile of Regular Kratom (Mitragyna speciosa Korth.) Users in the Community Setting. PLoS ONE 2020, 15, e0234639. [Google Scholar] [CrossRef]
- Purintrapiban, J.; Keawpradub, N.; Kansenalak, S.; Chittrakarn, S.; Janchawee, B.; Sawangjaroen, K. Study on Glucose Transport in Muscle Cells by Extracts from Mitragyna speciosa (Korth) and Mitragynine. Nat. Prod. Res. 2011, 25, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R. In vitro Effect of Mitragynine on Activity of Drug Metabolizing Enzymes, N-demethylase and Glutathuione S-transferase in Streptozotocin-Induced Diabetic Rats. Pharmacologyonline 2012, 1, 68–75. [Google Scholar]
- Lee, S.Y.; Mediani, A.; Nur Ashikin, A.H.; Azliana, A.B.S.; Abas, F. Antioxidant and α-Glucosidase Inhibitory Activities of the Leaf and Stem of Selected Traditional Medicinal Plants. Int. Food. Res. J. 2014, 21, 379–386. [Google Scholar]
- Chen, L.; Fei, S.; Olatunji, O.J. LC/ESI/TOF-MS Characterization, Anxiolytic and Antidepressant-like Effects of Mitragyna speciosa Korth Extract in Diabetic Rats. Molecules 2022, 27, 2208. [Google Scholar] [CrossRef] [PubMed]
- La-up, A.; Saengow, U.; Aramrattana, A. High Serum High-Density Lipoprotein and Low Serum Triglycerides in Kratom Users: A Study of Kratom Users in Thailand. Heliyon 2021, 7, e06931. [Google Scholar] [CrossRef]
- Vernon, L.S.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- John, B.; Sulaiman, C.T.; George, S.; Reddy, V.R.K. Spectrophotometric estimation of total alkaloids in selected justicia species. Int. J. Pharm. Pharm. Sci. 2014, 6, 647–648. [Google Scholar]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanyam, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and Pancreatic Lipase Inhibitory Activities and Glucose Uptake Stimulatory Effect of Phenolic Compounds from Dendrobium formosum. Rev. Bras. Farmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Prevete, E.; Kuypers, K.P.C.; Theunissen, E.L.; Corazza, C.; Bersani, G.; Ramaekers, J.G. A systematic review of (pre)clinical studies on the therapeutic potential and safety profile of kratom in humans. Hum. Psychopharmacol. Clin. Exp. 2021, 37, e2805. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, A.C.; Gassaway, M.M.; Kapoor, A.; Varadi, A.; Majumar, S.; Filizola, M.; Javitch, A.J.; Sames, D. Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators. J. Am. Chem. Soc. 2016, 138, 6754–6764. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Stewart, A.J.; Tao, Y.; Shao, Y.; Cui, Y.; Yue, H.; Pei, J.; Liu, Z.; Mei, L.; Yu, R.; et al. Erythritol Attenuates Postprandial Blood Glucose by Inhibiting α-Glucosidase. J. Agric. Food. Chem. 2018, 66, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Mateș, L.; Popa, D.S.; Rusu, M.E.; Fizeșan, I.; Leucuța, D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1412. [Google Scholar] [CrossRef]
- Veeramachaneni, G.K.; Raj, K.K.; Chalasani, L.M.; Annamraju, S.K.; Js, B.; Talluri, V.R. Shape Based Virtual Screening and Molecular Docking Towards Designing Novel Pancreatic Lipase Inhibitors. Bioinformation 2015, 11, 535–542. [Google Scholar] [CrossRef]
- Nguyen, P.T.V.; Huynh, H.A.; Truong, D.V.; Tran, T.-D.; Thi Vo, C.-V. Exploring Aurone Derivatives as Potential Human Pancreatic Lipase Inhibitors through Molecular Docking and Molecular Dynamics Simulations. Molecules. 2020, 25, 4657. [Google Scholar] [CrossRef]
- Swogger, M.T.; Smith, K.E.; Garcia-Romeu, A.; Grundmann, O.; Veltri, C.A.; Henningfield, J.E.; Busch, L.Y. Understanding Kratom Use: A Guide for Healthcare Providers. Front. Pharmacol. 2022, 13, 801855. [Google Scholar] [CrossRef]

| Samples | TPC (mg GAE/g) ± SD | TFC (mg QE/g) ± SD | TAC (mg ATR/g) ± SD |
|---|---|---|---|
| Ethanol | 252.92 ± 1.15 * | 26.07 ± 0.01 * | 88.04 ± 0.15 * |
| Methanol | 159.30 ± 2.01 | 13.15 ± 0.09 | 52.82 ± 0.85 |
| Aqueous | 130.58 ± 0.68 | 0.82 ± 0.02 | 5.61 ± 0.13 |
| Identification | Calculated m/z [M+H]+ | Precursor ion Experimental m/z [M+H]+ | Major Ion in MS/MS Spectra (Key Fragment Ions) | Ethanol RT, min | Methanol RT, min | Aqueous RT, min |
|---|---|---|---|---|---|---|
| Mitragynine | 399.2278 | 399.20 | 174.10 | 3.284 | 3.224 | 3.224 |
| 7-hydroxymitragynine | 415.2227 | 415.2 | 190.10 | 3.286 | 3.212 | 3.212 |
| Rutin | 611.1602 | 611.16 | 303.10 | 1.209 | 1.175 | 1.175 |
| Quercetin | 303.0508 | 303.05 | 229.00 | 1.217 | 1.223 | 1.223 |
| Compounds | Amount (mg/g) ± SD | ||
|---|---|---|---|
| Ethanol Extract | Methanol Extract | Aqueous Extract | |
| Mitragynine | 58.75 ± 0.21 * | 35.87 ± 1.01 | 3.85 ± 0.17 |
| Quercetin | 19.10 ± 0.85 * | 5.90 ± 0.14 | 1.28 ± 0.02 |
| Rutin | 11.36 ± 0.11 * | 3.19 ± 0.22 | 1.22 ± 0.05 |
| Samples | α-Glucosidase | Pancreatic Lipase | ||
|---|---|---|---|---|
| IC50 (µM) | IC50 (µg/mL) | IC50 (µM) | IC50 (µg/mL) | |
| Ethanol extract | - | 15.90 ± 1.34 * | - | 14.15 ± 1.71 * |
| Methanol extract | - | 42.12 ± 1.76 * | - | 28.38 ± 2.34 * |
| Aqueous extract | - | 69.48 ± 2.67 * | - | 41.43 ± 3.32 * |
| Mitragynine | 205.04 ± 15.11 * | 81.68 ± 1.70 * | 24.9 ± 1.38 * | 9.86 ± 0.45 * |
| Acarbose | 1121.09 ± 67.01 | 728.20 ± 7.01 | - | - |
| Orlistat | - | - | 0.84 ± 0.10 | 0.42 ± 0.05 |
| Inhibitors | α-Glucosidase | Pancreatic Lipase | ||
|---|---|---|---|---|
| Ki (mM) | Mode | Ki (µM) | Mode | |
| Mitragynine | 0.10 | noncompetitive | 14.94 | competitive |
| Acarbose | 0.28 | mixed-type | - | - |
| Orlistat | - | - | 0.24 | competitive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limcharoen, T.; Pouyfung, P.; Ngamdokmai, N.; Prasopthum, A.; Ahmad, A.R.; Wisdawati, W.; Prugsakij, W.; Warinhomhoun, S. Inhibition of α-Glucosidase and Pancreatic Lipase Properties of Mitragyna speciosa (Korth.) Havil. (Kratom) Leaves. Nutrients 2022, 14, 3909. https://doi.org/10.3390/nu14193909
Limcharoen T, Pouyfung P, Ngamdokmai N, Prasopthum A, Ahmad AR, Wisdawati W, Prugsakij W, Warinhomhoun S. Inhibition of α-Glucosidase and Pancreatic Lipase Properties of Mitragyna speciosa (Korth.) Havil. (Kratom) Leaves. Nutrients. 2022; 14(19):3909. https://doi.org/10.3390/nu14193909
Chicago/Turabian StyleLimcharoen, Thanchanok, Phisit Pouyfung, Ngamrayu Ngamdokmai, Aruna Prasopthum, Aktsar Roskiana Ahmad, Wisdawati Wisdawati, Woraanong Prugsakij, and Sakan Warinhomhoun. 2022. "Inhibition of α-Glucosidase and Pancreatic Lipase Properties of Mitragyna speciosa (Korth.) Havil. (Kratom) Leaves" Nutrients 14, no. 19: 3909. https://doi.org/10.3390/nu14193909
APA StyleLimcharoen, T., Pouyfung, P., Ngamdokmai, N., Prasopthum, A., Ahmad, A. R., Wisdawati, W., Prugsakij, W., & Warinhomhoun, S. (2022). Inhibition of α-Glucosidase and Pancreatic Lipase Properties of Mitragyna speciosa (Korth.) Havil. (Kratom) Leaves. Nutrients, 14(19), 3909. https://doi.org/10.3390/nu14193909