Trace Element Interactions, Inflammatory Signaling, and Male Sex Implicated in Reduced Growth Following Excess Oral Iron Supplementation in Pre-Weanling Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Necropsy and Hematology
2.3. Non-Heme Iron
2.4. Histopathology
2.5. Liver Gene Expression
2.6. Serum Chemokine/Cytokine Array
2.7. Zinc and Copper
2.8. Statistical Analysis
3. Results
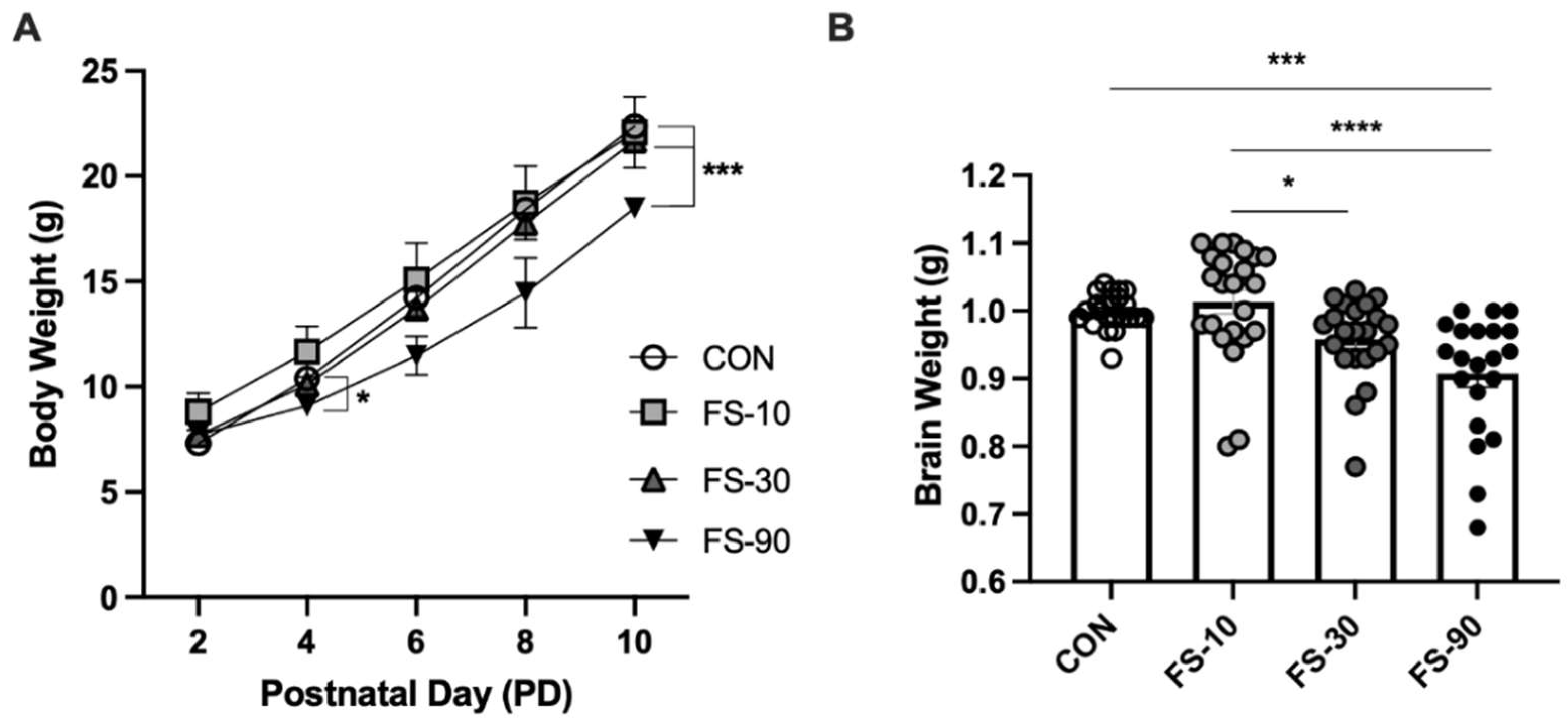
3.1. Excess FS Disrupts Growth
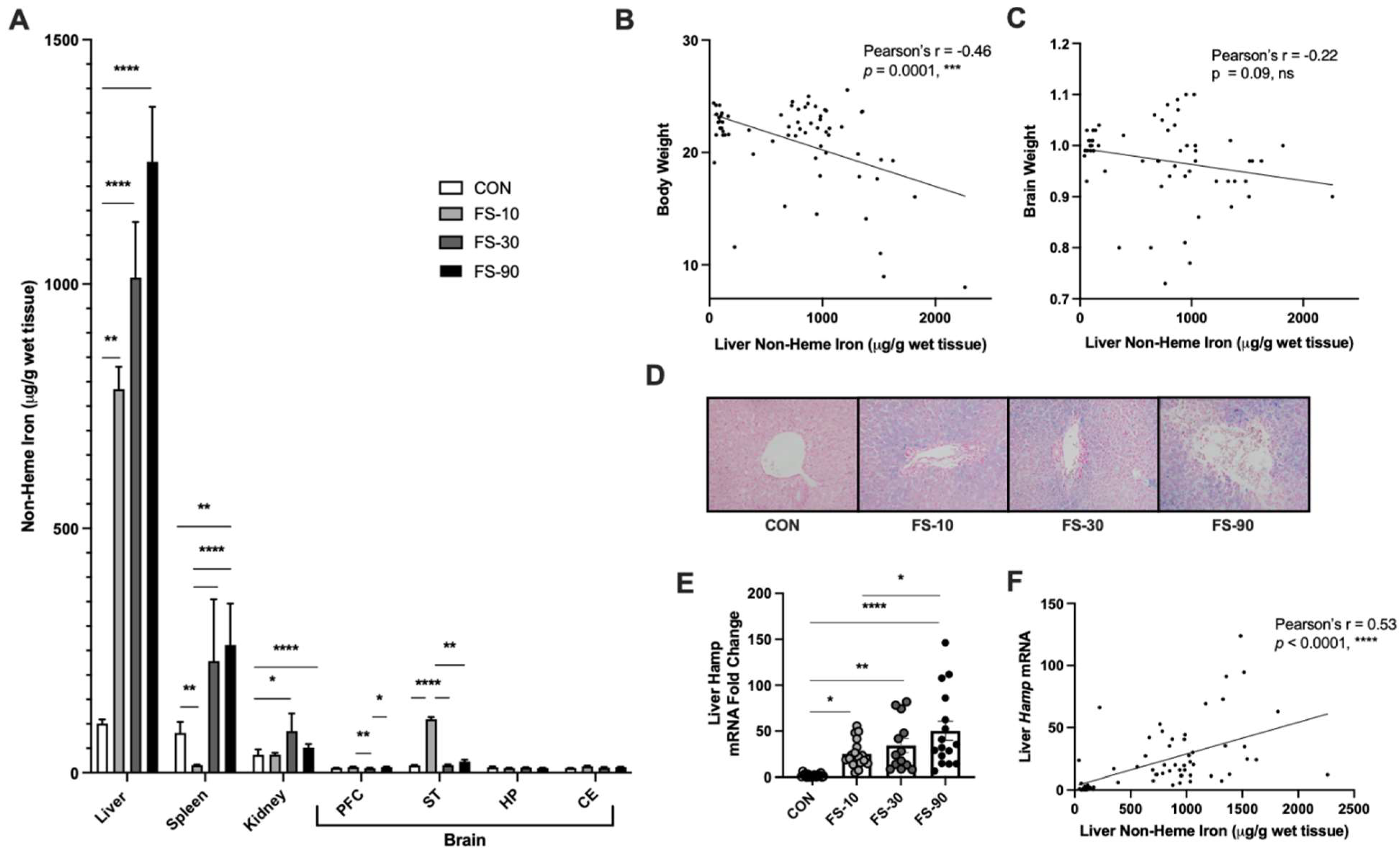
3.2. Liver Iron Loading from FS Dosing
3.3. Hematological Effects of Excess FS
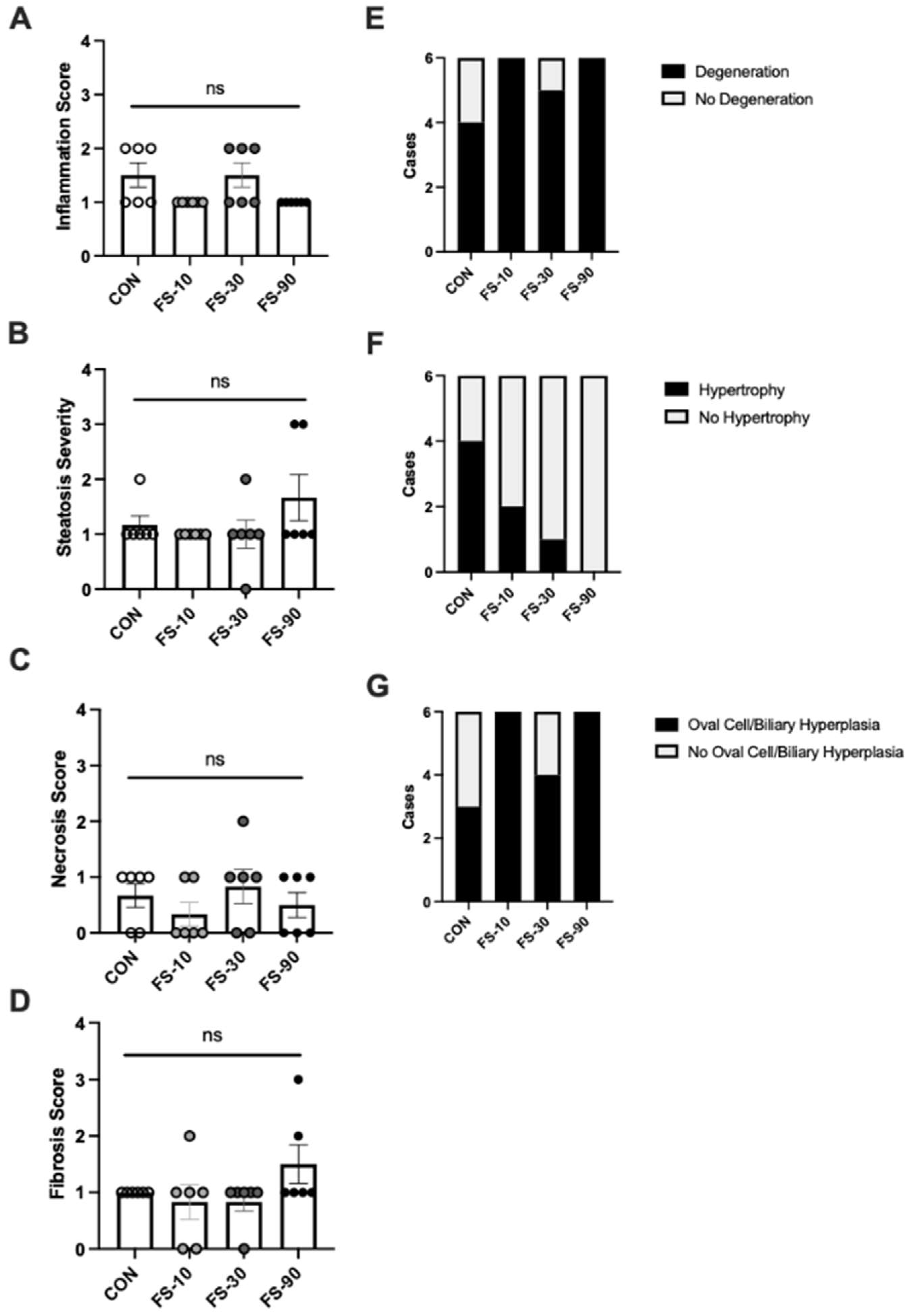
3.4. Liver Histopathology
3.5. Chemokine and Cytokine Response
3.6. Alterations to Liver Zinc and Copper rr
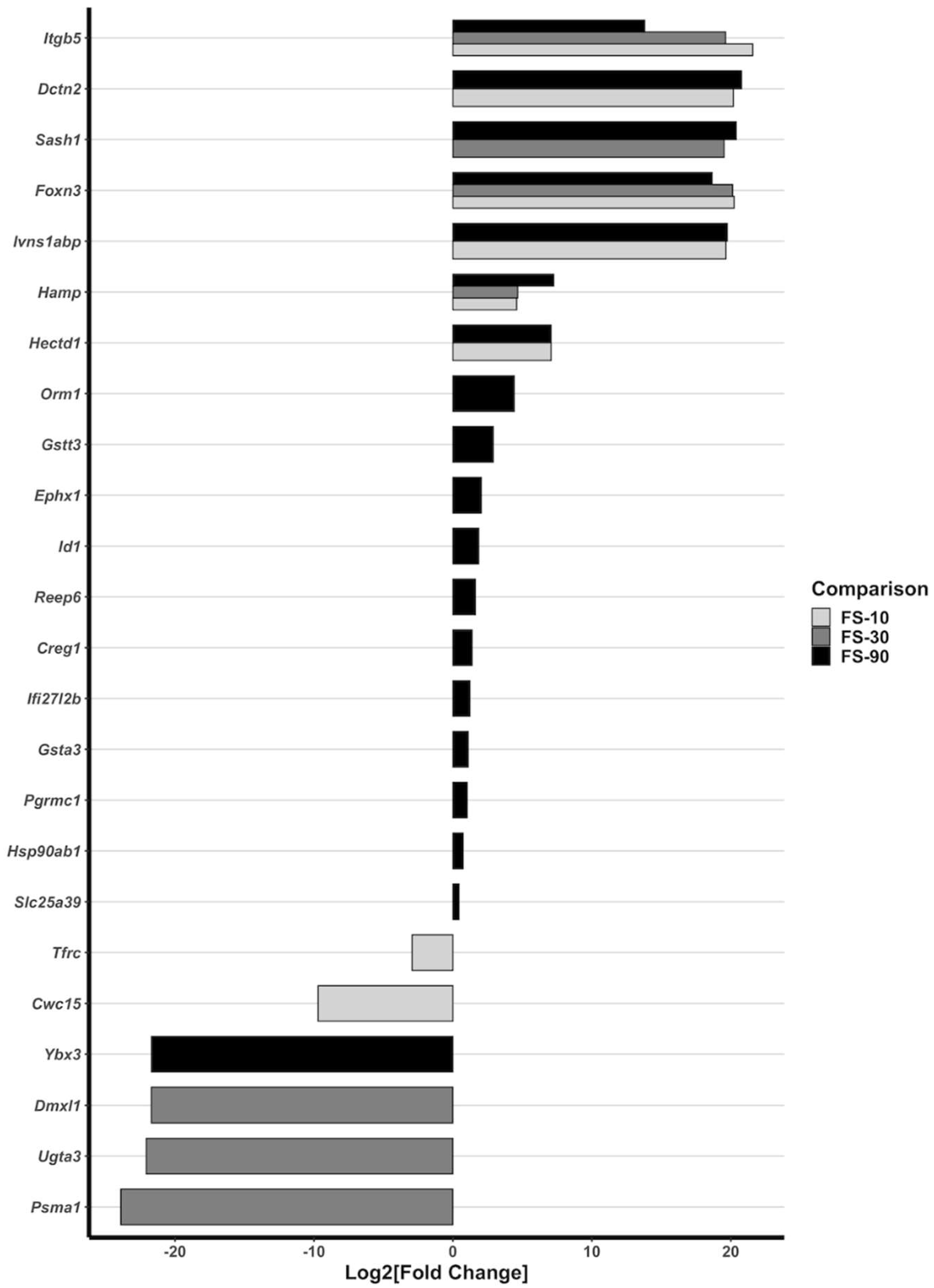
3.7. Alterations to Liver Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anemia; De Benoist, B., McLean, E., Egli, I., Cogswell, M.E., Eds.; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-159665-7. [Google Scholar]
- Burke, R.; Leon, J.; Suchdev, P. Identification, Prevention and Treatment of Iron Deficiency during the First 1000 Days. Nutrients 2014, 6, 4093–4114. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional Iron Deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- East, P.; Doom, J.R.; Blanco, E.; Burrows, R.; Lozoff, B.; Gahagan, S. Iron Deficiency in Infancy and Neurocognitive and Educational Outcomes in Young Adulthood. Dev. Psychol. 2021, 57, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Quigg, A.M.; Hurley, K.M.; Pepper, M.R. Iron Deficiency and Iron-Deficiency Anemia in the First Two Years of Life: Strategies to Prevent Loss of Developmental Potential: Nutrition Reviews©, Vol. 66, No. S1. Nutr. Rev. 2011, 69, S64–S70. [Google Scholar] [CrossRef]
- Shelov, S.P. American Academy of Pediatrics. Caring for Your Baby and Young Child: Birth to Age Five; Bantam: New York, NY, USA, 2009; ISBN 978-0-553-38630-1. [Google Scholar]
- Lonnerdal, B. Excess Iron Intake as a Factor in Growth, Infections, and Development of Infants and Young Children. Am. J. Clin. Nutr. 2017, 106, 1681S–1687S. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Excess Iron: Considerations Related to Development and Early Growth. Am. J. Clin. Nutr. 2017, 106, 1600S–1605S. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.-R.; Hayes, E.; Kalumba, K.; Biggs, B.-A. Effect of Daily Iron Supplementation on Health in Children Aged 4–23 Months: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet Glob. Health 2013, 1, e77–e86. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The Effects of Iron Fortification and Supplementation on the Gut Microbiome and Diarrhea in Infants and Children: A Review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef]
- Hare, D.J.; Cardoso, B.R.; Szymlek-Gay, E.A.; Biggs, B.-A. Neurological Effects of Iron Supplementation in Infancy: Finding the Balance between Health and Harm in Iron-Replete Infants. Lancet Child Adolesc. Health 2018, 2, 144–156. [Google Scholar] [CrossRef]
- Dewey, K.G.; Domellöf, M.; Cohen, R.J.; Landa Rivera, L.; Hernell, O.; Lönnerdal, B. Iron Supplementation Affects Growth and Morbidity of Breast-Fed Infants: Results of a Randomized Trial in Sweden and Honduras. J. Nutr. 2002, 132, 3249–3255. [Google Scholar] [CrossRef]
- Lind, T.; Seswandhana, R.; Persson, L.-A.; Lönnerdal, B. Iron Supplementation of Iron-Replete Indonesian Infants Is Associated with Reduced Weight-for-Age. Acta Paediatr 2008, 97, 770–775. [Google Scholar] [CrossRef]
- Lozoff, B. Iron-Fortified vs. Low-Iron Infant Formula: Developmental Outcome at 10 Years. Arch. Pediatr Adolesc. Med. 2012, 166, 208. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of Routine Prophylactic Supplementation with Iron and Folic Acid on Admission to Hospital and Mortality in Preschool Children in a High Malaria Transmission Setting: Community-Based, Randomised, Placebo-Controlled Trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Moya-Alvarez, V.; Cottrell, G.; Ouédraogo, S.; Accrombessi, M.; Massougbodgi, A.; Cot, M. High Iron Levels Are Associated with Increased Malaria Risk in Infants during the First Year of Life in Benin. Am. J. Trop Med. Hyg. 2017, 97, 497–503. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020; p. 786. [Google Scholar]
- Cai, C.; Granger, M.; Eck, P.; Friel, J. Effect of Daily Iron Supplementation in Healthy Exclusively Breastfed Infants: A Systematic Review with Meta-Analysis. Breastfeed Med. 2017, 12, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Brannon, P.M.; Stover, P.J.; Taylor, C.L. Integrating Themes, Evidence Gaps, and Research Needs Identified by Workshop on Iron Screening and Supplementation in Iron-Replete Pregnant Women and Young Children. Am. J. Clin. Nutr. 2017, 106, 1703S–1712S. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, I.; Paul, P.; Talib, V.H.; Ranga, S. The Effect of Iron Therapy on the Growth of Iron-Replete and Iron-Deplete Children. J. Trop Pediatr 2003, 49, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Olofin, I.; Boy, E.; Donahue Angel, M.; Rohner, F. The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 773. [Google Scholar] [CrossRef] [PubMed]
- Gahagan, S.; Yu, S.; Kaciroti, N.; Castillo, M.; Lozoff, B. Linear and Ponderal Growth Trajectories in Well-Nourished, Iron-Sufficient Infants Are Unimpaired by Iron Supplementation. J. Nutr. 2009, 139, 2106–2112. [Google Scholar] [CrossRef]
- Björmsjö, M.; Hernell, O.; Lönnerdal, B.; Berglund, S.K. Reducing Iron Content in Infant Formula from 8 to 2 Mg/L Does Not Increase the Risk of Iron Deficiency at 4 or 6 Months of Age: A Randomized Controlled Trial. Nutrients 2020, 13, 3. [Google Scholar] [CrossRef]
- Domellöf, M.; Dewey, K.G.; Cohen, R.J.; Lönnerdal, B.; Hernell, O. Iron Supplements Reduce Erythrocyte Copper-Zinc Superoxide Dismutase Activity in Term, Breastfed Infants. Acta Paediatr 2005, 94, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Berger, J.; Dijkhuizen, M.A.; Hidayat, A.; Ninh, N.X.; Utomo, B.; Wasantwisut, E.; Winichagoon, P. Combined Iron and Zinc Supplementation in Infants Improved Iron and Zinc Status, but Interactions Reduced Efficacy in a Multicountry Trial in Southeast Asia. J. Nutr. 2007, 137, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Haschke, F.; Ziegler, E.E.; Edwards, B.B.; Fomon, S.J. Effect of Iron Fortification of Infant Formula on Trace Mineral Absorption. J. Pediatric Gastroenterol. Nutr. 1986, 5, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Trace Element Nutrition of Infants--Molecular Approaches. J. Trace Elem. Med. Biol. 2005, 19, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Gahagan, S.; Delker, E.; Blanco, E.; Burrows, R.; Lozoff, B. Randomized Controlled Trial of Iron-Fortified versus Low-Iron Infant Formula: Developmental Outcomes at 16 Years. J. Pediatr 2019, 212, 124–130. [Google Scholar] [CrossRef]
- Ji, P.; Lonnerdal, B.; Kim, K.; Jinno, C.N. Iron Oversupplementation Causes Hippocampal Iron Overloading and Impairs Social Novelty Recognition in Nursing Piglets. J. Nutr. 2019, 149, 398–405. [Google Scholar] [CrossRef]
- Ji, P.; B Nonnecke, E.; Doan, N.; Lönnerdal, B.; Tan, B. Excess Iron Enhances Purine Catabolism Through Activation of Xanthine Oxidase and Impairs Myelination in the Hippocampus of Nursing Piglets. J. Nutr. 2019, 149, 1911–1919. [Google Scholar] [CrossRef]
- Hare, D.J.; Braat, S.; Cardoso, B.R.; Morgan, C.; Szymlek-Gay, E.A.; Biggs, B.-A. Health Outcomes of Iron Supplementation and/or Food Fortification in Iron-Replete Children Aged 4–24 Months: Protocol for a Systematic Review and Meta-Analysis. Syst. Rev. 2019, 8, 253. [Google Scholar] [CrossRef]
- McMillen, S.; Lönnerdal, B. Postnatal Iron Supplementation with Ferrous Sulfate vs. Ferrous Bis-Glycinate Chelate: Effects on Iron Metabolism, Growth, and Central Nervous System Development in Sprague Dawley Rat Pups. Nutrients 2021, 13, 1406. [Google Scholar] [CrossRef]
- National Research Council (US) Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of the Laboratory Rat. In Nutrient Requirements of Laboratory Animals, 4th ed.; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Torrance, J.D.; Bothwell, T.H. A Simple Technique for Measuring Storage Iron Concentrations in Formalinised Liver Samples. S. Afr. J. Med. Sci. 1968, 33, 9–11. [Google Scholar]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, P.; Chang, S.-Y.; Wu, Q.; Yu, P.; Xie, C.; Wu, W.; Zhao, B.; Gao, G.; Chang, Y.-Z. Hypobaric Hypoxia Regulates Brain Iron Homeostasis in Rats: H YPOBARIC H YPOXIA R EGULATES I RON H OMEOSTASIS. J. Cell. Biochem. 2017, 118, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Darshan, D.; Wilkins, S.J.; Frazer, D.M.; Anderson, G.J. Reduced Expression of Ferroportin-1 Mediates Hyporesponsiveness of Suckling Rats to Stimuli That Reduce Iron Absorption. Gastroenterology 2011, 141, 300–309. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing 2022. Available online: https://www.R-project.org (accessed on 24 May 2022).
- Love, M.I.; Soneson, C.; Hickey, P.F.; Johnson, L.K.; Pierce, N.T.; Shepherd, L.; Morgan, M.; Patro, R. Tximeta: Reference Sequence Checksums for Provenance Identification in RNA-Seq. PLoS Comput. Biol. 2020, 16, e1007664. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Babitt, J.L. Liver Iron Sensing and Body Iron Homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Nemes-Baran, A.D.; White, D.R.; DeSilva, T.M. Fractalkine-Dependent Microglial Pruning of Viable Oligodendrocyte Progenitor Cells Regulates Myelination. Cell Rep. 2020, 32, 108047. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the Interplay of Inflammation, Metabolism and Immune System Disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Persson, T.; Monsef, N.; Andersson, P.; Bjartell, A.; Malm, J.; Calafat, J.; Egesten, A. Expression of the Neutrophil-Activating CXC Chemokine ENA-78/CXCL5 by Human Eosinophils: CXCL5 Expression by Eosinophils. Clin. Exp. Allergy 2003, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Dauphinee, S.M.; Clayton, A.; Hussainkhel, A.; Yang, C.; Park, Y.-J.; Fuller, M.E.; Blonder, J.; Veenstra, T.D.; Karsan, A. SASH1 Is a Scaffold Molecule in Endothelial TLR4 Signaling. J. Immunol. 2013, 191, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.T.; Bolderson, E.; Adams, M.N.; Baird, A.-M.; Zhang, S.-D.; Gately, K.A.; Umezawa, K.; O’Byrne, K.J.; Richard, D.J. Activation and Cleavage of SASH1 by Caspase-3 Mediates an Apoptotic Response. Cell Death Dis. 2016, 7, e2469. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.-I.; Bowlus, C.L.; Tallkvist, J.; Lönnerdal, B. Iron Supplementation during Infancy—Effects on Expression of Iron Transporters, Iron Absorption, and Iron Utilization in Rat Pups. Am. J. Clin. Nutr. 2003, 78, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Wilkins, S.J.; Darshan, D.; Mirciov, C.S.G.; Dunn, L.A.; Anderson, G.J. Ferroportin Is Essential for Iron Absorption During Suckling, But Is Hyporesponsive to the Regulatory Hormone Hepcidin. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.C.; Gardier, R.W.; Robinson, V.B.; Bunde, C.A. Comparative Toxicology of Iron Compounds. Am. J. Med. Sci. 1961, 241, 262–302. [Google Scholar] [CrossRef]
- Whittaker, P.; Ali, S.F.; Imam, S.Z.; Dunkel, V.C. Acute Toxicity of Carbonyl Iron and Sodium Iron EDTA Compared with Ferrous Sulfate in Young Rats. Regul. Toxicol. Pharmacol. 2002, 36, 280–286. [Google Scholar] [CrossRef]
- Alexeev, E.E.; He, X.; Slupsky, C.M.; Lönnerdal, B. Effects of Iron Supplementation on Growth, Gut Microbiota, Metabolomics and Cognitive Development of Rat Pups. PLoS ONE 2017, 12, e0179713. [Google Scholar] [CrossRef] [PubMed]
- Schröder, N.; Fredriksson, A.; Vianna, M.R.; Roesler, R.; Izquierdo, I.; Archer, T. Memory Deficits in Adult Rats Following Postnatal Iron Administration. Behav. Brain Res. 2001, 124, 77–85. [Google Scholar] [CrossRef]
- Jenkins, K.J.; Hidiroglou, M. Effect of Excess Iron in Milk Replacer on Calf Performance. J. Dairy Sci. 1987, 70, 2349–2354. [Google Scholar] [CrossRef]
- Deugnier, Y. Pathology of Hepatic Iron Overload. WJG 2007, 13, 4755. [Google Scholar] [CrossRef] [PubMed]
- Sewald, K.; Mueller, M.; Buschmann, J.; Hansen, T.; Lewin, G. Development of Hematological and Immunological Characteristics in Neonatal Rats. Reprod. Toxicol. 2015, 56, 109–117. [Google Scholar] [CrossRef]
- Dubuque, S.H.; Dvorak, B.; Woodward, S.S.; McCuskey, R.S.; Kling, P.J. Iron-Deficient Erythropoiesis in Neonatal Rats. Biol. Neonate 2002, 81, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.-I.; Bowlus, C.L.; Tallkvist, J.; Lönnerdal, B. DMT1 and FPN1 Expression during Infancy: Developmental Regulation of Iron Absorption. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 285, G1153–G1161. [Google Scholar] [CrossRef] [PubMed]
- Perng, V.; Li, C.; Klocke, C.R.; Navazesh, S.E.; Pinneles, D.K.; Lein, P.J.; Ji, P. Iron Deficiency and Iron Excess Differently Affect Dendritic Architecture of Pyramidal Neurons in the Hippocampus of Piglets. J. Nutr. 2021, 151, 235–244. [Google Scholar] [CrossRef]
- Egeli, A.K.; Framstad, T. Effect of an Oral Starter Dose of Iron on Haematology and Weight Gain in Piglets Having Voluntary Access to Glutamic Acid-Chelated Iron Solution. Acta Vet. Scand 1998, 39, 359–365. [Google Scholar]
- Dong, Z.; Wan, D.; Li, G.; Zhang, Y.; Yang, H.; Wu, X.; Yin, Y. Comparison of Oral and Parenteral Iron Administration on Iron Homeostasis, Oxidative and Immune Status in Anemic Neonatal Pigs. Biol. Trace Elem. Res. 2020, 195, 117–124. [Google Scholar] [CrossRef]
- Eisa, A.M.A.; Elgebaly, L.S. Effect of Ferrous Sulphate on Haematological, Biochemical and Immunological Parameters in Neonatal Calves. Vet. Ital. 2010, 46, 329–335. [Google Scholar]
- Kohn, C.W.; Jacobs, R.M.; Knight, D.; Hueston, W.; Gabel, A.A.; Reed, S.M. Microcytosis, Hypoferremia, Hypoferritemia, and Hypertransferrinemia in Standardbred Foals from Birth to 4 Months of Age. Am. J. Vet. Res. 1990, 51, 1198–1205. [Google Scholar]
- Baeck, C.; Wehr, A.; Karlmark, K.R.; Heymann, F.; Vucur, M.; Gassler, N.; Huss, S.; Klussmann, S.; Eulberg, D.; Luedde, T.; et al. Pharmacological Inhibition of the Chemokine CCL2 (MCP-1) Diminishes Liver Macrophage Infiltration and Steatohepatitis in Chronic Hepatic Injury. Gut 2012, 61, 416–426. [Google Scholar] [CrossRef]
- Tacke, F.; Zimmermann, H.W.; Trautwein, C.; Schnabl, B. CXCL5 Plasma Levels Decrease in Patients with Chronic Liver Disease: CXCL5 Plasma Levels in Liver Disease. J. Gastroenterol. Hepatol. 2011, 26, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Liu, Y.; Dai, N.; Favara, M.; Greene, T.; Jeyaseelan, S.; Poncz, M.; Lee, J.S.; Worthen, G.S. CXCL5 Regulates Chemokine Scavenging and Pulmonary Host Defense to Bacterial Infection. Immunity 2010, 33, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, S.; Harada, K.; Niiro, H.; Taketomi, A.; Maehara, Y.; Tsuneyama, K.; Kikuchi, K.; Nakanuma, Y.; Mackay, I.R.; Gershwin, M.E.; et al. CX3CL1 (Fractalkine): A Signpost for Biliary Inflammation in Primary Biliary Cirrhosis. Hepatology 2010, 51, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.; Lönnerdal, B.; Stenlund, H.; Gamayanti, I.L.; Ismail, D.; Seswandhana, R.; Persson, L.-A. A Community-Based Randomized Controlled Trial of Iron and Zinc Supplementation in Indonesian Infants: Effects on Growth and Development. Am. J. Clin. Nutr. 2004, 80, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.; Lönnerdal, B.; Stenlund, H.; Ismail, D.; Seswandhana, R.; Ekström, E.-C.; Persson, L.-A. A Community-Based Randomized Controlled Trial of Iron and Zinc Supplementation in Indonesian Infants: Interactions between Iron and Zinc. Am. J. Clin. Nutr. 2003, 77, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Sánchez-Hernández, D.; Reza-López, S.A.; Huot, P.S.P.; Kim, Y.-I.; Anderson, G.H. High Folate Gestational and Post-Weaning Diets Alter Hypothalamic Feeding Pathways by DNA Methylation in Wistar Rat Offspring. Epigenetics 2013, 8, 710–719. [Google Scholar] [CrossRef]
- Ha, J.-H.; Doguer, C.; Wang, X.; Flores, S.R.; Collins, J.F. High-Iron Consumption Impairs Growth and Causes Copper-Deficiency Anemia in Weanling Sprague-Dawley Rats. PLoS ONE 2016, 11, e0161033. [Google Scholar] [CrossRef]
- Ha, J.-H.; Doguer, C.; Collins, J.F. Consumption of a High-Iron Diet Disrupts Homeostatic Regulation of Intestinal Copper Absorption in Adolescent Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G353–G360. [Google Scholar] [CrossRef]
- Vayenas, D.V.; Repanti, M.; Vassilopoulos, A.; Papanastasiou, D.A. Influence of Iron Overload on Manganese, Zinc, and Copper Concentration in Rat Tissues in Vivo: Study of Liver, Spleen, and Brain. Int. J. Clin. Lab. Res. 1998, 28, 183–186. [Google Scholar] [CrossRef]
- DiSilvestro, R.A.; Marten, J.T. Effects of Inflammation and Copper Intake on Rat Liver and Erythrocyte Cu-Zn Superoxide Dismutase Activity Levels. J. Nutr. 1990, 120, 1223–1227. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 Regulates the Zinc Transporter Zip14 in Liver and Contributes to the Hypozincemia of the Acute-Phase Response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.A.; Hidalgo, J.; Manso, Y.; Devisscher, L.; Laukens, D.; Lawrence, D.A. Metallothionein and Stress Combine to Affect Multiple Organ Systems. Cell Stress Chaperones 2014, 19, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 Is an Iron and Zinc Transporter Whose Cell-Surface Expression Is Up-Regulated by Cellular Iron Loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef]
- Arredondo, M.; Martínez, R.; Núñez, M.T.; Ruz, M.; Olivares, M. Inhibition of Iron and Copper Uptake by Iron, Copper and Zinc. Biol. Res. 2006, 39, 95–102. [Google Scholar] [CrossRef]
- Domellöf, M.; Lönnerdal, B.; Dewey, K.G.; Cohen, R.J.; Rivera, L.L.; Hernell, O. Sex Differences in Iron Status during Infancy. Pediatrics 2002, 110, 545–552. [Google Scholar] [CrossRef]
- Kong, W.-N.; Niu, Q.-M.; Ge, L.; Zhang, N.; Yan, S.-F.; Chen, W.-B.; Chang, Y.-Z.; Zhao, S.-E. Sex Differences in Iron Status and Hepcidin Expression in Rats. Biol. Trace Elem. Res. 2014, 160, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Bachman, E.; Li, M.; Roy, C.N.; Blusztajn, J.; Wong, S.; Chan, S.Y.; Serra, C.; Jasuja, R.; Travison, T.G.; et al. Testosterone Administration Inhibits Hepcidin Transcription and Is Associated with Increased Iron Incorporation into Red Blood Cells. Aging Cell 2013, 12, 280–291. [Google Scholar] [CrossRef]
- Beggs, L.A.; Yarrow, J.F.; Conover, C.F.; Meuleman, J.R.; Beck, D.T.; Morrow, M.; Zou, B.; Shuster, J.J.; Borst, S.E. Testosterone Alters Iron Metabolism and Stimulates Red Blood Cell Production Independently of Dihydrotestosterone. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E456–E461. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Trevisani, V.; Boncompagni, A.; Marrozzini, L.; Berardi, A.; Iughetti, L. Minipuberty: Looking Back to Understand Moving Forward. Front. Pediatr. 2021, 8, 612235. [Google Scholar] [CrossRef]
- Gabrielsen, J.S. Iron and Testosterone: Interplay and Clinical Implications. Curr. Sex Health Rep. 2017, 9, 5–11. [Google Scholar] [CrossRef]
- Armitage, A.E.; Agbla, S.C.; Betts, M.; Sise, E.A.; Jallow, M.W.; Sambou, E.; Darboe, B.; Worwui, A.; Weinstock, G.M.; Antonio, M.; et al. Rapid Growth Is a Dominant Predictor of Hepcidin Suppression and Declining Ferritin in Gambian Infants. Haematologica 2019, 104, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Patsenker, E.; Stickel, F. Role of Integrins in Fibrosing Liver Diseases. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G425–G434. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Hayman, G.T.; Wang, S.-J.; Laulederkind, S.J.F.; Hoffman, M.J.; Kaldunski, M.L.; Tutaj, M.; Thota, J.; Nalabolu, H.S.; Ellanki, S.L.R.; et al. The Year of the Rat: The Rat Genome Database at 20: A Multi-Species Knowledgebase and Analysis Platform. Nucleic Acids Res. 2020, 48, D731–D742. [Google Scholar] [CrossRef] [PubMed]
- Karanth, S.; Zinkhan, E.K.; Hill, J.T.; Yost, H.J.; Schlegel, A. FOXN3 Regulates Hepatic Glucose Utilization. Cell Rep. 2016, 15, 2745–2755. [Google Scholar] [CrossRef] [PubMed]
- Karanth, S.; Adams, J.D.; de los Angeles Serrano, M.; Quittner-Strom, E.B.; Simcox, J.; Villanueva, C.J.; Ozcan, L.; Holland, W.L.; Yost, H.J.; Vella, A.; et al. A Hepatocyte FOXN3-α Cell Glucagon Axis Regulates Fasting Glucose. Cell Rep. 2018, 24, 312–319. [Google Scholar] [CrossRef] [PubMed]

| Group Mean ± Std. Deviation | |||||
|---|---|---|---|---|---|
| Result (Units) 1 | CON | FS-10 | FS-30 | FS-90 | p-Value 2 |
| Hemoglobin (g/dL) | 8.95 ± 0.44 | 8.50 ± 0.60 | 9.15 ± 0.25 | 9.03 ± 0.31 | 0.3635 |
| Hematocrit (%) | 31.0 ± 2.9 | 28.3 ± 1.5 | 30.8 ± 0.50 | 29.8 ± 3.3 | 0.4288 |
| RBC (M/µL) | 3.62 ± 0.17 | 3.52 ± 0.23 | 3.50 ± 0.11 | 3.50 ± 0.13 | 0.7836 |
| MCV (fl) | 85.5 ± 5.1 | 80.9 ± 1.4 | 87.7 ± 2.4 | 90.4 ± 0.85 | 0.1436 |
| MCH (pg) 2 | 24.8 ± 0.17 a,b | 24.1± 0.25 a | 26.2 ± 0.22 b | 25.8 ± 0.15 a,b | <0.0001, **** |
| MCHC (g/dL) | 29.1 ± 1.8 | 29.8 ± 0.60 | 29.8 ± 0.71 | 28.6 ± 0.30 | 0.4185 |
| RDW (%) | 20.8 ± 2.2 | 18.0 ± 1.0 | 17.3 ± 0.50 † | 17.0 ± 1.0 † | 0.0080, ** |
| WBC/µL | 2938 ± 18 | 4170 ± 110 | 3403 ± 630 | 4210 ± 1300 | 0.1355 |
| WBC/µL (corrected) 3 | 2874 ± 19 a | 3910 ± 77 a,b | 3300 ± 640 a,b | 4540 ± 620 b | 0.0095, ** |
| Monocytes (%) | 4.75 ± 2.8 | 6.00 ± 2.7 | 6.25 ± 2.6 | 7.25 ± 4.3 | 0.8003 |
| Monocytes (count) | 137 ± 78 | 244 ± 130 | 204 ± 81 | 310 ± 190 | 0.4937 |
| Lymphocytes (%) | 80.0 ± 3.7 | 78.3 ± 2.5 | 63.3 ± 30 | 68.3 ± 6.6 | 0.0976 |
| Lymphocytes (count) | 2300 ± 200 | 3060 ± 570 | 2150 ± 1200 | 2700 ± 610 | 0.3158 |
| Neutrophils (%) | 14.5 ± 2.1 | 15.0 ± 1.0 | 29.8 ± 30 | 23.0 ± 6.9 | 0.0689 |
| Neutrophils (count) | 416 ± 56 | 589 ± 130 | 924 ± 82 | 948 ± 480 † | 0.0486, * |
| Name of | Group Mean (pg/mL) ± Std. Deviation | ||||
|---|---|---|---|---|---|
| Protein | CON | FS-10 | FS-30 | FS-90 | p-Value 1 |
| G-CSF | 23.5 ± 5.2 | 23.2 ± 5.9 | 23.8 ± 4.9 | 24.1 ± 8.7 | 0.9907 |
| CCL11 | 5.44 ± 4.8 | 5.32 ± 3.9 | 5.91 ± 4.5 | 7.19 ± 5.3 | 0.8525 |
| GM-CSF 2 | 9.87 ± 12 a,b | 0.00 ± 0.0 a | 3.98 ± 8.4 a,b | 17.4 ± 21 b | 0.0184, * |
| IL-1α | 226 ± 200 | 164 ± 120 | 200 ± 74 | 289 ± 240 | 0.4027 |
| Leptin2 | 39,800 ± 5500 a,b | 28,800 ± 9200 a | 55,500 ± 11,000 b | 46,300 ± 38,000 a,b | 0.0445, * |
| CCL3 | 63.5 ± 10 | 49.2 ± 7.7 | 58.2 ± 11 | 51.0 ± 18 | 0.0533 |
| IL-4 | 0.770 ± 1.6 | 0.00 ± 0.0 | 0.385 ± 1.2 | 1.75 ± 3.2 | 0.2649 |
| IL-1β | 23.6 ± 7.2 | 18.8 ± 6.4 | 20.4 ± 8.7 | 144 ± 320 | 0.0594 |
| IL-2 | 11.8 ± 14 | 18.2 ± 24 | 23.4 ± 12 | 24.5 ± 30 | 0.1524 |
| IL-6 | 58.6 ± 190 | 0.00 ± 0.0 | 58.6 ± 190 | 319 ± 740 | 0.0856 |
| EGF | 37.2 ± 42 | 22.7 ± 20 | 14.8 ± 11 | 34.0 ± 42 | 0.7772 |
| IL-13 | 0.669 ± 1.1 | 0.858 ± 1.9 | 1.20 ± 3.1 | 0.599 ± 1.9 | 0.7739 |
| IL-10 | 78.0 ± 29 | 62.1 ± 22 | 55.0 ± 16 | 254 ± 540 | 0.2956 |
| IL-12p70 | 22.7 ± 18 | 15.8 ± 14 | 32.7 ± 17 | 19.2 ± 28 | 0.1264 |
| IFNγ | 289 ± 110 | 209 ± 140 | 311 ± 95 | 180 ± 95 † | 0.0359, * |
| IL-5 | 31.9 ± 16 | 27.8 ± 17 | 43.1 ± 21 | 48.6 ± 26 | 0.1014 |
| IL-17A | 14.2 ± 2.7 | 11.6 ± 3.2 | 12.5 ± 2.6 | 14.1 ± 7.7 | 0.3261 |
| IL-18 | 900 ± 320 | 987 ± 290 | 986 ± 400 | 1310 ± 400 | 0.0654 |
| CCL22 | 1640 ± 190 a,b | 1330 ± 190 a | 1640 ± 320 a,b | 1820 ± 620 b | 0.0274, * |
| IP-10 | 361 ± 40 | 343 ± 38 | 320 ± 60 | 341 ± 81 | 0.1078 |
| CXCL1 | 145 ± 140 | 116 ± 140 | 153 ± 73 | 79.3 ± 100 | 0.3601 |
| VEGF 2 | 211 ± 21 a | 192 ± 34 a,b | 167 ± 18 b | 193 ± 44 a,b | 0.0138, * |
| CX3CL1 2 | 227 ± 18 a | 209 ± 22 a,b | 188 ± 22 b | 190 ± 25 b | 0.0018, ** |
| CXCL5 2 | 10,000 ± 2100 a | 8670 ± 1400 a,b | 8480 ± 1500 a,b | 7150 ± 1500 b | 0.0047, ** |
| CXCL2 2 | 24.2 ± 19 a | 4.46 ± 10 a,b | 0.00 ± 0.0 b | 18.1 ± 27 a,b | 0.0076, ** |
| TNFα | 4.94 ± 1.5 | 5.06 ± 1.2 | 4.58 ± 1.5 | 4.08 ± 1.1 | 0.3831 |
| CCL5 | 57,800 ± 12,000 | 62,500 ± 19,000 | 52,800 ± 9100 | 49,200 ± 16,000 | 0.2522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMillen, S.A.; Nonnecke, E.B.; Lönnerdal, B. Trace Element Interactions, Inflammatory Signaling, and Male Sex Implicated in Reduced Growth Following Excess Oral Iron Supplementation in Pre-Weanling Rats. Nutrients 2022, 14, 3913. https://doi.org/10.3390/nu14193913
McMillen SA, Nonnecke EB, Lönnerdal B. Trace Element Interactions, Inflammatory Signaling, and Male Sex Implicated in Reduced Growth Following Excess Oral Iron Supplementation in Pre-Weanling Rats. Nutrients. 2022; 14(19):3913. https://doi.org/10.3390/nu14193913
Chicago/Turabian StyleMcMillen, Shasta A., Eric B. Nonnecke, and Bo Lönnerdal. 2022. "Trace Element Interactions, Inflammatory Signaling, and Male Sex Implicated in Reduced Growth Following Excess Oral Iron Supplementation in Pre-Weanling Rats" Nutrients 14, no. 19: 3913. https://doi.org/10.3390/nu14193913
APA StyleMcMillen, S. A., Nonnecke, E. B., & Lönnerdal, B. (2022). Trace Element Interactions, Inflammatory Signaling, and Male Sex Implicated in Reduced Growth Following Excess Oral Iron Supplementation in Pre-Weanling Rats. Nutrients, 14(19), 3913. https://doi.org/10.3390/nu14193913






