Infant Feeding Choices during the First Post-Natal Months and Anthropometry at Age Seven Years: Follow-Up of a Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Measurements
2.2. Statistics
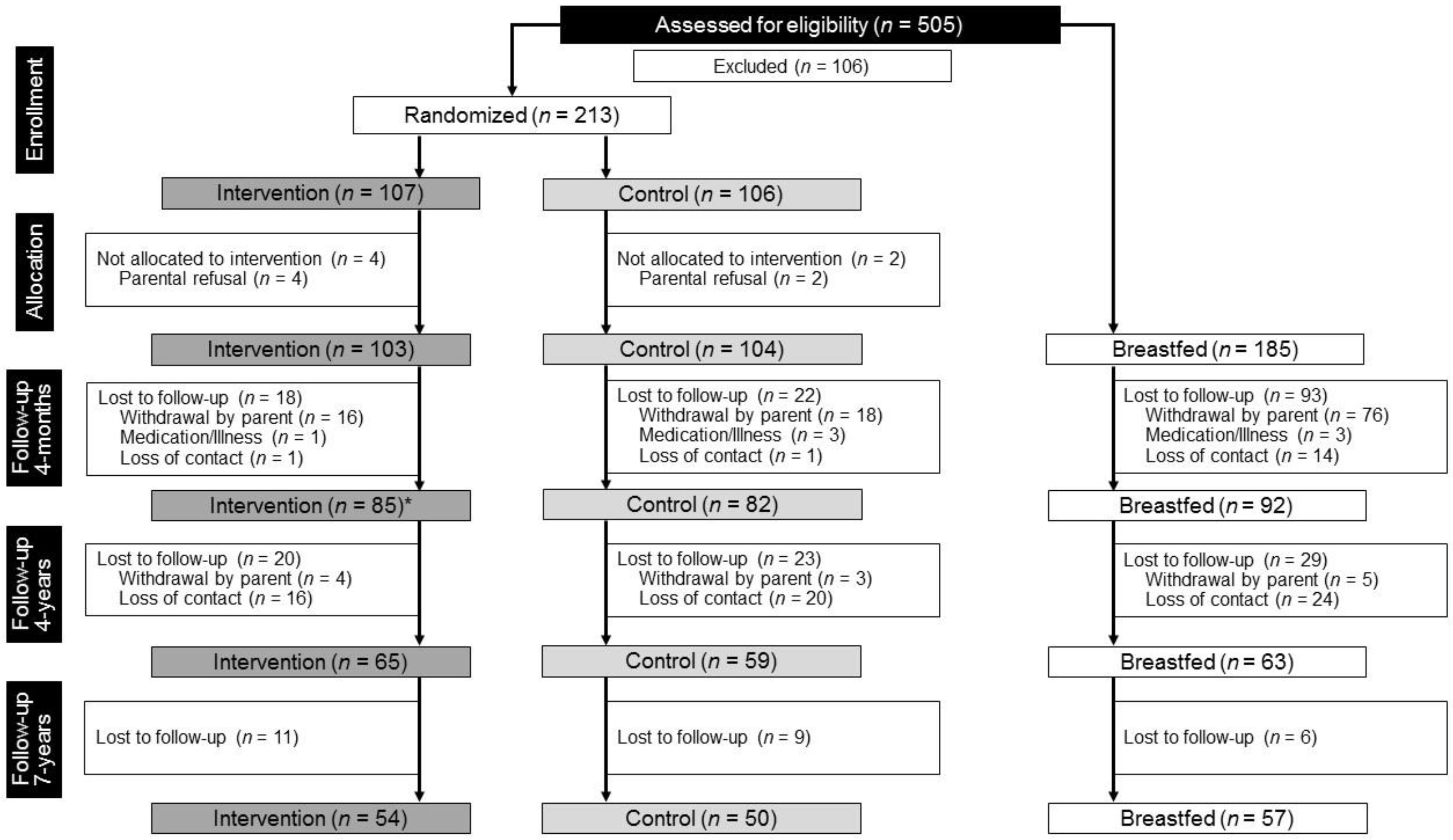
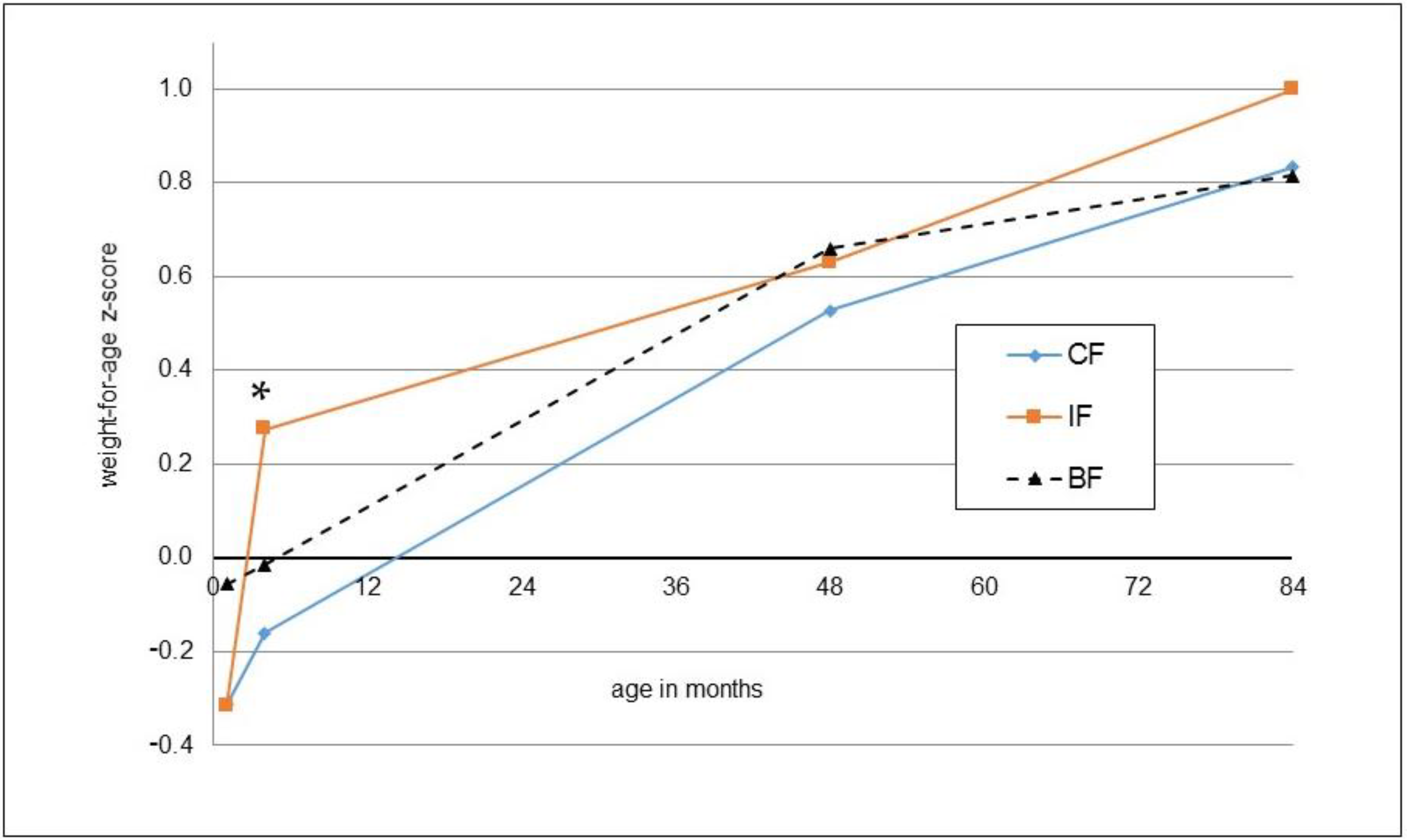
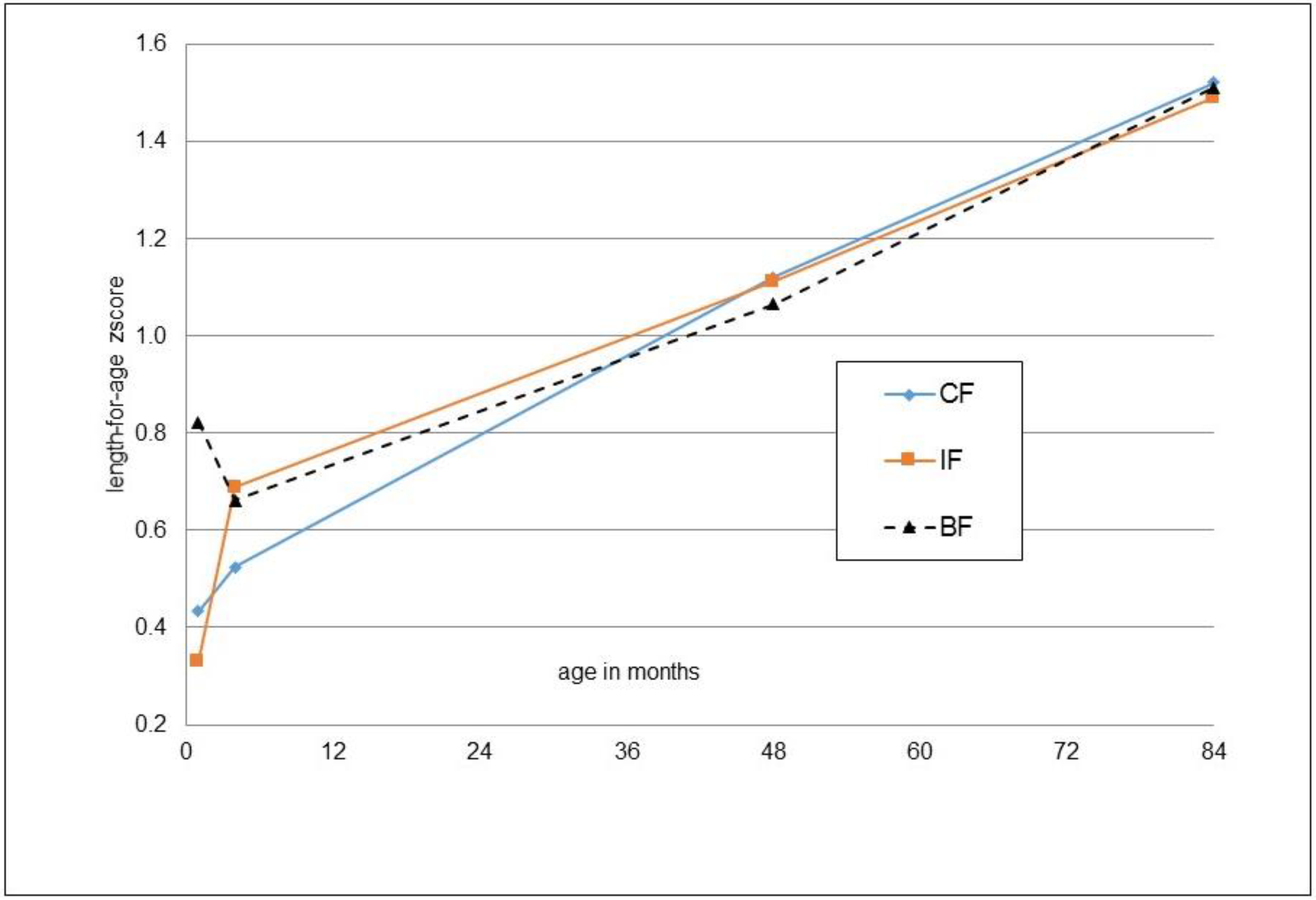
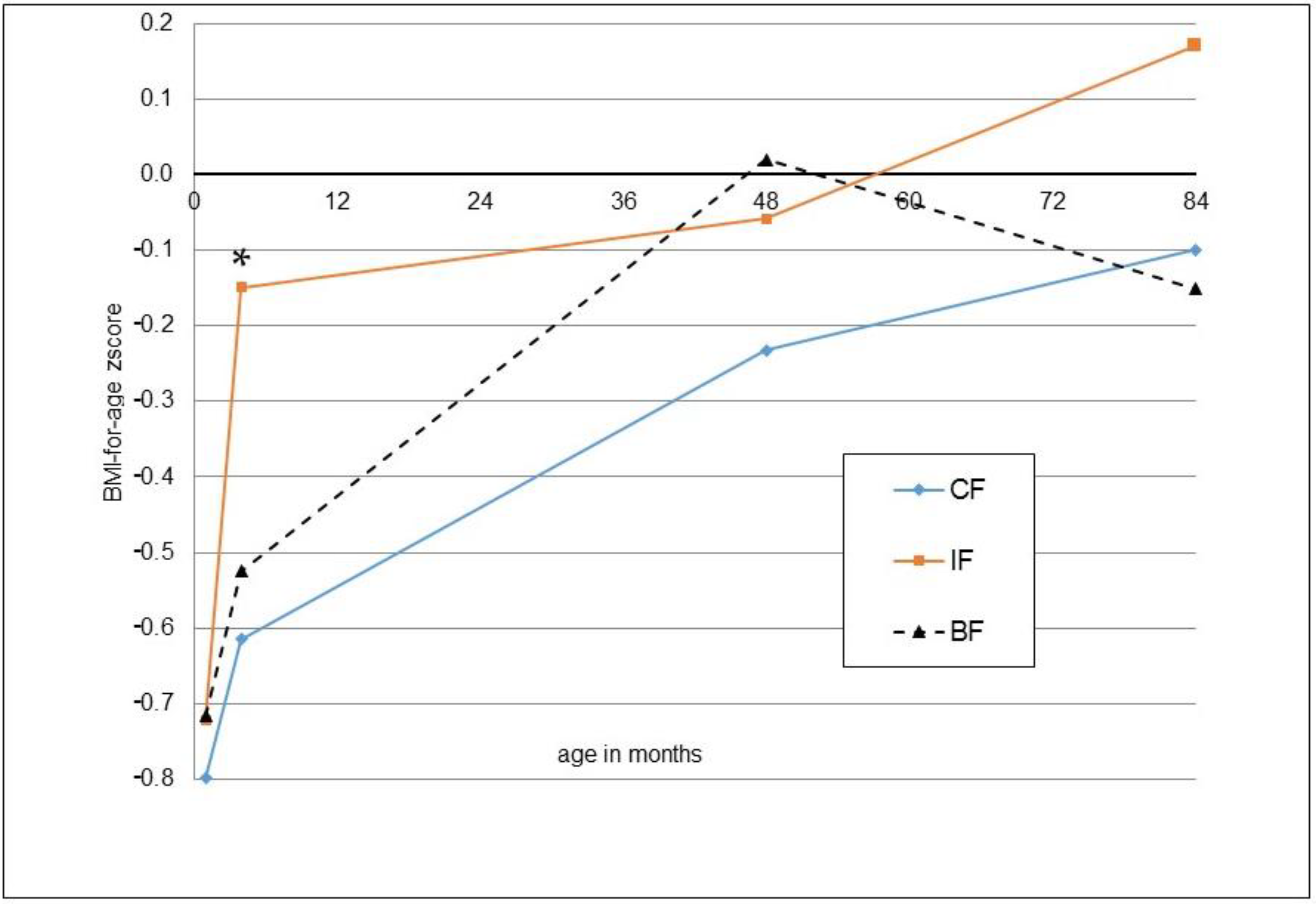
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lamb, K.E.; Grimes, C.; Laws, R.; Bolton, K.; Ong, K.K.; Campbell, K. Rapid weight gain during infancy and subsequent adiposity: A systematic review and meta-analysis of evidence. Obes. Rev. 2018, 19, 321–332. [Google Scholar] [CrossRef]
- Zheng, M.B.; Hesketh, K.D.; Vuillermin, P.; Dodd, J.; Wen, L.M.; Baur, L.A.; Taylor, R.; Byrne, R.; Mihrshahi, S.; Sly, P.D.; et al. Determinants of rapid infant weight gain: A pooled analysis of seven cohorts. Pediatr. Obes. 2022, 17, e12928. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, A.; Bergman, S.; Alm, B.; Bremander, A.; Dahlgren, J.; Roswall, J.; Staland-Nyman, C.; Almquist-Tangen, G. Nutrition- and feeding practice-related risk factors for rapid weight gain during the first year of life: A population-based birth cohort study. BMC Pediatr. 2020, 20, 507. [Google Scholar] [CrossRef]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Totzauer, M. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin. Perinatol. 2019, 43, 8. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Barebring, L.; Nwaru, B.; Dierkes, J.; Ramel, A.; Akesson, A. Protein intake in children and growth and risk of overweight or obesity: A systematic review and meta-analysis. Food Nutr. Res. 2022, 66, 1–23. [Google Scholar] [CrossRef]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef]
- Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.; Demmelmair, H.; Closa-Monasterolo, R.; Escribano Subias, J.; Scaglioni, S.; Verduci, E.; Dain, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94, 1776S–1784S. [Google Scholar]
- Rzehak, P.; Hellmuth, C.; Uhl, O.; Kirchberg, F.F.; Peissner, W.; Harder, U.; Grote, V.; Weber, M.; Xhonneux, A.; Langhendries, J.P.; et al. Rapid growth and childhood obesity are strongly associated with lysoPC(14:0). Ann. Nutr. Metab. 2014, 64, 294–303. [Google Scholar] [CrossRef]
- Fleddermann, M.; Demmelmair, H.; Grote, V.; Nikolic, T.; Trisic, B.; Koletzko, B. Infant formula composition affects energetic efficiency for growth: The BeMIM study, a randomized controlled trial. Clin. Nutr. 2014, 33, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Fleddermann, M.; Demmelmair, H.; Grote, V.; Bidlingmaier, M.; Grimminger, P.; Bielohuby, M.; Koletzko, B. Role of selected amino acids on plasma IGF-I concentration in infants. Eur. J. Nutr. 2017, 56, 613–620. [Google Scholar] [CrossRef]
- Fleddermann, M.; Demmelmair, H.; Hellmuth, C.; Grote, V.; Trisic, B.; Nikolic, T.; Koletzko, B. Association of infant formula composition and anthropometry at 4 years: Follow-up of a randomized controlled trial (BeMIM study). PLoS ONE 2018, 13, e0199859. [Google Scholar] [CrossRef]
- Brands, B.; Demmelmair, H.; Koletzko, B.; EarlyNutrition, P. How growth due to infant nutrition influences obesity and later disease risk. Acta Paediatr. 2014, 103, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Adair, L.S.; Fall, C.H.D.; Osmond, C.; Stein, A.D.; Martorell, R.; Ramirez-Zea, M.; Sachdev, H.S.; Dahly, D.L.; Bas, I.; Norris, S.A.; et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: Findings from five birth cohort studies. Lancet 2013, 382, 525–534. [Google Scholar] [CrossRef]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Vanloan, M.D.; Bemben, D.A. Skinfold Equations for Estimation of Body Fatness in Children and Youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar]
- de Castro, J.A.C.; de Lima, T.R.; Silva, D.A.S. Body composition estimation in children and adolescents by bioelectrical impedance analysis: A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Davey-Smith, G.; Gillman, M.W.; Cook, D.G. The effect of breastfeeding on mean body mass index throughout life: A quantitative review of published and unpublished observational evidence. Am. J. Clin. Nutr. 2005, 82, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Oddy, W.H.; Mearin, M.L.; Grote, V.; Mori, T.A.; Szajewska, H.; Shamir, R.; Koletzko, S.; Weber, M.; Beilin, L.J.; et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am. J. Clin. Nutr. 2017, 106, 568–580. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, D.; Belojevic, G. Prevalence of obesity among children aged 6-7 years in South-East Serbia. Obes. Rev. 2009, 10, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Jaksic, M.; Martinovic, M.; Belojevic, G.; Kavaric, N.; Asanin, B.; Samardzic, M.; Pantovic, S.; Boljevic, J. Prevalence of and contributing factors to overweight and obesity among schoolchildren of Podgorica, Montenegro. Srp. Arh. Celok. Lek. 2017, 145, 20–25. [Google Scholar] [CrossRef]
- Pavlica, T.; Rakic, R.; Sironjic, T. Changes in Morphological Characteristics During the Period 2005-2014 in a Sample of Serbian 7-Year-Old Children. Int. J. Morphol. 2017, 35, 691–697. [Google Scholar] [CrossRef]
- Totzauer, M.; Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Verduci, E.; ReDionigi, A.; Hoyos, J.; Langhendries, J.P.; Gruszfeld, D.; Socha, P.; et al. Effect of Lower Versus Higher Protein Content in Infant Formula Through the First Year on Body Composition from 1 to 6 Years: Follow-Up of a Randomized Clinical Trial. Obesity 2018, 26, 1203–1210. [Google Scholar] [CrossRef]
- Madsen, A.L.; Larnkjaer, A.; Molgaard, C.; Michaelsen, K.F. IGF-I and IGFBP-3 in healthy 9 month old infants from the SKOT cohort: Breastfeeding, diet, and later obesity. Growth Horm. IGF Res. 2011, 21, 199–204. [Google Scholar] [CrossRef]
- Larque, E.; Labayen, I.; Flodmark, C.E.; Lissau, I.; Czernin, S.; Moreno, L.A.; Pietrobelli, A.; Widhalm, K. From conception to infancy-early risk factors for childhood obesity. Nat. Rev. Endocrinol. 2019, 15, 456–478. [Google Scholar] [CrossRef]
- Ejlerskov, K.T.; Larnkjaer, A.; Pedersen, D.; Ritz, C.; Molgaard, C.; Michaelsen, K.F. IGF-I at 9 and 36 months of age-relations with body composition and diet at 3 years-the SKOT cohort. Growth Horm. IGF Res. 2014, 24, 239–244. [Google Scholar] [CrossRef]
- Ong, K.K.; Langkamp, M.; Ranke, M.B.; Whitehead, K.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B. Insulin-like growth factor I concentrations in infancy predict differential gains in body length and adiposity: The Cambridge Baby Growth Study. Am. J. Clin. Nutr. 2009, 90, 156–161. [Google Scholar] [CrossRef]
- Newton-Tanzer, E.; Demmelmair, H.; Horak, J.; Holdt, L.; Koletzko, B.; Grote, V. Acute Metabolic Response in Adults to Toddler Milk Formulas with Alternating Higher and Lower Protein and Fat Contents, a Randomized Cross-Over Trial. Nutrients 2021, 13, 3022. [Google Scholar] [CrossRef]
- Handakas, E.; Lau, C.H.; Alfano, R.; Chatzi, V.L.; Plusquin, M.; Vineis, P.; Robinson, O. A systematic review of metabolomic studies of childhood obesity: State of the evidence for metabolic determinants and consequences. Obes. Rev. 2022, 23, e13384. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, D.; Zeisel, S.H.; Orenstein, D.; German, J.B.; Field, C.J.; Teerdhala, S.; Knezevic, A.; Patil, S.; Donovan, S.M.; Lonnerdal, B. Effects of a novel high-quality protein infant formula on energetic efficiency and tolerance: A randomized trial. J. Pediatr. Gastroenterol. Nutr. 2022; ahead of print. [Google Scholar] [CrossRef]
| IF | CF | BF | |
|---|---|---|---|
| Male sex n (%) | 28 (51.9) | 28 (56.0) | 31 (54.4) |
| Maternal education * Basic/additional/tertiary n (%) | 1/38/15 (1.9/70.4/27.8) | 2/33/15 (4.0/66.0/30.0) | -/27/30 (0/47.4/52.6) |
| Mother smoked at 7-year FU n (%) | 20 (37.0) | 19 (38.0) | 16 (28.1) |
| Age mother at 7-year FU (years, mean ± SD) | 38.5 ± 5.0 | 39.0 ± 4.9 | 38.8 ± 4.5 |
| BMI mother at 7-year FU (kg/m2, mean ± SD) | 23.7 ± 3.5 | 23.6 ± 4.0 | 22.9 ± 4.2 |
| Father smoked at 7-year FU n (%) | 21 (38.9) | 16 (32.0) | 22 (38.6) |
| Age father at 7-year FU (years, mean ± SD) | 41.3 ± 5.4 | 41.8 ± 5.9 | 41.7 ± 6.3 |
| BMI father at 7-year FU (kg/m2, mean ± SD) | 27.5 ± 5.0 | 26.8 ± 3.6 | 28.0 ± 4.8 |
| IF (n = 54) | CF (n = 50) | IF vs. CF 1 | IF vs. CF 2 | BF (n = 57) | IF vs. BF 1 | CF vs. BF 1 | |
|---|---|---|---|---|---|---|---|
| Weight (kg) | 27.0 ± 5.2 | 26.2 ± 4.0 | 0.377 | 0.64 [−1.08; 2.36], p = 0.465 | 26.2 ± 5.1 | 0.382 | 0.956 |
| Weight-for-age (z-score) | 1.00 ± 1.13 | 0.83 ± 1.01 | 0.434 | 0.13 [−0.26; 0.52], p = 0.502 | 0.82 ± 1.16 | 0.402 | 0.930 |
| Height (cm) | 129.2 ± 5.3 | 129.5 ± 4.4 | 0.819 | −0.11 [−1.90; 1.69], p = 0.907 | 129.4 ± 5.2 | 0.901 | 0.914 |
| Height-for-age (z-score) | 1.49 ± 0.99 | 1.52 ± 0.83 | 0.860 | −0.01 [−0.34; 0.33], p = 0.973 | 1.51 ± 0.96 | 0.905 | 0.957 |
| Head circumference (cm) | 52.6 ± 1.8 | 52.8 ± 1.4 | 0.626 | −0.10 [−0.64; 0.44], p = 0.705 | 52.6 ± 1.3 | 0.855 | 0.431 |
| BMI (kg/m2) | 16.1 ± 2.6 | 15.6 ± 1.7 | 0.210 | 0.41 [−0.42; 1.24], p = 0.328 | 15.6 ± 2.5 | 0.268 | 0.996 |
| BMI-for-age (z-score) | 0.17 ± 1.34 | −0.10 ± 1.19 | 0.280 | 0.18 [−0.28; 0.64], p = 0.433 | −0.15 ± 1.44 | 0.227 | 0.840 |
| Body fat from BIA (%) | 15.5 ± 7.5 | 14.7 ± 6.3 | 0.523 | 0.66 [−2.01; 3.32], p = 0.626 | 13.0 ± 8.3 | 0.101 | 0.256 |
| Triceps (mm) | 12.1 ± 4.8 | 11.4 ± 3.8 | 0.425 | 0.43 [−1.11; 1.97], p = 0.582 | 11.0 ± 3.7 | 0.193 | 0.597 |
| Subscapular (mm) | 7.9 ± 4.1 | 6.8 ± 1.9 | 0.102 | 0.90 [−0.31; 2.11], p = 0.142 | 7.3 ± 3.4 | 0.424 | 0.391 |
| Body fat from skinfolds (%) 3 | 18.3 ± 6.3 | 17.2 ± 4.5 | 0.278 | 0.86 [−1.13; 2.84], p = 0.393 | 17.2 ± 5.1 | 0.295 | 0.992 |
| n | IF | n | CF | IF vs. CF a | n | BF | IF vs. BF a | CF cv. BF a | |
|---|---|---|---|---|---|---|---|---|---|
| Weight gain (g/d) | |||||||||
| 7 years–1 month | 54 | 9.0 ± 2.0 | 50 | 8.7 ± 1.6 | 0.365 | 57 | 8.6 ± 1.9 | 0.275 | 0.790 |
| 7 years–4 months | 54 | 8.2 ± 2.0 | 50 | 8.0 ± 1.6 | 0.585 | 57 | 8.0 ± 2.0 | 0.495 | 0.854 |
| 7 years–4 years | 52 | 8.1 ± 3.2 | 48 | 7.7 ± 2.5 | 0.572 | 57 | 7.4 ± 3.0 | 0.310 | 0.593 |
| Height gain (mm/d) | |||||||||
| 7 years–1 month | 54 | 0.295 ± 0.020 | 50 | 0.295 ± 0.017 | 1.000 | 57 | 0.291 ± 0.019 | 0.298 | 0.264 |
| 7 years–4 months | 54 | 0.266 ± 0.020 | 50 | 0.268 ± 0.017 | 0.615 | 57 | 0.266 ± 0.018 | 0.928 | 0.656 |
| 7 years–4 years | 52 | 0.192 ± 0.026 | 48 | 0.195 ± 0.024 | 0.485 | 57 | 0.196 ± 0.024 | 0.367 | 0.856 |
| Head circumference gain (mm/d) | |||||||||
| 7 years–1 month | 54 | 0.062 ± 0.006 | 50 | 0.062 ± 0.005 | 0.854 | 57 | 0.060 ± 0.007 | 0.152 | 0.094 |
| 7 years–4 months | 54 | 0.045 ± 0.005 | 50 | 0.046 ± 0.005 | 0.518 | 57 | 0.045 ± 0.006 | 0.881 | 0.444 |
| 7 years–4 years | 52 | 0.014 ± 0.009 | 48 | 0.015 ± 0.007 | 0.327 | 57 | 0.011 ± 0.009 | 0.082 | 0.004 |
| IF | CF | IF vs. CF a | BF | IF vs. BF a | CF cv. BF a | |
|---|---|---|---|---|---|---|
| Overweight acc. to Cole n (%) | 12 (22%) | 5 (10%) | 0.115 | 9 (16%) | 0.470 | 0.407 |
| Obesity acc. to Cole n (%) | 3 (6%) | 0 (0%) | 0.244 | 3 (5%) | 1.000 | 0.246 |
| BMI z-score > +1 | 14 (26%) | 9 (18%) | 0.355 | 11 (19%) | 0.497 | 1.000 |
| Outcome Weight-for-Age z-Score at Seven Years | |||||
| Considering maternal pre-pregnancy BMI, one-month weight z-score and diet groups (R2, corr: 0.16) | Considering maternal pre-pregnancy BMI, one-month weight z-score, diet groups and IGF-1 at the age of four months (R2, corr: 0.21) | ||||
| Beta coefficient ± SE | p-value | Beta coefficient ± SE | p-value | ||
| WZS 1 at 1 month | 0.473 ± 0.111 | 3.7 × 10−5 | WZS 1 at 1 month | 0.554 ± 0.111 | 2.0 × 10−6 |
| BMI mother | 0.072 ± 0.026 | 0.006 | BMI mother | 0.069 ± 0.027 | 0.010 |
| CF * | - | 0.140 | CF * | - | 0.986 |
| IF * | - | 0.611 | IF * | - | 0.756 |
| IGF-1 | 0.015 ± 0.004 | 0.001 | |||
| Outcome Height-for-Age z-Score at Seven Years | |||||
| Considering maternal pre-pregnancy BMI, one-month height z-score and diet groups (R2, corr: 0.14) | Considering maternal pre-pregnancy BMI, one-month height z-score, diet groups and IGF-1 at the age of four months (R2, corr: 0.21) | ||||
| HZS 2 at 1 month | 0.372 ± 0.073 | 8.8 × 10−7 | HZS 2 at 1 month | 0.421 ± 0.073 | 5.2 × 10−8 |
| BMI mother | - | 0.082 | BMI mother | 0.049 ± 0.022 | 0.027 |
| CF * | - | 0.366 | CF * | - | 0.488 |
| IF * | - | 0.429 | IF * | - | 0.733 |
| IGF-1 | 0.013 ± 0.004 | 0.001 | |||
| Outcome BMI-for-Age z-score at Seven Years | |||||
| Considering maternal pre-pregnancy BMI, one-month BMI 1 z-score and diet groups (R2, corr: 0.12) | Considering maternal pre-pregnancy BMI, one-month BMI z-score, diet groups and IGF-1 at the age of four months (R2, corr: 0.13) | ||||
| BMIZS 3 at 1 month | 0.430 ± 0.131 | 0.001 | BMIZS 3 at 1 month | 0.456 ± 0.131 | 0.001 |
| BMI mother | 0.088 ± 0.032 | 0.007 | BMI mother | 0.081 ± 0.034 | 0.017 |
| CF * | 0.871 | CF * | - | 0.764 | |
| IF * | 0.207 | IF * | - | 0.795 | |
| IGF-1 | 0.012 ± 0.006 | 0.035 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmelmair, H.; Fleddermann, M.; Koletzko, B. Infant Feeding Choices during the First Post-Natal Months and Anthropometry at Age Seven Years: Follow-Up of a Randomized Clinical Trial. Nutrients 2022, 14, 3900. https://doi.org/10.3390/nu14193900
Demmelmair H, Fleddermann M, Koletzko B. Infant Feeding Choices during the First Post-Natal Months and Anthropometry at Age Seven Years: Follow-Up of a Randomized Clinical Trial. Nutrients. 2022; 14(19):3900. https://doi.org/10.3390/nu14193900
Chicago/Turabian StyleDemmelmair, Hans, Manja Fleddermann, and Berthold Koletzko. 2022. "Infant Feeding Choices during the First Post-Natal Months and Anthropometry at Age Seven Years: Follow-Up of a Randomized Clinical Trial" Nutrients 14, no. 19: 3900. https://doi.org/10.3390/nu14193900
APA StyleDemmelmair, H., Fleddermann, M., & Koletzko, B. (2022). Infant Feeding Choices during the First Post-Natal Months and Anthropometry at Age Seven Years: Follow-Up of a Randomized Clinical Trial. Nutrients, 14(19), 3900. https://doi.org/10.3390/nu14193900






