Abstract
Purpose: this systematic review aimed to assess the effects of dietary liberalization following tetrahydrobiopterin (BH4) treatment on anthropometric measurements, nutritional biomarkers, quality of life, bone density, mental health and psychosocial functioning, and burden of care in PKU patients. Methods: the PubMed, Cochrane, and Embase databases were searched on 7 April 2022. We included studies that reported on the aforementioned domains before and after dietary liberalization as a result of BH4 treatment in PKU patients. Exclusion criteria were: studies written in a language other than English; studies that only included data of a BH4 loading test; insufficient data for the parameters of interest; and wrong publication type. Both within-subject and between-subject analyses were assessed, and meta-analyses were performed if possible. Results: twelve studies containing 14 cohorts and 228 patients were included. Single studies reported few significant differences. Two out of fifteen primary meta-analyses were significant; BMI was higher in BH4-treated patients versus controls (p = 0.02; standardized mean difference (SMD) (95% confidence interval (CI)) = −0.37 (−0.67, −0.06)), and blood cholesterol concentrations increased after starting BH4 treatment (p = 0.01; SMD (CI) = −0.70 (−1.26, −0.15)). Conclusion: there is no clear evidence that dietary liberalization after BH4 treatment has a positive effect on anthropometric measurements, nutritional biomarkers, or quality of life. No studies could be included for bone density, mental health and psychosocial functioning, and burden of care.
1. Introduction
Phenylketonuria (PKU; OMIM 261600) is an inborn error of phenylalanine (Phe) metabolism, caused by a deficiency of the Phe hydroxylase (PAH; EC 1.14.16.1) enzyme [1]. Due to this deficiency, the conversion of Phe into tyrosine is impaired, resulting in high Phe concentrations in the blood and brain. These high Phe concentrations ultimately cause severe neurological impairment that typically characterizes the phenotype of untreated PKU. Fortunately, early institution of a life-long dietary treatment that limits intake of Phe by reducing natural protein consumption is very effective in preventing severe complications. Nevertheless, even in early-treated PKU patients, outcomes still appear to be suboptimal, for example, when it comes to cognition and mental health [2], white matter [2], nutrient status [3], bone density [4], overweight and obesity [5], and growth [6].
Tetrahydrobiopterin (BH4, prescribed as sapropterin dihydrochloride) is a pharmacological treatment option that aims to further improve outcomes by increasing residual PAH activity in a subset of PKU patients. Through this mechanism, daily administration of BH4 in BH4-responsive patients may result in lower blood Phe concentrations, and possibly in the increased stability of blood Phe concentrations and/or increased dietary Phe tolerance [7,8,9,10,11]. In BH4-responsive patients with suboptimal blood Phe concentrations, BH4 can thus be used to improve metabolic control. In already well-controlled BH4-responsive patients, however, BH4 treatment is used to partially or completely replace dietary treatment, resulting in significant dietary changes [12]. Typically, patients who respond to BH4 have a milder phenotype compared to BH4-unresponsive patients [13]. Their milder phenotype is caused by a higher level of residual PAH activity, which means that these patients generally require less natural protein restriction, even without BH4 treatment.
It has been hypothesised that relaxation of the diet following the start of BH4 treatment, accompanied by a reduced need for protein substitutes, may improve many outcomes in PKU patients. It is often said that dietary liberalization may improve quality of life in patients, by decreasing the burden of the strict diet [14,15]. Furthermore, it has been hypothesized that dietary changes with BH4 treatment improve growth [16] and nutrition [17]. Possible other clinical benefits of dietary relaxation relate to mental health, psychosocial functioning, burden of care (i.e., the burden experienced by parents and caregivers), and bone health. However, even though BH4 has currently been available for more than ten years in many countries, the effect of dietary relaxation associated with BH4 treatment on these outcomes requires examination. Therefore, we performed a systematic review and meta-analyses to assess the effects of dietary liberalization following BH4 treatment on anthropometric measurements, nutritional biomarkers, quality of life, bone density, mental health and psychosocial functioning, and burden of care in patients with PKU.
2. Materials and Methods
2.1. Use of Guidelines
This manuscript was written using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [18]. Compliance with these guidelines is described in Supplementary File S1. The review was not registered and the study protocol was not published.
2.2. Search Strategy
The PubMed, Cochrane, and Embase databases were searched up to 7 April 2022. Both medical subject headings and text words were used. The search strategy was generated together with a librarian. The full search criteria are available in Supplementary File S2.
2.3. Eligibility Criteria
The online tool Rayyan® was used to manage the screening process [19]. After deleting duplicate references, studies were screened to select eligible studies for the systematic review. To be eligible for inclusion, studies needed to report on: (1) patients with PKU; (2) treatment with BH4 in responsive patients, resulting in dietary liberalization (as measured by an increase in dietary Phe or natural protein intake); and (3) anthropometric measurements, nutritional biomarkers, quality of life, bone density, mental health and psychosocial functioning, and/or burden of care, both before and after starting BH4 treatment. Studies were excluded based on the following exclusion criteria: (1) written in a language other than English; (2) containing data only on a short-term BH4 loading test for determining (potential) BH4 responsiveness; (3) insufficient data for outcome parameters (lacking means with standard deviations (SDs) and lacking any statistical between-subject or within-subject analyses); and (4) case reports, case series, and/or conference abstracts.
2.4. Study Selection
Study selection for the systematic review was conducted in two steps. First, titles and/or abstracts were screened. Second, possible eligible studies were screened by reading the full text. Each step was independently performed by two reviewers (R.A.F.E. and A.M.J.v.W.). Agreement was compared after each step, and disagreements were resolved through discussion until full consensus was reached. After inclusion in the systematic review, studies could be included in one or more meta-analyses if they reported sufficient data on the outcome parameters.
2.5. Data Collection and Data Items
Following the study selection, we collected all data that related to the outcome domains of interest. In addition to outcome data, we collected study and cohort characteristics (study country, study design, type of control group, number of patients, gender, age, length of follow-up, change in blood Phe concentrations, change in dietary Phe intake, change in protein substitute intake). Data from the included studies were collected from their main manuscripts, Supplementary Materials, and online trial data registers (e.g., clinicaltrials.gov, accessed on 11 August 2021). Study authors were contacted for additional information when necessary. Data extraction for meta-analyses was performed by two independent researchers (R.A.F.E. and A.M.J.v.W.) using data collection forms.
2.6. Data Analysis
With respect to our objective, two types of analyses were relevant: (1) within-subject analyses, i.e., comparisons between the outcome at baseline (before the start of BH4 treatment) and the outcome after a certain follow-up period of BH4 treatment; and (2) between-subject analyses, i.e., comparisons between the outcome in BH4-treated patients versus the outcome in a control group (e.g., PKU patients not treated with BH4), after a certain follow-up period. These analyses are discussed separately in this manuscript.
Meta-analyses were performed if at least two studies or cohorts had similar outcome parameters and reported on those parameters in a manner suitable for a meta-analysis (i.e., reporting a mean, standard deviation, and number of patients/controls). A random-effects model was used for all meta-analyses. For anthropometric outcomes specifically, only data presented in the form of age-corrected z-scores (or SD-scores) were used. All meta-analyses were performed using RevMan 5.
For our primary meta-analyses (Table 1), we used the outcome data from the last point of follow-up, since this study was focused on longer-term effects. In addition, we performed secondary meta-analyses using only short-term data (≤6 months of BH4 treatment, using the closest data point up to 6 months) and using only long-term data (≥5 years of BH4 treatment, using the last data point). All meta-analyses were additionally stratified for different age groups: <12 years old, 12 to 18 years old, and ≥18 years old. The results of the secondary meta-analyses and stratifications according to age are shown in the Supplementary File S3; when these analyses showed additional significant results (compared to the primary meta-analysis with all age groups combined), it is reported in this manuscript. Furthermore, forest plots of all primary meta-analyses are displayed in Supplementary File S4.

Table 1.
Overview of the results from the primary meta-analyses. Means for cohort characteristics are weighted by the number of patients per cohort, and are based on full cohort data as presented in Table 2. ↑, higher after BH4 treatment (within-subject analysis) or higher in BH4-treated patients after follow-up compared to a control group (between-subject analysis). N/r, not reported.
2.7. Risk of Bias and Certainty of Evidence
The risk of bias in individual studies was assessed using the Quality In Prognosis Studies (QUIPS) tool [20], which defines six possible areas of bias: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analyses and reporting. Risk of bias can be scored as low, moderate, or high for each area. Two researchers (R.A.F.E. and A.M.J.v.W.) independently assessed the risk of bias in each paper for each general outcome type, after which agreement was compared and disagreements were resolved through discussion.
In addition to evaluating the risk of bias in individual studies, reporting bias was also assessed. This was achieved by evaluating the funnel plots of the primary meta-analyses and considering any signs of bias.
Certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [21]. Each individual outcome was assessed and scored as having either ‘very low’, ‘low’, ‘moderate’, or ‘high’ certainty of evidence. This evaluation was conducted by two independent researchers (R.A.F.E. and A.M.J.v.W.) in a process similar to that used with the QUIPS tool.
3. Results
3.1. Study Selection
After removing duplicate publications, a total of 1276 publications were screened (Figure 1). Full texts of 79 reports were ultimately assessed for eligibility, of which 67 were excluded (Supplementary File S5), including 3 papers with overlapping data sets [22,23,24].
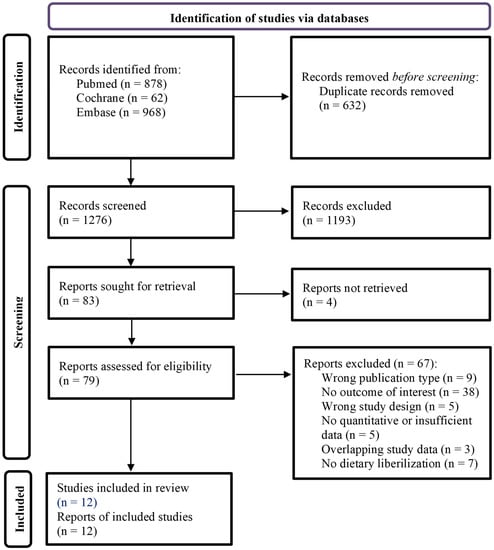
Figure 1.
Flowchart of the study inclusion.
3.2. Study Characteristics
Twelve studies were included (Table 2). These studies contained fourteen cohorts, since two studies both described two separate cohorts that were analysed individually. First, the study from Aldámiz-Echevarría et al. described two separate cohorts with respect to follow-up time (two years versus five years) [25]. Second, the extension study from Muntau et al. reported on two cohorts with different treatment strategies relating to a previous randomized controlled trial; this difference manifested itself especially with regard to the change in dietary Phe intake after BH4 treatment [24,26].
The included studies contained patients from 12 different countries. Ten of the twelve studies were prospective, and seven studies included a control group that consisted of PKU patients who were not treated with BH4. In total, the 14 cohorts included 228 patients. The median number of patients per cohort was 14 (range: 6–36). The median percentage of female patients per cohort was 45 (range: 0–67). Mean or median age (reported in 11 cohorts) was below 12 years in seven cohorts, and 12 to 18 years in four cohorts. The median follow-up time after starting BH4 treatment was 24 months (range: 3–62 months).
Following the start of BH4 treatment, blood Phe concentrations (reported in 12 cohorts) decreased in seven cohorts and increased in five, although most changes were not significant. Dietary Phe intake increased in all 14 cohorts, reaching significance in 10 cohorts. The mean change in protein substitute intake decrease in all nine cohorts for which this was reported; in three cohorts, this change was found to be significant.

Table 2.
1 Last available moment of follow-up was used. 2 Or natural protein intake. 3 Body weight not taken into account. 4 Body weight taken into account. 5 Numbers not reported in manuscript; read from graph/figure. 6 Mean follow-up time (different follow-up times for different patients). Underlined results denote significant changes within the group of BH4-treated patients; if not underlined, there was either no significant change, or statistical analysis was not performed or reported. N/r, not reported. P, prospective. R, retrospective.
Table 2.
1 Last available moment of follow-up was used. 2 Or natural protein intake. 3 Body weight not taken into account. 4 Body weight taken into account. 5 Numbers not reported in manuscript; read from graph/figure. 6 Mean follow-up time (different follow-up times for different patients). Underlined results denote significant changes within the group of BH4-treated patients; if not underlined, there was either no significant change, or statistical analysis was not performed or reported. N/r, not reported. P, prospective. R, retrospective.
| Study (Reference) | Main Study Characteristics | Characteristics of BH4-Treated Patients at Baseline | Direct Effects of BH4 Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Study Design | Type of Control Group | Patient Number | Gender (% Female) | Age, Mean ± SD (Years) | Follow-Up Time (Months) | Change 1 in Blood Phe Concentrations | Change 1 in Dietary Phe Intake 2 | Change 1 in Protein Substitute Intake | |
| Lambruschini 2005 [27] | Spain | P | None | 11 | 64 | 5.0 ± 4.2 | 12 | 16% increase | 4.3-fold increase 3 | 100% decrease |
| Singh 2010 [28] | USA | P | None | 6 | 0 | n/r | 24 | 10% decrease 5 | 3.3-fold increase 4 | 84% decrease |
| Ziesch 2012 [29] | Germany | P | Non-BH4-treated PKU patients | 8 | 50 | 11.1 ± 4.4 | 3 | 7% increase | 3.4-fold increase 3 | n/r |
| Aldámiz-Echevarría 2013 (1) [25] | Spain | R | Non-BH4-treated PKU patients | 36 | 50 | 5.0 ± 4.6 | 24 | 43% increase | 1.4-fold increase 4 | 44% decrease |
| Aldámiz-Echevarría 2013 (2) [25] | 10 | 40 | 5.2 ± 3.1 | 60 | 42% increase | 1.2-fold increase 4 | 57% decrease | |||
| Demirdas 2013 [30] | The Netherlands | P | Non-BH4-treated PKU patients | 10 | n/r | 13.8 ± 9.7 | n/r | n/r | 4.1-fold increase 3 | n/r |
| Douglas 2013 [31] | USA | P | Non-BH4-treated PKU patients | 11 | n/r | n/r | 12 | 33% decrease 5 | 3.8-fold increase 3,5 | 85% decrease5 |
| Scala 2015 [32] | Italy | P | None | 17 | n/r | n/r | 62 6 | 53% increase | 1.7-fold increase 3 | n/r |
| Tansek 2016 [33] | Slovenia | P | None | 9 | n/r | 6.2 ± 3.1 | 24 | 5% decrease | 3.2-fold increase 3 | 93% decrease |
| Feldmann 2017 [34] | Germany | P | Non-BH4-treated PKU patients | 20 | 35 | 12.5 | 6 | n/r | 2.6-fold increase 4 | 42% decrease |
| Brantley 2018 [35] | USA | P | Healthy controls and non-BH4-treated PKU patients | 18 | 44 | 16.6 ± 10.3 | 12 | 23% decrease | 1.5-fold increase | 66% decrease |
| Evers 2018 [36] | The Netherlands | R | Non-BH4-treated PKU patients | 21 | 67 | 13.1 ± 9.2 | 60 | 3% decrease 5 | 1.5-fold increase 4 | 68% decrease |
| Muntau 2021 (1) [26] | Austria, Belgium, Czech Republic, Germany, Italy, The Netherlands, Slovakia, Turkey, UK | P | None | 25 | 40 | 1.7 ± 1.0 | 36 | 6% decrease 5 | 2.0-fold increase5 | n/r |
| Muntau 2021 (2) [26] | 26 | 46 | 1.7 ± 1.0 | 36 | 12% decrease 5 | 1.1-fold increase 5 | n/r | |||
3.3. Results of the Systematic Review and Meta-Analyses
3.3.1. Anthropometric Measurements
Results of Individual Studies
Anthropometric measurements were investigated in nine cohorts. In total, 5 out of 41 analyses revealed significant results. Weight [36], BMI [32], height [28], and brachial adipose area [27] were found to have significantly increased in single cohorts (Table 3). In contrast, in one cohort, weight was lower in BH4-treated patients after the follow-up than in non-BH4-treated PKU patients [25].

Table 3.
Results of within-subject (baseline versus last point of follow-up during BH4 treatment) and between-subject (BH4-treated group versus control group at last point of follow-up) analyses for different parameters regarding anthropometric measurements. Cohort characteristics are taken from Table 2. =, no significant change (within-subject analyses) of no significant difference (between-subject analyses); ↑, significant increase or significantly higher in BH4-treated group (if not already significantly higher at baseline); ↓, significant decrease or significantly lower in BH4-treated group (if not already significantly lower at baseline). N/r, not reported.
Results from Meta-Analyses
One out of nine of the primary meta-analyses performed showed a significant result: BMI was significantly higher following BH4 treatment compared to non-BH4-treated PKU patients (Table 1). Furthermore, a secondary meta-analysis for short-term between-subject differences showed a significant (p = 0.01) result that indicated higher weight in BH4-treated patients, whereas the primary and long-term meta-analyses for this parameter revealed no significant results.
3.3.2. Nutritional Biomarkers
Results of Individual Studies
Nutritional biomarkers (not including blood Phe concentrations) were assessed in five cohorts. Out of 43 analyses, 10 were significant (Table 4). Significant increases were found in cholesterol [36], transthyretin [28], selenium [27], and haematocrit [28] (all single cohorts), and haemoglobin [28,36] (two cohorts). One cohort showed decreases in methylmalonic acid [36] (indicating better intracellular vitamin B12 status) and phosphate [36]. Brantley et al. reported significant differences for vitamin B12, but only for BH4-treated patients <18 years, in whom vitamin B12 concentrations significantly dropped after starting BH4 treatment and were also lower when compared to non-BH4-treated PKU patients [35].

Table 4.
Results of within-subject (baseline versus last point of follow-up during BH4 treatment) and between-subject (BH4-treated group versus control group at last point of follow-up) analyses for different parameters regarding anthropometric measurements. Cohort characteristics are taken from Table 2. =, no significant change (within-subject analyses) of no significant difference (between-subject analyses); ↑, significant increase or significantly higher in BH4-treated group; ↓, significant decrease or significantly lower in BH4-treated group. * No significant difference for patients > 18 years, but a significant difference for patients < 18 years. N/r, not reported.
Results from Meta-Analyses
One of the three primary meta-analyses was significant (Table 1). This analysis indicated an increase in cholesterol in BH4-treated patients.
3.3.3. Quality of Life
Results of Individual Studies
Quality of life was investigated in four cohorts. Studies investigating quality of life using generic questionnaires found no significant differences between total scores (Table 5) [29,30,34]. With quality of life subscales, only one significant result was reported in one study: self-esteem (proxy report) was significantly higher in BH4-treated patients compared to non-BH4-treated PKU patients (p = 0.030) [29].

Table 5.
Results of within-subject (baseline versus last point of follow-up during BH4 treatment) and between-subject (BH4-treated group versus control group at last point of follow-up) analyses for total scores of quality of life questionnaires. Cohort characteristics are taken from Table 2. =, no significant change (within-subject analyses) of no significant difference (between-subject analyses); ↑, significant increase or significantly higher in BH4-treated group (if not already significantly higher at baseline). N/r, not reported.
Demirdas et al. additionally measured quality of life with questionnaire for patients with a chronic illness, but this did not demonstrate a significant within-subject or between-subject difference in quality of life [30]. Only Douglas et al. used a PKU-specific questionnaire, and reported an increase in quality of life after 12 months of BH4 treatment [31]. Furthermore, this study also reported significant within-subject improvement in the subscales ‘impact’ and ‘satisfaction’ [31]. These improvements were significantly associated with increased Phe tolerance.
One study assessed parental quality of life (Table 5) [34]. This study performed a between-subject analysis but found no difference in the total scores, although it was reported that the subscale ‘emotional stability’ was significantly higher in the BH4 group (p = 0.037).
Results from Meta-Analyses
Meta-analyses could only be performed with data from studies that assessed quality of life through generic questionnaires. None of the four meta-analyses showed significant results (Table 1).
3.3.4. Bone Density, Mental Health and Psychosocial Functioning, and Burden of Care
No studies could be included for bone density, mental health and psychosocial functioning, and burden of care.
3.4. Assessment of Risk of Bias Assessment and Certainty of Evidence
3.4.1. Risk of Bias in Individual Studies
Risk of bias was low or moderate for most areas in most studies (Supplementary File S6). Of the 108 total ratings, 53 were low (49.1%), 54 were moderate (50.0%), and one was high (0.9%). Moderate risks of bias often existed for the domains ‘study participation’ and ‘study confounding’, whereas bias ‘statistical analysis and reporting’ was usually low.
3.4.2. Risk of Reporting Bias
The funnel plots of the primary meta-analyses are given in Supplementary File S7. Although interpretation is hindered due to the low number of publications for most topics, we did not find clear signs of reporting bias.
3.4.3. Certainty of Evidence
Certainty of evidence, assessed using the GRADE method, was “low” or “very low” for all parameters. This was due to the mostly observational study designs, low sample sizes, and inconsistent outcomes for some parameters (Supplementary File S8).
4. Discussion
A synthetic form of BH4, sapropterin dihydrochloride (Kuvan™), was the first pharmaceutical treatment option approved for PKU. Following its approval by the FDA [37] and EMA [38], and its subsequent recommendations by the American and European guidelines for use in responsive patients [15,39], BH4 has become part of standard PKU care. In many BH4-responsive patients, BH4 treatment results in a higher dietary Phe tolerance. While it has previously been hypothesized that such dietary relaxation has beneficial effects, this systematic review found no clear evidence for improvements in anthropometric measurements, nutritional biomarkers, quality of life, bone density, mental health and psychosocial functioning, and burden of care.
We will first address the strengths and limitations of the methodology we used for this systematic review. Since we aimed to collect evidence on all of the possibly relevant parameters, we assessed all data related to the outcome domains from the included studies. While this clearly resulted in a less focused systematic review, we considered it our only option for giving a broad overview of the evidence on the effects of BH4 treatment. In line with these considerations, our inclusion and exclusion criteria were broad (e.g., we did not select studies based on follow-up time or patients’ age), creating a rather heterogenous sample of cohorts. While we have homogenized our results by performing several types of secondary meta-analysis, the method behind our study selection prevents us from coming to more specific or detailed conclusions.
In this systematic review, we assessed outcomes related to six domains: anthropometric measurements, nutritional biomarkers, quality of life, bone density, mental health and psychosocial functioning, and burden of care. Although outcomes are, for the most part, comparable to those of a healthy population, problems have previously been noted in this domains among PKU patients [2,3,4,6,40], thus leaving room for improvement. However, the findings of this systematic review indicate that (1) for many domains of interest, insufficient data exist; and (2) for domains with sufficient data, few signs of improvement following dietary relaxation due to BH4 treatment are seen (Table 6). Considering this latter finding, only two primary meta-analyses showed significant results. However, these analyses indicated a higher BMI compared to non-BH4-treated PKU patients and an increase in blood cholesterol concentrations following BH4 treatment, which are not positive changes.

Table 6.
Overview of our main findings, and recommendations for care and future research on dietary liberalization, resulting from adjuvant treatment options such as BH4, in PKU patients. BMI, body mass index; DEXA, dual-energy X-ray ab-sorptiometry; PKU, phenylketonuria; QoL, quality of life.
For anthropometric measurements, it has been hypothesized that an increase in intact protein intake could impact height and growth [16]. However, this was not found in our meta-analysis. As was the case with other outcomes, this could be due to limitations in the data, such as small sample sizes, mixed age groups, and follow-up periods that were too short. However, it may also indicate that dietary liberalization does not improve this outcome, since these measurements were generally already normal prior to starting BH4 treatment. For weight and BMI, the meta-analysis found significantly higher BMI but not weight in BH4-treated PKU patients compared to non-BH4-treated PKU patients. This discrepancy appears to be at least partly driven by the first cohort in the study from Aldamiz et al., in which BH4-treated patients had a higher BMI z-score but a lower weight z-score compared to dietary-treated PKU patients, in contrast to the other cohorts that showed higher BMI and weight in BH4-treated patients [25]. A secondary meta-analysis for short-term effects showed that weight was significantly higher compared to non-BH4-treated PKU patients, but this was not observed in the long-term analysis. With regard to within-subject studies, one study reported an increase in BMI after BH4 treatment, but this was not the case in other studies and was hence not replicated in our within-subject meta-analysis. Despite the significant findings for weight and BMI, mean/median weight and mean/median BMI in BH4-treated patients typically remained within one standard deviation of age- and gender-adjusted reference data, with the exception of two cohorts (weight z-score of 1.21 [36]; BMI z-score of 1.27 [33]). Although these results are somewhat conflicting, it could be hypothesized that relaxing the diet in BH4-treated patients may result in unhealthy food choices in some patients, especially in the short term [41,42], and therefore influences body weight. However, most of the studies included in this systematic review lack the specific dietary data necessary to test this hypothesis. Only Singh et al. reported relevant dietary data: in line with the fact that weight remained stable in their cohort, intake of total calories, fats and proteins had not significantly changed in BH4-treated patients [28]. Other studies, not included in this systematic review, similarly showed no increase in intake of total proteins, carbohydrates, fats, or calories [41,42]. Nevertheless, our findings do underline the importance of continued, or possibly even intensified, nutritional counselling and education to ensure a balanced diet following BH4-related dietary relaxation.
Regarding nutritional biomarkers, many were only assessed in single studies and meta-analyses could thus often not be performed. For cholesterol, however, a within-subject meta-analysis could be performed, which indicated increased blood cholesterol concentrations in BH4-treated patients. This is somewhat in line with the results we found for weight and BMI, and may result from a less healthy diet following BH4 treatment. Furthermore, while several studies did report lower dietary intake, in some cases even the below recommended amounts, of several micronutrients [35,41,42], these dietary findings were generally not mirrored by changes in the biomarker concentrations of these micronutrients. In fact, the significant results found in single studies typically indicated an improvement, although these changes are possibly related to ageing effects (e.g., haemoglobin generally increases in children).
Quality of life is probably one of the most important outcome measures with regard to dietary relaxation with the use of BH4. In contrast to experiences in practice, the meta-analysis did not show any improvement in quality of life following BH4 treatment. There are several possible explanations for this. First, this observation might be an example of hedonic adaptation, i.e., the psychological phenomenon describing the relative stability of someone’s happiness, irrespective of both positive and negative life events [43]. Moreover, it may also be explained by a ceiling effect: BH4 treatment is most effective in patients with a relatively mild phenotype [13], in whom quality of life is already very good [44]. In addition, many BH4-treated patients cannot fully liberalize their diet, meaning that they may still experience the negative consequences of dietary treatment. Lastly, these counterintuitive results may be caused by a methodological problem relating to the sensitivity of quality of life questionnaires. The only study that used a PKU-specific questionnaire actually found an increase in quality of life after 12 months of BH4 treatment, which was related to increased Phe tolerance [31]. Since this result is in line with reported patient experiences in clinical practice, it can be concluded that general quality of life questionnaires are not always sensitive enough for PKU patients, as they do not seem to capture the daily burden of a strict diet. While general questionnaires may suffice to detect relatively large differences, the use of a PKU-specific questionnaire is recommended over non-specific questionnaires to be able to find relatively subtle changes in quality of life [45].
For bone density, mental health and psychosocial functioning, and burden of care, we could not include any studies that assessed the effects of BH4 treatment in a longitudinal manner. Although some of these domains may partly overlap with quality of life, no conclusions can be drawn for these outcomes. For example, burden of care refers in particular to emotional, financial, and social problems faced by caregivers. Although these problems may in part be covered by parental quality of life, for which we could include one study, the effect of BH4 treatment on this domain specifically deserves attention in the future. In line with the scarce data for some other parameters, the complete lack of studies for bone density, mental health and psychosocial functioning, and burden of care underline the need for additional research on the effects of dietary liberalization.
Although this parameter lay outside of the scope of our study, two of the included studies investigated IQ and reported similar IQ levels after dietary relaxation [26,27]. This was expected, since blood Phe concentrations did not change dramatically after BH4 treatment in these patients (382 to 442 µmol/L in Lambruschini et al. [27]; approximately 270 to 255 µmol/L and 330 to 290 µmol/L, respectively, in the two cohorts in Muntau et al. [26]). Relatedly, there are several studies in which BH4 treatment was used to decrease blood Phe concentrations rather than adjusting natural protein intake. It is noteworthy that some of these studies reported improved outcomes on executive functioning and neuro-imaging findings when Phe levels decreased with BH4 treatment (Supplementary File S9).
Since there is currently no strong evidence for improved outcomes after BH4-induced dietary relaxation, and the costs of BH4 treatment are high (data from the United States indicate that average annual costs are approximately $67,000 and $122,000 for children and adults, respectively) there are questions as to whether dietary relaxation justifies the use of BH4 in all responsive patients, especially when they still need to use protein substitutes. However, as mentioned, most quality of life reports used non-specific questionnaires that do not seem to accurately capture current practice experiences. Thus, the lack of findings in this systematic review, especially for quality of life but also for the other outcomes, could be explained by a lack of reliable data, which is partly reflected by the ‘low’ and ‘very low’ GRADE ratings. The low number of studies also prevented us from conducting more detailed analyses to control for differences in the increase in natural protein intake. Therefore, our findings do not argue against the use of BH4 to increase natural protein intake, but rather underscore the need for additional understanding of the effects of BH4-related dietary relaxation, especially on quality of life. PKU-specific quality of life questionnaires, which already are available [44,46], could be used for this purpose. Other recommendations for future research on dietary liberalization in PKU patients are summarized in Table 6.
5. Conclusions
Even though BH4 treatment can result in higher dietary Phe tolerance, this systematic review shows that there is a lack of evidence that dietary relaxation leads to improvements in anthropometric measurements, nutritional biomarkers, and quality of life. Furthermore, no studies could be included for bone density, mental health and psychosocial functioning, and burden of care. The results for quality of life are especially surprising, since they are in sharp contrast with observations from clinical practice. Overall, these results necessitate future studies on BH4 and other potential drugs that aim to allow dietary liberalization in PKU patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14183874/s1, Supplementary File S1: PRISMA checklist, Supplementary File S2: Search details, Supplementary File S3: Meta-analyses, Supplementary File S4: Forest plot of primary meta-analyses, Supplementary File S5: Excluded studies, Supplementary File S6: Risk of bias in individual studies, Supplementary File S7: Funnel plots, Supplementary File S8: GRADE ratings, Supplementary File S9: Studies with lower Phe levels.
Author Contributions
Conceptualization: R.A.F.E., A.M.J.v.W., F.J.v.S. Formal analysis: R.A.F.E., A.M.J.v.W. Investigation: R.A.F.E., A.M.J.v.W., A.M., S.C.J.H., V.L., F.J.v.S. Writing—original draft: R.A.F.E., A.M.J.v.W. Writing—review and editing: A.M., S.C.J.H., V.L.; F.J.v.S. Supervision: F.J.v.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Elmie Peters (Radboudumc Health Academy, Nijmegen, The Netherlands) for her help with the search strategy. Furthermore, we would like to acknowledge Luis Aldámiz-Echevarría, Shawn Christ, and María L. Couce for their help with the interpretation of their publications and providing additional information, and Nenad Blau and Annet Bosch for critically reading the manuscript and their suggestions.
Conflicts of Interest
R.A.F.E. has received financial support from Biomarin for attending symposia. A.M.J.v.W. is a member of the Vitaflo Dietitians Advisory Board, has received a research grant from Nutricia, honoraria from Biomarin as a speaker, and travel grants from Nutricia and Vitaflo. F.J.v.S. has received research grants, advisory board fees, and/or speaker’s honoraria from Agios, Alexion, Applied Pharma Research, Arla Food Int., Biomarin, Beatrix Research Fund, Codexis, Eurocept, ESPKU, Homology, Lucane Nestle-Codexis Alliance, Moderna, MendeliKABS, Nutricia, NPKUA, NPKUV, Orphan Europe, Pluvia Biotech, Rivium Medical BV, Sobi, Tyrosinemia Foundation, Vitaflo, Vivet, and ZONMW. A.M., S.C.J.H., and V.L declare no conflicts of interest.
References
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Prim. 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Burlina, A.P.; Lachmann, R.H.; Manara, R.; Cazzorla, C.; Celato, A.; van Spronsen, F.J.; Burlina, A. The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: A systematic review. J. Inherit. Metab. Dis. 2019, 42, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Montoya Parra, G.A.; Singh, R.H.; Cetinyurek-Yavuz, A.; Kuhn, M.; MacDonald, A. Status of nutrients important in brain function in phenylketonuria: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2018, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.J.; de Lamas, C.; Sánchez-Pintos, P.; González-Lamuño, D.; Couce, M.L. Bone Status in Patients with Phenylketonuria: A Systematic Review. Nutrients 2020, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pinto, A.; Faria, A.; Teixeira, D.; van Wegberg, A.M.J.; Ahring, K.; Feillet, F.; Calhau, C.; MacDonald, A.; Moreira-Rosário, A.; et al. Is the Phenylalanine-Restricted Diet a Risk Factor for Overweight or Obesity in Patients with Phenylketonuria (PKU)? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3443. [Google Scholar] [CrossRef]
- Ilgaz, F.; Pinto, A.; Gökmen-Özel, H.; Rocha, J.C.; Van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Karabulut, E.; Macdonald, A. Long-term growth in phenylketonuria: A systematic review and meta-analysis. Nutrients 2019, 11, 2070. [Google Scholar] [CrossRef]
- Lindegren, M.L.; Krishnaswami, S.; Reimschisel, T.; Fonnesbeck, C.; Sathe, N.A.; McPheeters, M.L. A systematic review of BH4 (Sapropterin) for the adjuvant treatment of phenylketonuria. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8, pp. 109–119. [Google Scholar]
- Somaraju, U.R.; Merrin, M. Sapropterin dihydrochloride for phenylketonuria. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Qu, J.; Yang, T.; Wang, E.; Li, M.; Chen, C.; Ma, L.; Zhou, Y.; Cui, Y. Efficacy and safety of sapropterin dihydrochloride in patients with phenylketonuria: A meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2019, 85, 893–899. [Google Scholar] [CrossRef]
- Burton, B.K.; Bausell, H.; Katz, R.; LaDuca, H.; Sullivan, C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU). Mol. Genet. Metab. 2010, 101, 110–114. [Google Scholar] [CrossRef]
- Lotz-Havla, A.S.; Weiß, K.; Schiergens, K.; Regenauer-Vandewiele, S.; Parhofer, K.G.; Christmann, T.; Böhm, L.; Havla, J.; Maier, E.M. Optical Coherence Tomography to Assess Neurodegeneration in Phenylalanine Hydroxylase Deficiency. Front. Neurol. 2021, 12, 2196. [Google Scholar] [CrossRef]
- Ilgaz, F.; Marsaux, C.; Pinto, A.; Singh, R.; Rohde, C.; Karabulut, E.; Gökmen-özel, H.; Kuhn, M.; Macdonald, A. Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1040. [Google Scholar] [CrossRef]
- Garbade, S.F.; Shen, N.; Himmelreich, N.; Haas, D.; Trefz, F.K.; Hoffmann, G.F.; Burgard, P.; Blau, N. Allelic phenotype values: A model for genotype-based phenotype prediction in phenylketonuria. Genet. Med. 2018, 21, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Röschinger, W.; Habich, M.; Demmelmair, H.; Hoffmann, B.; Sommerhoff, C.P.; Roscher, A.A. Tetrahydrobiopterin as an Alternative Treatment for Mild Phenylketonuria. N. Engl. J. Med. 2002, 347, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; Motzfeldt, K.; Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Bélanger-Quintana, A.; MacDonald, A. Optimising growth in phenylketonuria: Current state of the clinical evidence base. Clin. Nutr. 2012, 31, 16–21. [Google Scholar] [CrossRef]
- Singh, R.H.; Cunningham, A.C.; Mofidi, S.; Douglas, T.D.; Frazier, D.M.; Hook, D.G.; Jeffers, L.; McCune, H.; Moseley, K.D.; Ogata, B.; et al. Updated, web-based nutrition management guideline for PKU: An evidence and consensus based approach. Mol. Genet. Metab. 2016, 118, 72–83. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Longo, N.; Siriwardena, K.; Feigenbaum, A.; Dimmock, D.; Burton, B.K.; Stockler, S.; Waisbren, S.; Lang, W.; Jurecki, E.; Zhang, C.; et al. Long-term developmental progression in infants and young children taking sapropterin for phenylketonuria: A two-year analysis of safety and efficacy. Genet. Med. 2015, 17, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Llarena, M.; Andrade, F.; Blasco, J.; Alcalde, C.; et al. 6R-tetrahydrobiopterin treated PKU patients below 4years of age: Physical outcomes, nutrition and genotype. Mol. Genet. Metab. 2015, 115, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Burlina, A.; Eyskens, F.; Freisinger, P.; De Laet, C.; Leuzzi, V.; Rutsch, F.; Sivri, H.S.; Vijay, S.; Bal, M.O.; et al. Efficacy, safety and population pharmacokinetics of sapropterin in PKU patients <4 years: Results from the SPARK open-label, multicentre, randomized phase IIIb trial. Orphanet J. Rare Dis. 2017, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Andrade, F.; Llarena, M.; Blasco, J.; Alcalde, C.; et al. Tetrahydrobiopterin therapy vs phenylalanine-restricted diet: Impact on growth in PKU. Mol. Genet. Metab. 2013, 109, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Burlina, A.; Eyskens, F.; Freisinger, P.; Leuzzi, V.; Sivri, H.S.; Gramer, G.; Pazdírková, R.; Cleary, M.; Lotz-Havla, A.S.; et al. Long-term efficacy and safety of sapropterin in patients who initiated sapropterin at <4 years of age with phenylketonuria: Results of the 3-year extension of the SPARK open-label, multicentre, randomised phase IIIb trial. Orphanet J. Rare Dis. 2021, 16, 341. [Google Scholar] [CrossRef]
- Lambruschini, N.; Pérez-Dueñas, B.; Vilaseca, M.A.; Mas, A.; Artuch, R.; Gassió, R.; Gómez, L.; Gutiérrez, A.; Campistol, J. Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol. Genet. Metab. 2005, 86, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.H.; Quirk, M.E.; Douglas, T.D.; Brauchla, M.C. BH4 therapy impacts the nutrition status and intake in children with phenylketonuria: 2-Year follow-up. J. Inherit. Metab. Dis. 2010, 33, 689–695. [Google Scholar] [CrossRef]
- Ziesch, B.; Weigel, J.; Thiele, A.; Mütze, U.; Rohde, C.; Ceglarek, U.; Thiery, J.; Kiess, W.; Beblo, S. Tetrahydrobiopterin (BH4) in PKU: Effect on dietary treatment, metabolic control, and quality of life. J. Inherit. Metab. Dis. 2012, 35, 983–992. [Google Scholar] [CrossRef]
- Demirdas, S.; Maurice-Stam, H.; Boelen, C.C.A.; Hofstede, F.C.; Janssen, M.C.H.; Langendonk, J.G.; Mulder, M.F.; Rubio-Gozalbo, M.E.; van Spronsen, F.J.; de Vries, M.; et al. Evaluation of quality of life in PKU before and after introducing tetrahydrobiopterin (BH4); a prospective multi-center cohort study. Mol. Genet. Metab. 2013, 110, S49–S56. [Google Scholar] [CrossRef]
- Douglas, T.D.; Ramakrishnan, U.; Kable, J.A.; Singh, R.H. Longitudinal quality of life analysis in a phenylketonuria cohort provided sapropterin dihydrochloride. Health Qual. Life Outcomes 2013, 11, 218. [Google Scholar] [CrossRef]
- Scala, I.; Concolino, D.; Della Casa, R.; Nastasi, A.; Ungaro, C.; Paladino, S.; Capaldo, B.; Ruoppolo, M.; Daniele, A.; Bonapace, G.; et al. Long-term follow-up of patients with phenylketonuria treated with tetrahydrobiopterin: A seven years experience. Orphanet J. Rare Dis. 2015, 10, 14. [Google Scholar] [CrossRef]
- Tansek, M.Z.; Groselj, U.; Kelvisar, M.; Kobe, H.; Lampret, B.R.; Battelino, T. Long-term BH4 (sapropterin) treatment of children with hyperphenylalaninemia—Effect on median Phe/Tyr ratios. J. Pediatr. Endocrinol. Metab. 2016, 29, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.; Wolfgart, E.; Weglage, J.; Rutsch, F. Sapropterin treatment does not enhance the health-related quality of life of patients with phenylketonuria and their parents. Acta Paediatr. Int. J. Paediatr. 2017, 106, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Brantley, K.D.; Douglas, T.D.; Singh, R.H. One-year follow-up of B vitamin and Iron status in patients with phenylketonuria provided tetrahydrobiopterin (BH4). Orphanet J. Rare Dis. 2018, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Evers, R.A.F.; van Wegberg, A.M.J.; van Dam, E.; de Vries, M.C.; Janssen, M.C.H.; van Spronsen, F.J. Anthropomorphic measurements and nutritional biomarkers after 5 years of BH 4 treatment in phenylketonuria patients. Mol. Genet. Metab. 2018, 124, 238–242. [Google Scholar] [CrossRef]
- Drug Approval Package: Kuvan (Sapropterin Dihydrochloride) NDA #022181. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022181TOC.cfm (accessed on 11 August 2021).
- Kuvan|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kuvan (accessed on 11 August 2021).
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Walkowiak, D.; Kaluzny, L.; Bukowska-Posadzy, A.; Oltarzewski, M.; Staszewski, R.; Moczko, J.A.; Musielak, M.; Walkowiak, J. Overweight in classical phenylketonuria children: A retrospective cohort study. Adv. Med. Sci. 2019, 64, 409–414. [Google Scholar] [CrossRef]
- Thiele, A.G.; Weigel, J.F.; Ziesch, B.; Rohde, C.; Mütze, U.; Ceglarek, U.; Thiery, J.; Müller, A.S.; Kiess, W.; Beblo, S. Nutritional changes and micronutrient supply in patients with phenylketonuria under therapy with tetrahydrobiopterin (BH4). In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9, pp. 31–40. [Google Scholar]
- Thiele, A.G.; Rohde, C.; Mütze, U.; Arelin, M.; Ceglarek, U.; Thiery, J.; Baerwald, C.; Kiess, W.; Beblo, S. The challenge of long-term tetrahydrobiopterin (BH4) therapy in phenylketonuria: Effects on metabolic control, nutritional habits and nutrient supply. Mol. Genet. Metab. Rep. 2015, 4, 62–67. [Google Scholar] [CrossRef]
- Diener, E.; Lucas, R.E.; Scollon, C.N. Beyond the hedonic treadmill: Revising the adaptation theory of well-being. Am. Psychol. 2006, 61, 305–314. [Google Scholar] [CrossRef]
- Bosch, A.M.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Koledova, E.; Benmedjahed, K.; Regnault, A. Assessment of the impact of phenylketonuria and its treatment on quality of life of patients and parents from seven European countries. Orphanet J. Rare Dis. 2015, 10, 80. [Google Scholar] [CrossRef]
- Huijbregts, S.C.J.; Bosch, A.M.; Simons, Q.A.; Jahja, R.; Brouwers, M.C.G.J.; De Sonneville, L.M.J.; De Vries, M.C.; Hofstede, F.C.; Hollak, C.E.M.; Janssen, M.C.H.; et al. The impact of metabolic control and tetrahydrobiopterin treatment on health related quality of life of patients with early-treated phenylketonuria: A PKU-COBESO study. Mol. Genet. Metab. 2018, 125, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Benmedjahed, K.; Bosch, A.M. Development and psychometric validation of measures to assess the impact of phenylketonuria and its dietary treatment on patients’ and parents’ quality of life: The phenylketonuria—Quality of life (PKU-QOL) questionnaires. Orphanet J. Rare Dis. 2015, 10, 59. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).